Introduction
The presence of lymph node metastasis is considered
a risk factor for lymph node recurrence or distant metastasis in
patients with thyroid cancer (1). The
success of surgery for thyroid cancer depends on accurate
preoperative imaging, which enables complete clearance of
metastatic lymph nodes (2,3). Ultrasound remains the most important
imaging modality in the evaluation of thyroid cancer (3). In recent years, the clinical
applications using positron emission tomography (PET) have
increased significantly. PET with 18F-fluorodeoxyglucose
(FDG) is a non-invasive whole-body imaging technique used to
evaluate various types of malignancies, including thyroid cancer
(1,3–7), in terms
of tumor staging, restaging, detection of recurrence and monitoring
treatment response (8,9). However, there are limited data regarding
the role of FDG-PET in preoperative staging of thyroid cancer
(3,7,10). Only a
limited number of previous studies have evaluated the accuracy of
PET in detecting preoperative lymph node metastasis, and it has
been reported that PET does not improve the management or outcome
of thyroid cancer (3,11–13). For
the evaluation of affected lymph nodes in thyroid cancer, an
understanding of FDG avidity is important. Several studies
evaluated factors associated with the FDG avidity of the primary
thyroid tumor in cases with thyroid cancer, and the thyroid tumor
size has been reported to be associated with a higher likelihood of
positive FDG uptake (14,15). However, to date, there has been no
study assessing the factors associated with FDG avidity of the
affected lymph nodes. The aim of this study was to evaluate the
usefulness of FDG-PET for detecting metastatic lymph nodes in
differentiated thyroid cancer. Furthermore, we investigated whether
certain factors, including the size of metastasis to the lymph
nodes, were associated with FDG avidity.
Patients and methods
Patients
A total of 22 consecutive patients with
differentiated thyroid cancer who underwent FDG-PET preoperatively
were enrolled in this study. All the patients underwent
thyroidectomy at the Department of Surgical Science, Graduate
School of Medicine, Gunma University (Maebashi, Japan) from January
2008 to December 2014. Patients with incomplete clinical
information were excluded. None of the patients had distant
metastasis.
Thyroid cancer detection and
evaluation
Most cases of thyroid cancer in this study were
detected by PET during evaluation for other cancers. PET images
were qualitatively examined by expert nuclear radiologists. Maximum
standardized uptake values (SUVmax) were calculated according to a
routine clinical method. Thyroid nodule size, size of metastatic
foci to the lymph nodes, age, and serum levels of
thyroid-stimulating hormone (TSH), thyroglobulin and C-reactive
protein (CRP) were investigated as possible predictors of lymph
node metastasis.
Statistical analysis
The Fisher's exact test, χ2 test and
Student's t-test were used to compare benign and malignant groups.
Differences were considered to be statistically significant when
P<0.05.
Results
Measures of the effectiveness of
preoperative FDG-PET in the prediction of lymph node status
The mean SUVmax of metastatic lymph nodes was 4.53
(range, 0–23.5). As shown in Table I,
the sensitivity, specificity, overall accuracy and false-negative
rates for FDG uptake in the prediction of lymph node status were
40.0, 100, 72.7 and 60.0%, respectively. The false-positive rate of
FDG-PET evaluation for lymph node status was 0%.
 | Table I.Measures of the effectiveness of
preoperative positron emission tomography with
18F-fluorodeoxyglucose in the prediction of lymph node
status. |
Table I.
Measures of the effectiveness of
preoperative positron emission tomography with
18F-fluorodeoxyglucose in the prediction of lymph node
status.
| Measures | No./total (%) |
|---|
| Sensitivity | 4/10 (40.0) |
| Specificity | 12/12 (100.0) |
| Accuracy | 16/22 (72.7) |
| False-negative
rate | 6/10 (60.0) |
Patient and clinicopathological
characteristics associated with lymph node metastasis and FDG
uptake
The mean age of the patients was 58.6±13.8 years and
4 of the 22 patients were men. The mean size of the thyroid nodules
was 15.8±8.3 mm. Lymph node metastasis was diagnosed in the final
pathology in 10 of the 22 patients (45.5%). The 22 cases with
differentiated thyroid cancer were divided into two groups based on
lymph node metastasis. The patient characteristics and the results
of the univariate analysis conducted to determine the association
between the clinicopathological variables and lymph node metastasis
are shown in Table II. These
clinicopathological variables, apart from the FDG uptake of
metastatic lymph nodes, were not predictors of lymph node
metastasis from thyroid cancer. The 10 cases with lymph node
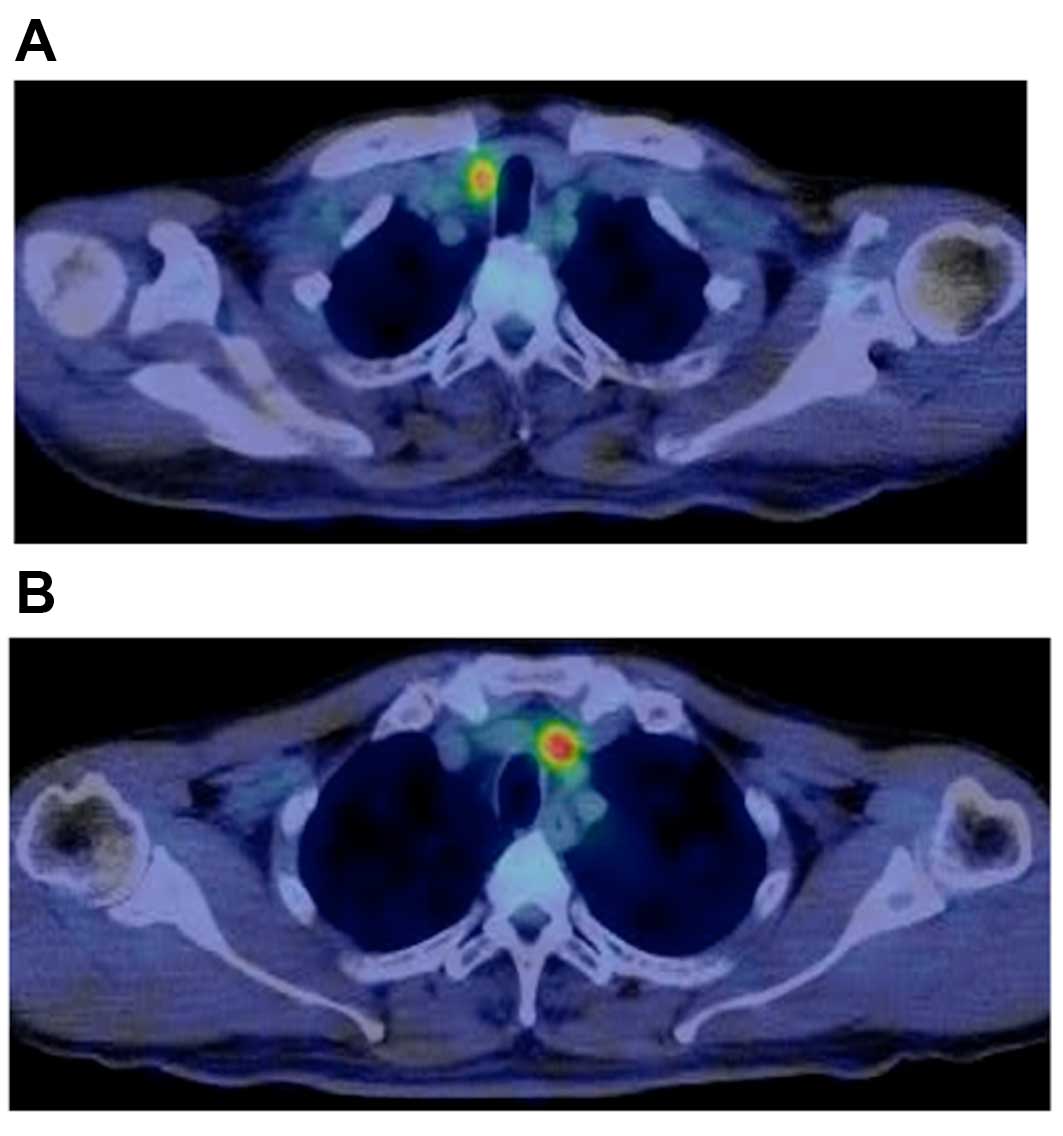
metastasis were divided into two groups based on the presence of
FDG uptake in the lymph nodes (Fig.
1). The patient characteristics and the results of the
univariate analysis conducted to determine the association between
the clinicopathological variables and FDG uptake in the lymph nodes
are shown in Table III. None of the
clinicopathological characteristics of the primary tumor, including
size and SUVmax, were significantly associated with FDG uptake.
However, the clinicopathological characteristics of the metastatic
lymph nodes were significantly associated with FDG uptake in the
lymph nodes. The analysis revealed that the size of the node
metastasis was a statistically significant factor, although the
number of lymph node metastases was not statistically
significant.
 | Table II.Patient and clinicopathological
characteristics associated with lymph node metastasis. |
Table II.
Patient and clinicopathological
characteristics associated with lymph node metastasis.
|
| Lymph node
metastasis |
|
|---|
|
|
|
|
|---|
| Characteristics | Absent (n=12) | Present (n=10) | P-value |
|---|
| Age, years | 59.0±14.5 | 58.1±13.1 | 0.792 |
| Gender |
|
| 0.956 |
| Male | 1 | 3 |
|
|
Female | 11 | 7 |
|
| Primary tumor size,
mm | 18.7±7.8 | 15.8±8.3 | 0.311 |
| SUVmax of primary
tumor | 12.5±12.9 | 4.4±4.4 | 0.634 |
| FDG uptake in lymph
nodes, n (%) | 0 (0.0) | 4 (40.0) | 0.157 |
| TSH | 1.25±0.47 | 1.81±1.00 | 0.224 |
| Tg | 170.9±421.9 | 109.1±134.5 | 0.450 |
| CRP | 0.10±0.21 | 0.17±0.35 | 0.721 |
 | Table III.Patient and clinicopathological
characteristics associated with FDG uptake in the lymph nodes. |
Table III.
Patient and clinicopathological
characteristics associated with FDG uptake in the lymph nodes.
|
| FDG uptake in
axillary lymph nodes |
|
|---|
|
|
|
|
|---|
| Characteristics | Present (n=4) | Absent (n=6) | P-value |
|---|
| Age, years | 67.8±15.4 | 51.7±6.5 | 0.024 |
| Gender |
|
| 0.333 |
| Male | 2 | 1 |
|
|
Female | 2 | 5 |
|
| TSH | 1.67±1.08 | 1.81±1.04 | 0.637 |
| Tg | 145.4±176.6 | 84.9±110.0 | 0.259 |
| CRP | 0.06±0.04 | 0.22±0.42 | 0.723 |
| Primary tumor |
|
|
|
|
Histology |
|
| – |
|
Papillary
carcinoma | 4 | 9 |
|
| Tumor
size, mm | 12.5±10.7 | 18.0±6.3 | 0.834 |
|
SUVmax | 5.8±6.7 | 3.6±2.2 | 0.236 |
| Extrathyroidal
extention, n | 1 | 1 | 0.667 |
| Lymph node
metastasis |
|
|
|
| Tumor
size, mm | 23.0±9.2 | 10.7±4.5 | 0.011 |
| Number of
node metastases | 3.8±2.8 | 5.2±2.9 | 0.717 |
FDG-PET results and size of lymph node
metastasis
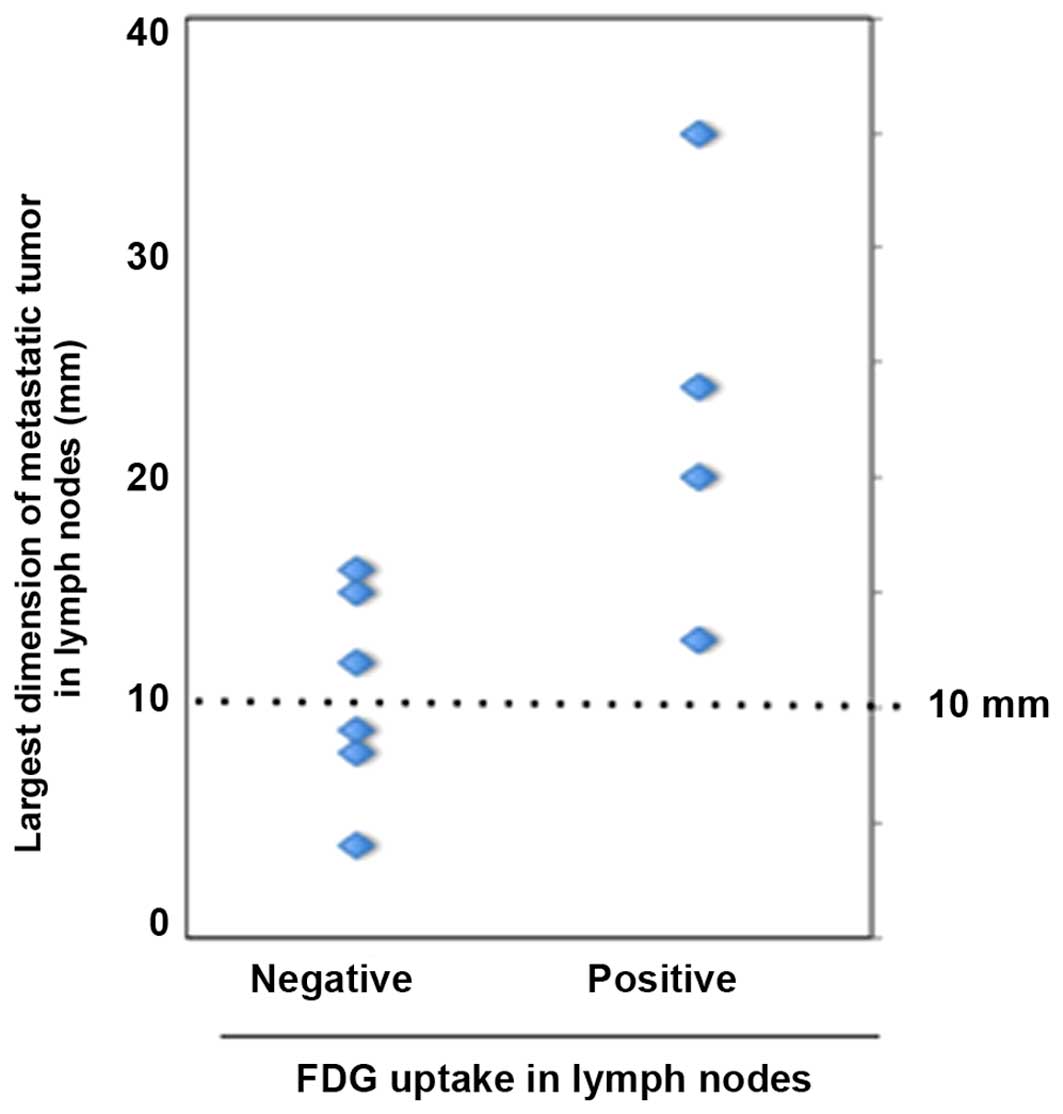
The association of metastatic tumor size in the
lymph nodes and FDG-PET evaluation results (i.e., positive or
negative) is shown in Fig. 2. The
mean largest dimension of metastatic tumors was 23.0 mm for
FDG-positive and 10.9 mm for FDG-negative cases. Thus, a
significantly larger size of metastatic tumors was observed in
FDG-positive nodes compared with that in FDG-negative nodes
(P<0.01). However, despite this marked difference in the size of
the metastases, the false-negative rate was still 50.0% in patients
with node metastases sized >10 mm.
Discussion
FDG-PET has been widely used for diagnosing,
staging, or detecting recurrence in various types of cancer;
however, its diagnostic usefulness for thyroid cancer is
controversial (1,3–8,14,15).
Regarding thyroid nodules, there are several reports of
preoperative evaluation with FDG-PET, and it is generally
considered that FDG-PET is of limited value in predicting thyroid
cancer outcome (3–8,14,15). Furthermore, there are limited data
regarding the role of FDG-PET in detecting preoperative lymph node
metastasis of thyroid cancer (3,11–13). Clinically, FDG-PET is not generally
used for the primary diagnosis of thyroid cancer. However, as
FDG-PET is becoming a commonly used imaging modality, the number of
thyroid lesions incidentally detected by FDG-PET is increasing. We
previously demonstrated that the risk of thyroid cancer in patients
with PET incidentaloma was relatively high (5). Previous studies evaluated the factors
associated with the FDG avidity of the primary tumor in thyroid
cancer (14,15), but there has been no study assessing
the factors associated with FDG avidity of the affected lymph
nodes. Thus, the present study was undertaken to assess the
accuracy of FDG-PET evaluation of lymph node metastases for
patients with thyroid cancer. The key observations made in this
study may be summarized as follows: i) The sensitivity,
specificity, overall accuracy and false-negative rates of
preoperative FDG-PET evaluation in the prediction of lymph node
status were 40.0, 100, 72.7 and 60.0%, respectively; ii) the size
of node metastasis, but not their number, was associated with FDG
uptake in the lymph nodes; and iii) the false-positive rate of
FDG-PET evaluation of lymph node metastasis was 0%; however, even
in the patient group with node metastasis sized >10 mm, the
false-negative rate was 50%.
SUVmax is used as a semi-quantitative indicator of
FDG uptake, but it is sometimes difficult to obtain a reliable
value with only one FDG-PET imaging, as SUVmax is affected by
several factors, including glucose transporter expression, viable
cell number, tumor perfusion and inflammatory cells (5,16,17). Several studies have reported that
SUVmax is correlated with the size of the thyroid nodule to a
certain extent (14,15), according to the resolution of the PET
scanner, known as the partial volume effect (14,18).
However, there has been no study assessing the factors associated
with FDG avidity of affected lymph nodes. A few previous studies
have evaluated the diagnostic accuracy of PET in lymph node
metastasis. In this study, we evaluated the association between the
size of lymph node metastasis and the FDG avidity of lymph nodes.
There was a significant correlation between FDG uptake and the size
of lymph node metastasis; however, even in the patient group with
node metastasis >10 mm, the false-negative rate was 50%.
Therefore, FDG-PET evaluation of lymph node metastasis is not
predictive of small metastasis or micrometastasis.
On the other hand, in the present study, the
false-positive rate of FDG-PET evaluation of lymph node metastasis
was 0%. Thus, in cases with FDG uptake by the lymph nodes,
macrometastasis to the lymph node is highly suspected. However, the
size of lymph node metastases does not always reflect lymphatic
spread; thus, FDG-PET imaging was not sufficient for the evaluation
of lymphatic spread. This study has potential limitations, the
major one being that it was a retrospective analysis and the number
of cases was relatively small. However, the clinical implications
of the data we obtained on FDG avidity are very important. However,
additional research is required to elucidate this putative
association between FDG-PET evaluation and lymph node
metastasis.
Inflammation also increases FDG uptake and,
therefore, SUVmax (14). CRP is an
acknowledged marker of inflammation reflecting a systemic
inflammatory response, and the measurement of serum CRP levels is
an easily available test. However, recent clinical evidence
suggests that FDG-PET is more accurate in detecting thyroid cancer
at high rather than at low TSH levels (19). In this study, there was no correlation
between SUVmax and either CRP or TSH level in lymph node
metastasis.
In conclusion, we demonstrated that preoperative
FDG-PET evaluation of lymph nodes is not effective in predicting
node status. Even in cases with relatively large (>10 mm) node
metastases, FDG-PET imaging was not sufficient for the evaluation
of lymph node status. The positive predictive value is high, but
our findings suggest that preoperative FDG-PET evaluation of lymph
node is not predictive of the final pathology.
Acknowledgements
The authors would like to thank Saitoh Y, Yano T,
Matsui Y, Ishida A and Yamamoto A for their secretarial
assistance.
References
|
1
|
Byun BH, Jeong UG, Hong SP, Min JJ, Chong
A, Song HC and Bom HS: Prediction of central lymph node metastasis
from papillary thyroid microcaprcinoma by
18F-fluorodeoxyglucose PET/CT and ultrasonography. Ann
Nucl Med. 26:471–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeh MW, Bauer AJ, Bernet VA, Ferris RL,
Loevner LA, Mandel SJ, Orloff LA, Randolph GW and Steward DL:
American Thyroid Association Surgical Affairs Committee Writing
Task Force: American Thyroid Association statement on preoperative
imaging for thyroid cancer surgery. Thyroid. 25:3–14. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pak K, Kim SJ, Kim IJ, Kim BH, Kim SS and
Jeon YK: The role of 18F-fluorodeoxyglucose positron emission
tomography in differentiated thyroid cancer before surgery. Endocr
Relat Cancer. 20:R203–R213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kresnik E, Gallowitsch HJ, Mikosch P,
Stettner H, Igerc I, Gomez I, Kumnig G and Lind P:
Fluorine-18-fluorodeoxyglucose positron emission tomography in the
preoperative assessment of thyroid nodules in an endemic goiter
area. Surgery. 133:294–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujii T, Yajima R, Yamaguchi S, Tsutsumi
S, Asao T and Kuwano H: Is it possible to predict malignancy in
cases with focal thyroid incidentaloma identified by
18F-fluorodeoxyglucose positron emission tomography? Am
Surg. 78:141–143. 2012.PubMed/NCBI
|
|
6
|
Wu YJ, Wu HS, Yen RF, Shen YY and Kao CH:
Detecting metastatic neck lymph nodes in papillary thyroid
carcinoma by 18F-2-deoxyglucose positron emission
tomography and Tc-99m tetrofosmin single photon emission computed
tomography. Anticancer Res. 23:2973–2976. 2003.PubMed/NCBI
|
|
7
|
Marcus C, Whitworth PW, Surasi DS, Pai SI
and Subramaniam RM: PET/CT in the management of thyroid cancers.
AJR Am J Roentgenol. 202:1316–1329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y: Clinical significance of thyroid
uptake on F18-fluorodeoxyglucose positron emission tomography. Ann
Nucl Med. 23:17–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fletcher JW, Djulbegovic B, Soares H,
Siegel BA, Lowe VJ, Lyman GH, Coleman RE, Wahl R, Paschold JC,
Avril N, et al: Recommendations on the use of 18F-FDG
PET in oncology. J Nucl Med. 49:480–508. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Urhan M, Velioglu M, Rosenbaum J, Basu S
and Alavi A: Imaging for the diagnosis of thyroid cancer. Expert
Opin Med diagn. 3:237–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi JW, Yoon YH, Yoon YH, Kim SM and Koo
BS: Characteristics of primary papillary thyroid carcinoma with
false-negative findings on initial 18F-FDG PET/CT. Ann
Surg Oncol. 18:1306–1311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeong HS, Baek CH, Son YI, Choi JY, Kim
HJ, Ko YH, Chung JH and Baek HJ: 18F-FDG PET/CT for the
initial evaluation of cervical node level of patients with
papillary thyroid carcinoma: comparison with ultrasound and
contrast-enhanced CT. Clin Endocrinol (Oxf). 65:402–407. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morita S, Mizoguchi K, Suzuki M and Iizuka
K: The accuracy of [18 F]-fluoro-2-deoxy
D-glucose-positron emission tomography/computed tomography,
ultrasonography, and enhanced computed tomography alone in the
preoperative diagnosis of cervical lymph node metastasis in
patients with papillary thyroid carcinoma. World J Surg.
34:2564–2569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohba K, Nishizawa S, Matsushita A,
Inubushi M, Nagayama K, Iwaki H, Matsunaga H, Suzuki S, Sasaki S,
Oki Y, et al: High incidence of thyroid cancer in focal thyroid
incidentaloma detected by 18F-fluorodeoxyglucose
[corrected] positron emission tomography in relatively young
healthy subjects: Results of 3-year follow-up. Endocr J.
57:395–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bae JS, Chae BJ, Park WC, Kim JS, Kim SH,
Jung SS and Song BJ: Incidental tyroid lesions detected by
FDG-PET/CT: Prevalence and risk of thyroid cancer. World J Surg
Oncol. 7:632009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi JY, Lee KS, Kim HJ, Shim YM, Kwon OJ,
Park K, Baek CH, Chung JH, Lee KH and Kim BT: Focal thyroid lesions
incidentally identified by integrated 18F-FDG PET/CT:
Clinical significance and improved characterization. J Nucl Med.
47:609–615. 2006.PubMed/NCBI
|
|
17
|
Matsuzu K, Segade F, Matsuzu U, Carter A,
Bowden DW and Perrier ND: Differential expression of glucose
transporters in normal and pathologic thyroid tissue. Thyroid.
14:806–812. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoffman EJ, Huang SC and Phelps ME:
Quantitation in positron emission computed tomography: 1. Effect of
object size. J Comput Assist Tomogr. 3:299–308. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deichen JT, Schmidt C, Prante O, Maschauer
S, Papadopoulos T and Kuwert T: Influence of TSH on uptake of
[18F]fluorodeoxyglucose in human thyroid cells in vitro.
Eur J Nucl Med Mol Imaging. 31:507–512. 2004. View Article : Google Scholar : PubMed/NCBI
|