Introduction
The combination of leucovorin, 5-fluorouracil
(5-FU), and oxaliplatin (FOLFOX) and bevacizumab (bev) has been
extensively used as first-line treatment for metastatic colorectal
cancer (1,2). However, infusion or injection of 5-FU
with leucovorin has certain disadvantages, including increased
cost, inconvenience and morbidity associated with the use of a
portable infusion pump and central venous catheter (3). The use of oral anticancer drugs has
helped to overcome problems associated with continuous infusion,
infection and thrombosis. S-1 is an oral anticancer drug that
combines the prodrug tegafur with two modulators of 5-FU
metabolism, gimeracil and oteracil (4). Several phase III clinical trials on
metastatic colorectal cancer have demonstrated that oral S-1 may
substitute infusional 5-FU (5,6).
Furthermore, a phase III trial of first-line treatments in patients
with metastatic colorectal cancer (SOFT trial) demonstrated that
the combination of S-1 and oxaliplatin with bevacizumab (SOX+bev)
was as efficacious as FOLFOX6+bev with respect to progression-free
survival (PFS) (7). Therefore, the
aim of the present study was to evaluate the efficacy and safety of
SOX+bev for patients with advanced recurrent colorectal cancer.
Patients and methods
Patients
A total of 36 patients with advanced recurrent
colorectal cancer who were administered SOX+bev chemotherapy
between January, 2010 and December, 2013 at the Department of
Surgery, Hachioji Digestive Disease Hospital (Tokyo, Japan) were
included in this retrospective study. All patients provided written
informed consent prior to treatment initiation. All the patients
had sufficient oral drug intake. The patient inclusion criteria
were as follows: i) Colorectal cancer diagnosed by pathological
examination or radiography; ii) unresectable, locally advanced
disease, or metastatic/recurrent disease; iii) Eastern Cooperative
Oncology Group performance status of 0–2; iv) at least one
measurable lesion according to the Response Evaluation Criteria in
Solid Tumours [RECIST (8)]; v) no
limit of age and life expectancy; AND vi) adequate organ function,
as indicated by white blood cell count ≥3,000/µl, haemoglobin
levels of 8 g/dl, platelet count ≥100,000/mm3, total
bilirubin levels ≤2.0 mg/dl, aspartate aminotransferase (AST) and
alanine aminotransferase levels not exceeding three times the upper
limit of normal, creatinine ≤1.5 mg/dl, blood urea nitrogen ≤25
mg/dl and creatinine clearance ≥50 ml/min. The exclusion criteria
were as follows: i) Severe complications, including ACTIVE
infection, cardiac or renal disease, marked pleural effusion or
ascites; ii) severe drug hypersensitivity; iii) an active
concomitant malignancy; and iv) pregnanCY or lactation.
Treatment
The SOX+bev regimen consisted of administration of
intravenous oxaliplatin (85 mg/m2) on days 1 and 14,
bevacizumab (5 mg/kg) on day 1 and co-administration of oral S-1
twice daily on days 1–14. The total daily S-1 dosage was calculated
according to the body surface area (BSA): Patients with a BSA of
<1.25 m2 received 80 mg/day, those with a BSA
1.25–1.5 m2 received 100 mg/day and those with a BSA
≥1.5 m2 received 120 mg/day. The drug regimen was
repeated every 4 weeks. The tumour response was measured by
computed tomography in accordance with the RECIST every two
courses, or more frequently in patients with suspected disease
progression. Toxicities associated with the drug regimen were
evaluated according to the Common Terminology Criteria for Adverse
Events [CTCAE; version 3.0 (9)].
Survival Analysis
Overall survival (OS) was calculated from the day of
treatment initiation to either the date of the last patient
follow-up or death, and PFS was calculated from the day of
treatment initiation to the identification of disease progression,
death or the censored date. Median OS and PFS were estimated using
Kaplan-Meier analysis and compared using the log-rank test.
Results
Patient characteristics
A total of 36 patients were enrolled in this study.
Patient characteristics are summarized in Table I. The median patient age was 67.4
years (range, 44–84 years) and the male:female ratio was 23:13. The
primary tumour was located in the colon in 26 patients (72.2%) and
the rectum in 10 patients (27.8%). Within this cohort, SOX+bev was
the first-line chemotherapy regimen administered to 27 patients
(75%), while only 9 patients (25%) had received previous
chemotherapy. Of these 9 patients, 6 had received a course of
S-1+irinotecan (IRIS) prior to SOX+bev, while the remaining 3
patients received courses of IRIS and FOLFOX prior to initiation of
SOX+bev therapy.
 | Table I.Characteristics of patients
(n=36). |
Table I.
Characteristics of patients
(n=36).
| CharacteristicS | Patients, n (%) |
|---|
| Age (years) |
|
| Median
(range) | 67.4 (44–86) |
| Gender |
|
| Male | 23 (63.9) |
|
Female | 13 (36.1) |
| Primary location
(%) |
|
|
Colon | 26 (72.2) |
|
Rectum | 10 (27.8) |
| Previous
chemotherapy |
|
| No | 27 (75.0) |
| Yes | 9 (25.0) |
| Metastatic sites |
|
|
Liver | 8 |
|
Peritoneum | 7 |
| Local
recurrence | 4 |
| Lung | 4 |
|
Lung/lymph nodes | 4 |
|
Liver/lymph nodes | 3 |
| Lymph
nodes | 2 |
|
Other | 4 |
| Number of treatment
courses, MEAN (range) | 5.7 (1–18) |
| Response rate
(%) | 45.2 |
Efficacy
The median number of chemotherapy courses was 5.7
(range, 1–18). Although a response to chemotherapy could not be
determined in 5 patients, 14/31 patients achieved a partial
response to SOX+bev treatment, resulting in a response rate (RR) of
45.2%. Stable disease was observed in an additional 8 patients,
resulting in a disease control rate (DCR) of 71%. The RR and DCR of
patients who received SOX+bev as first-line chemotherapy were the
same as those calculated for the complete cohort.
Survival
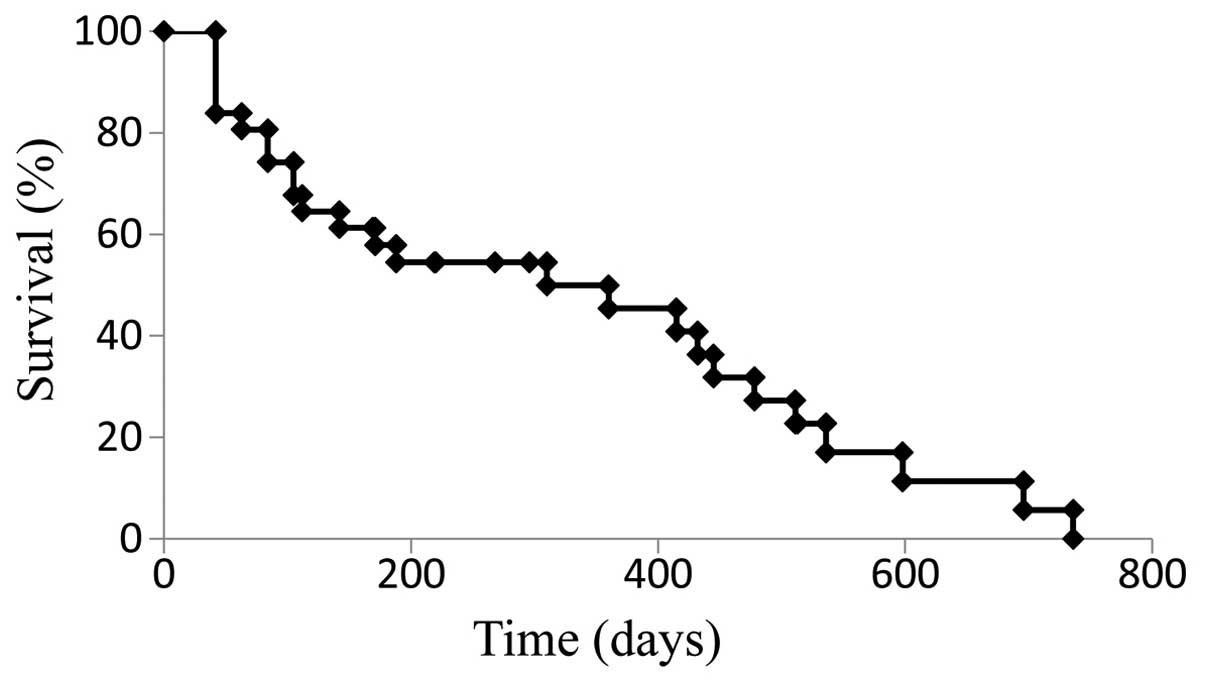
The median PFS for the cohort treated with SOX+bev
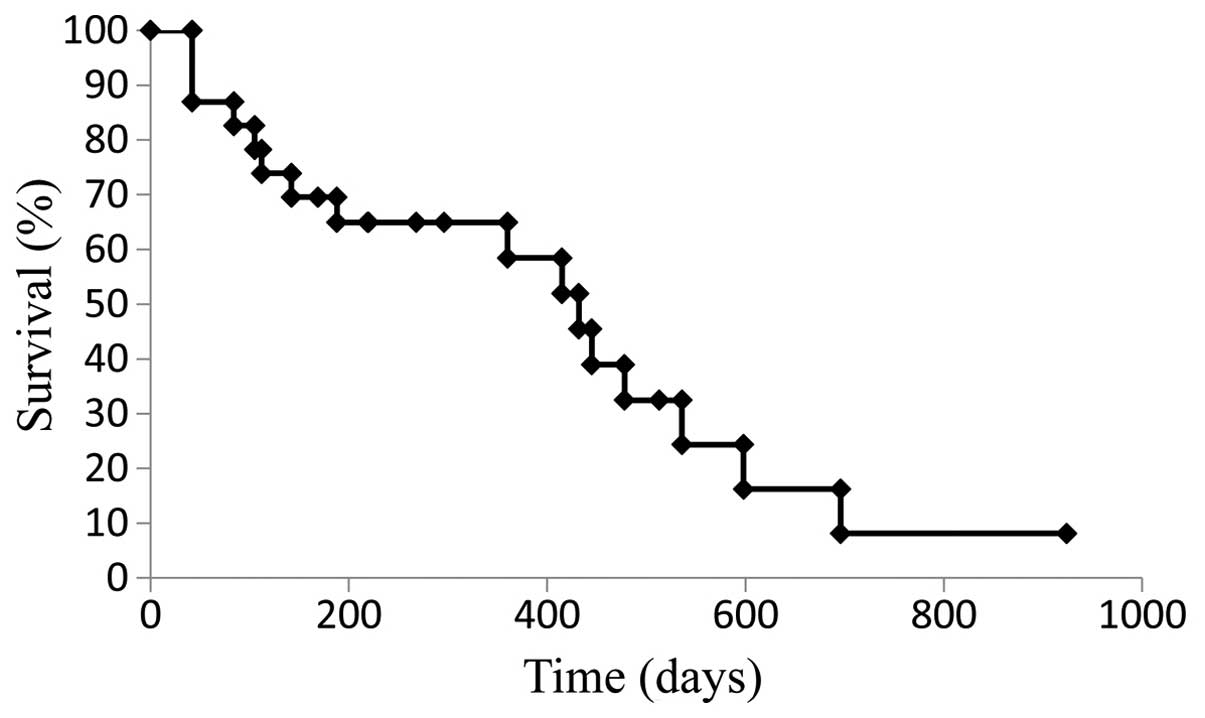
was 9.9 months (Fig. 1), while the
median OS was 21.9 months (Fig. 2).
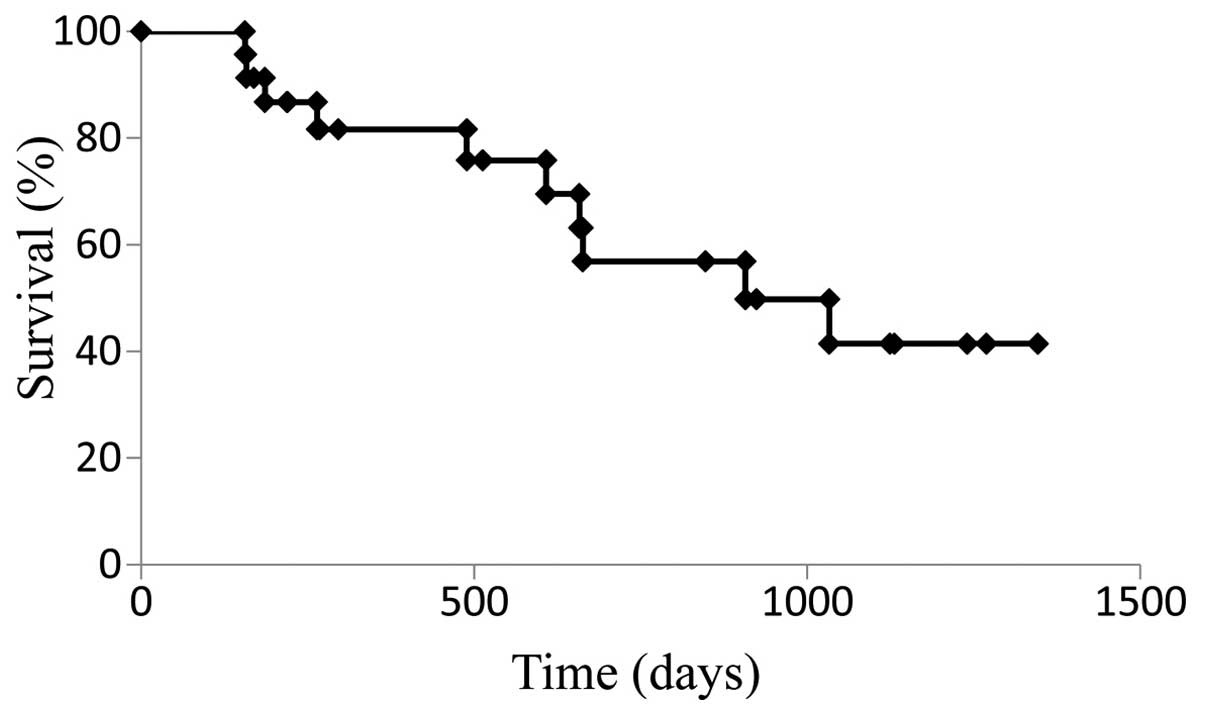
For patients who received SOX+bev exclusively as first-line
treatment, the median PFS (13.8 months) (Fig. 3) and the median OS (28.2 months)
(Fig. 4) were increased compared with
those for the entire cohort.
Toxicity
Adverse events, which were potentially due to
drug-related toxicity of SOX+bev, are summarized in Table II. Certain adverse events were
frequently observed, including sensory neuropathy (n=22; 61.1%),
thrombocytopenia (n=13; 36.1%), anorexia (n=9; 25%) and leukopenia
(n=7; 19.4%). More severe toxicities (grade 3 and 4), including
anaemia, thrombocytopenia, anorexia, diarrhea, sensory neuropathy,
increased AST levels, rash and infusion reaction, were observed in
8 patients. No adverse events were observed in patients who had
completed other chemotherapies prior to the SOX+bev regimen.
 | Table II.Adverse events in the ORAL S-1,
oxaliplatin and bevacizumab group. |
Table II.
Adverse events in the ORAL S-1,
oxaliplatin and bevacizumab group.
|
| Grade (n) |
|
|---|
|
|
|
|
|---|
| Adverse eventS | 1 | 2 | 3 | 4 | Patients with grade
3/4 toxicity, n (%) |
|---|
| Leukopenia | 3 | 4 | 0 | 0 | 0 (0.0) |
| Anemia | 1 | 2 | 1 | 0 | 1 (2.8) |
| Thrombocytopenia | 9 | 4 | 0 | 1 | 1 (2.8) |
| Anorexia | 8 | 2 | 1 | 0 | 1 (2.8) |
| Diarrhea | 3 | 3 | 1 | 0 | 1 (2.8) |
| Sensory
neuropathy | 7 | 14 | 1 | 0 | 1 (2.8) |
| Nausea | 0 | 2 | 0 | 0 | 0 (0.0) |
| Alopecia | 1 | 0 | 0 | 0 | 0 (0.0) |
| Increased alanine
aminotransferase | 0 | 3 | 0 | 0 | 0 (0.0) |
| Increased aspartate
aminotransferase | 0 | 1 | 2 | 0 | 2 (5.5) |
| Stomatitis | 1 | 0 | 0 | 0 | 0 (0.0) |
| Rash | 1 | 0 | 1 | 0 | 1 (2.8) |
| Elevated blood
pressure | 2 | 0 | 0 | 0 | 0 (0.0) |
Discussion
A combinatorial approach to chemotherapy using
targeted agents and complementary drug combinations, such as
oxaliplatin and 5-FU, is widely used for THE first-line treatment
of patients with advanced and recurrent colorectal cancer (10,11).
Combinations of folinic acid and 5-FU (either bolus or infusional)
with either oxaliplatin or irinotecan (FOLFOX and FOLFIRI,
respectively), are considered the gold standard regimens for the
first-line treatment of advanced and recurrent colorectal cancer
(12–14). In Japan, oral S-1 has been widely used
for the treatment of gastrointestinal cancers. In phase II studies,
S-1 alone elicited an RR of 19–40% with tolerable adverse events in
patients with metastatic colorectal cancer (15–17).
Furthermore, several trials using SOX regimens have reported RRs of
47–54% and DCRs of 81–90% (6,18,19). In
thOse studies, the median PFS of patients receiving SOX regimens
was 6.5–8.5 months, while the median OS was 21.2–27.2 months,
highlighting the efficacy and feasibility of SOX as first-line
chemotherapy for metastatic colorectal cancer (6,18,19). As mentioned above, the SOFT trial
reported that the SOX+bev combination was as effective as
MFOLFOX6+bev in terms of PFS in patients with metastatic colorectal
cancer (7). To determine whether the
continuous 5-FU infusion in the FOLFOX regimen can be replaced with
oral S-1, the present clinical study was performed with the SOX+bev
regimen administered TO patients with advanced and recurrent
colorectal cancer. In the present study, the dose of intravenous
oxaliplatin on days 1 and 14 and that of bevacizumab was based on
the FOLFOX6 regimen PRIOR TO the SOFT trial. In the cohort of the
present study, an RR of 45.2%, a DCR of 71%, a median PFS of 9.9
months and a median OS of 21.9 months were observed. The increased
median PFS of 13.8 months and median OS of 28.2 months in patients
undergoing first-line chemotherapy with SOX+bev suggests that this
regimen may effective as a first-line chemotherapeutic regimen. The
RR, DCR, median PFS and median OS observed in the present study
were similar to those obtained with the combination of oxaliplatin
and S-1. Furthermore, OUR SOX+bev first-line chemotherapy DATA were
similar to those presented in the SOFT trial (7). However, it should be mentioned that the
27 patients who received SOX+bev as first-line chemotherapy
subsequently received second-line chemotherapy with IRIS+bev,
indicating that our data require further evaluation. In addition,
third-line and subsequent treatments included the addition of
experimental molecular-targetED agents (cetuximab+irinotecan),
which may have contributed to the survival prolongation observed in
our cohort.
The SOFT trial also reported a significantly lower
incidence of grade ≥3 neutropenia and leukopenia with SOX+bev,
compared with mFOLFOX6+bev treatment (7). Other studies also reported grade 3–4
leukopenia in 0–2% patients following administration of SOX
(6,7,18).
Thrombocytopenia is one of the main haematological abnormalities
caused by oxaliplatin, with 13–28% of patients exhibiting grade ≥3
thrombocytopenia in previous SOX therapy studies (6,7,18). It has been suggested that
oxaliplatin-induced thrombocytopenia may be associated with bone
marrow suppression, as well as liver damage due to sinusoidal
obstruction syndrome (20–22). In addition, it has been reported that
0–10% of patients treated with SOX experienced grade ≥3 diarrhea;
however, grade 3–4 thrombocytopenia and diarrhea were only observed
in 2.8% of our cohort at the SOX dose administered. Of note, the
most frequently observed adverse event in trials using SOX was
sensory neuropathy, which occurred at a frequency of 75–91%, with
high-grade neuropathy occurring in 0–10% of the patients (6,7,18). In the present study, sensory
neuropathy of any grade was also the most frequent adverse event
observed, occurring at a frequency of 61.1%, while grade 3–4
sensory neuropathy occurred in 2.8% of the patients. The reduced
frequency of sensory neuropathy in the present study compared with
that in previous studies may be the result of the revised criteria
for dose reduction.
In conclusion, the results of the present study
suggested that SOX+bev combination chemotherapy is safe and
feasible for patients with advanced recurrent colorectal
cancer.
References
|
1
|
Goldberg RM, Sargent DJ, Morton RF, Fuchs
CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC and Alberts
SR: A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tournigand C, Andrú T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Watanabe K, Kawahara H, Enomoto H, Toyama
Y, Akiba T and Yanaga K: Feasibility study of oxaliplatin with oral
S-1 or capecitabine as first-line therapy for patients with
metastases from colorectal cancer. Anticancer Res. 33:4029–4032.
2013.PubMed/NCBI
|
|
4
|
Shirasaka T: Development history and
concept of an oral anticancer agent S-1 (TS-1): Its clinical
usefulness and future vistas. J Clin Oncol. 39:2–15. 2009.
|
|
5
|
Muro K, Boku N, Shimada Y, Tsuji A,
Sameshima S, Baba H, Satoh T, Denda T, Ina K, Nishina T, et al:
Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid
plus irinotecan (FOLFIRI) as second-line chemotherapy for
metastatic colorectal cancer: A randomised phase 2/3
non-inferiority study (FIRIS study). Lancet Oncol. 11:853–860.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong YS, Park YS, Lim HY, Lee J, Kim TW,
Kim KP, Kim SY, Baek JY, Kim JH, Lee KW, et al: S-1 plus
oxaliplatin versus capecitabine plus oxaliplatin for first-line
treatment of patients with metastatic colorectal cancer: A
randomised, non-inferiority phase 3 trial. Lancet Oncol.
13:1125–1132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamada Y, Takahari D, Matsumoto H, Baba H,
Nakamura M, Yoshida K, Yoshida M, Iwamoto S, Shimada K, Komatsu Y,
et al: Leucovorin, fluorouracil and oxaliplatin plus bevacizumab
versus S-1 and oxaliplatin plus bevacizumab in patients with
metastatic colorectal cancer (SOFT): An open-label,
non-inferiority, randomised phase 3 trial. Lancet Oncol.
14:1278–1286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Glabbeke MV, van
Oosterom AT, Christian MC, et al: New guidelines to evaluate the
response to treatment in solid tumors. European Organization for
Research and Treatment of Cancer, National Cancer Institute of the
United States, National Cancer Center Institute of Canada. J Natl
Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimoyama M: The Japanese edition of the
National Cancer Institute-common toxicity criteria. Jpn J Cancer
Chemother. 26:1084–1144. 1999.(In Japanese).
|
|
10
|
Cassidy J, Tabernero J, Twelves C, Brunet
R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N, et
al: XELOX (capecitabine plus oxaliplatin): Active first-line
therapy for patients with metastatic colorectal cancer. J Clin
Oncol. 22:2084–2091. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
deGramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, CortesFunes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000.PubMed/NCBI
|
|
12
|
Giacchetti S, Perpoint B, Zidani R, Le
Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y,
Coudert B, et al: Phase III multicenter randomized trial of
oxaliplatin added to chronomodulated fluorouracil-leucovorin as
first-line treatment of metastatic colorectal cancer. J Clin Oncol.
18:136–147. 2000.PubMed/NCBI
|
|
13
|
Douillard JY, Cunningham D, Roth AD,
Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J,
Alakl M, et al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: A multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meyerhardt JA and Mayer RJ: Systemic
therapy for colorectal cancer. N Engl J Med. 352:476–487. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohtsu A, Baba H, Sakata Y, Mitachi Y,
Horikoshi N, Sugimachi K and Taguchi T: Phase II study of S-1, a
novel oral fluoropyrimidine derivative, in patients with metastatic
colorectal carcinoma. S-1 Cooperative Colorectal Carcinoma Study
Group. Br J Cancer. 83:141–145. 2000.PubMed/NCBI
|
|
16
|
Van den Brande J, Schöffski P, Schellens
JH, Roth AD, Duffaud F, Weigang-Köhler K, Reinke F, Wanders J, de
Boer RF, Vermorken JB, et al: EORTC Early Clinical Studies Group
early phase II trial of S-1 in patients with advanced or metastatic
colorectal cancer. Br J Cancer. 88:648–653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shirao K, Ohtsu A, Takada H, Mitachi Y,
Hirakawa K, Horikoshi N, Okamura T, Hirata K, Saitoh S, Isomoto H,
et al: Phase II study of oral S-1 for treatment of metastatic
colorectal carcinoma. Cancer. 100:2355–2361. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zang DY, Lee BH, Park HC, Song HH, Kim HJ,
Jung JY, Kim JH, Kim HY, Kwon JH, Hwang SW, et al: Phase II study
with oxaliplatin and S-1 for patients with metastatic colorectal
cancer. Ann Oncol. 20:892–896. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamada Y, Tahara M, Miya T, Satoh T,
Shirao K, Shimada Y, Ohtsu A, Sasaki Y and Tanigawara Y: Phase I/II
study of oxaliplatin with oral S-1 as FIRST-line therapy for
patients with metastatic colorectal cancer. Br J Cancer.
98:1034–1038. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Overman MJ, Maru DM, Charnsangavej C,
Loyer EM, Wang H, Pathak P, Eng C, Hoff PM, Vauthey JN, Wolff RA,
et al: Oxaliplatin-mediated increase in spleen size as a biomarker
for the development of hepatic sinusoidal injury. J Clin Oncol.
28:2549–2555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
RubbiaBrandt L, Audard V, Sartoretti P,
Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane
O, Chaussade S, et al: Severe hepatic sinusoidal obstruction
associated with oxaliplatin-based chemotherapy in patients with
metastatic colorectal cancer. Ann Oncol. 15:460–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tournigand C, Cervantes A, Figer A, Lledo
G, Flesch M, Buyse M, Mineur L, Carola E, Etienne PL, Rivera F, et
al: OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with
oxaliplatin in a stop-and-go fashion in advanced colorectal canceR
- A GERCOR study. J Clin Oncol. 24:394–400. 2006. View Article : Google Scholar : PubMed/NCBI
|