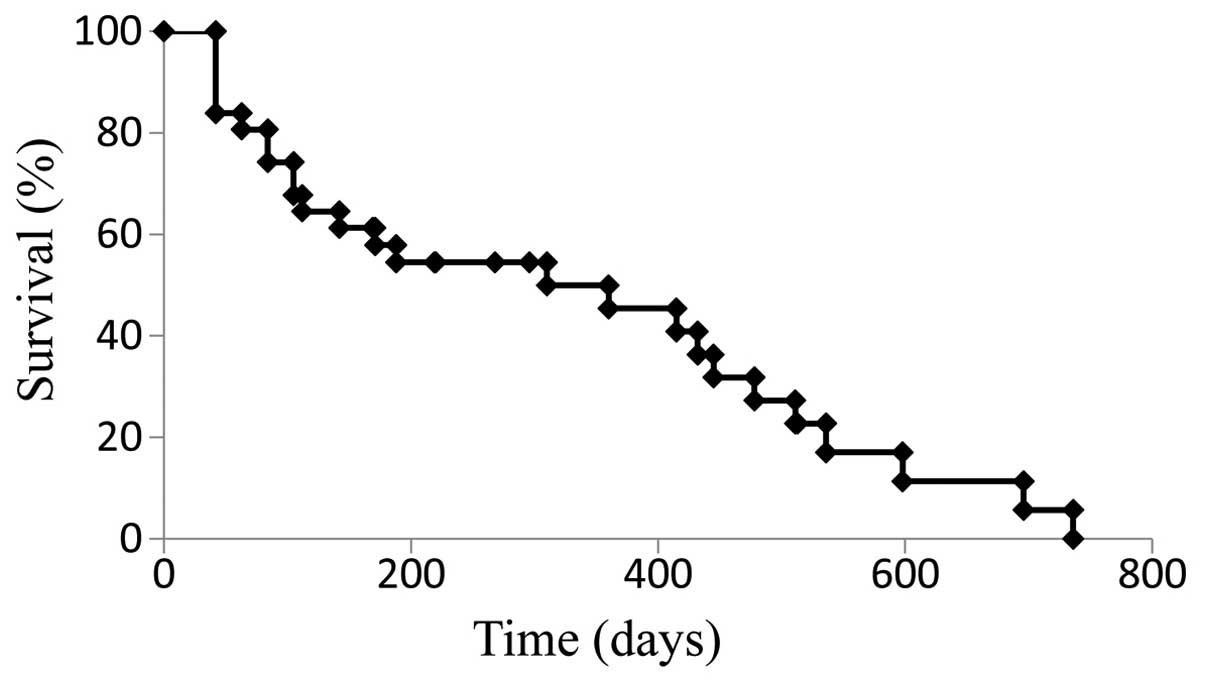
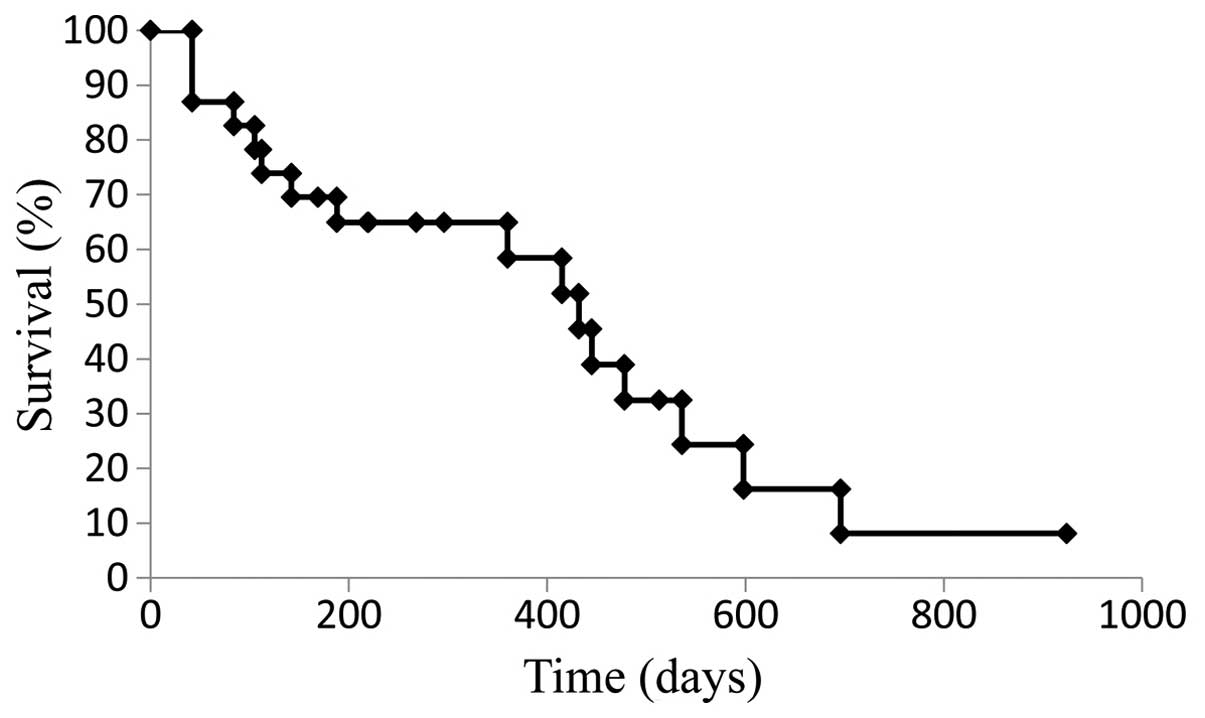
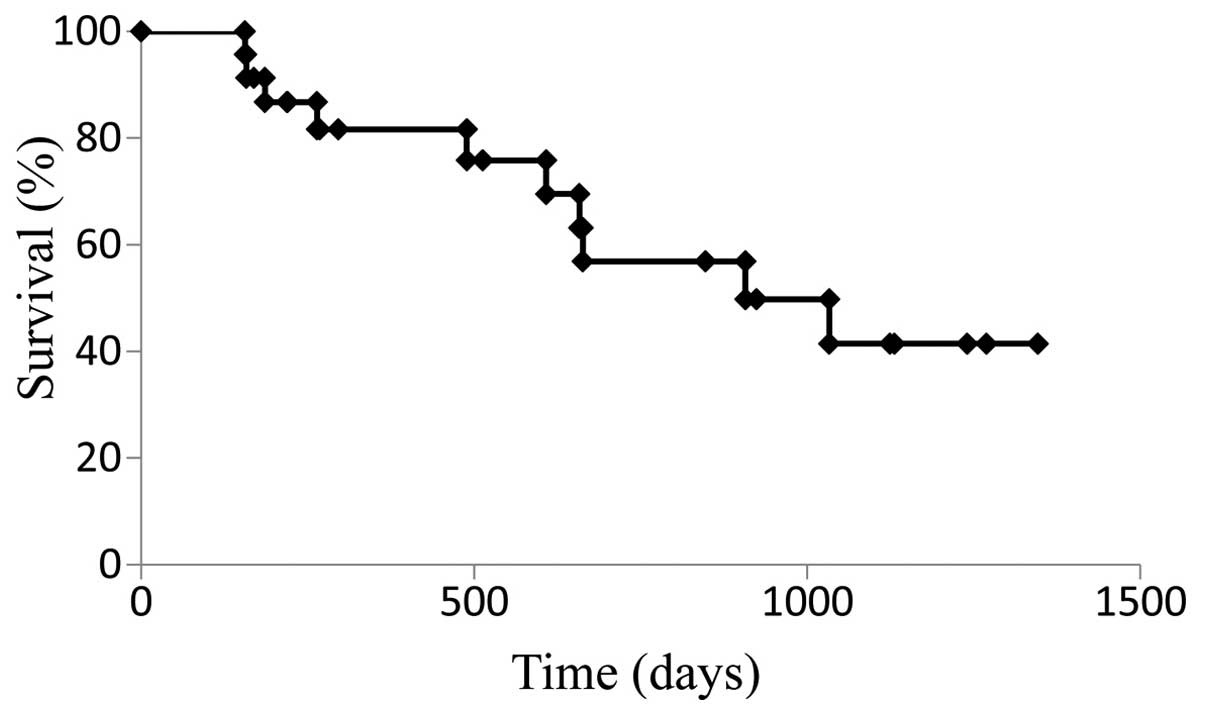
|
1
|
Goldberg RM, Sargent DJ, Morton RF, Fuchs
CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC and Alberts
SR: A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tournigand C, Andrú T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Watanabe K, Kawahara H, Enomoto H, Toyama
Y, Akiba T and Yanaga K: Feasibility study of oxaliplatin with oral
S-1 or capecitabine as first-line therapy for patients with
metastases from colorectal cancer. Anticancer Res. 33:4029–4032.
2013.PubMed/NCBI
|
|
4
|
Shirasaka T: Development history and
concept of an oral anticancer agent S-1 (TS-1): Its clinical
usefulness and future vistas. J Clin Oncol. 39:2–15. 2009.
|
|
5
|
Muro K, Boku N, Shimada Y, Tsuji A,
Sameshima S, Baba H, Satoh T, Denda T, Ina K, Nishina T, et al:
Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid
plus irinotecan (FOLFIRI) as second-line chemotherapy for
metastatic colorectal cancer: A randomised phase 2/3
non-inferiority study (FIRIS study). Lancet Oncol. 11:853–860.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong YS, Park YS, Lim HY, Lee J, Kim TW,
Kim KP, Kim SY, Baek JY, Kim JH, Lee KW, et al: S-1 plus
oxaliplatin versus capecitabine plus oxaliplatin for first-line
treatment of patients with metastatic colorectal cancer: A
randomised, non-inferiority phase 3 trial. Lancet Oncol.
13:1125–1132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamada Y, Takahari D, Matsumoto H, Baba H,
Nakamura M, Yoshida K, Yoshida M, Iwamoto S, Shimada K, Komatsu Y,
et al: Leucovorin, fluorouracil and oxaliplatin plus bevacizumab
versus S-1 and oxaliplatin plus bevacizumab in patients with
metastatic colorectal cancer (SOFT): An open-label,
non-inferiority, randomised phase 3 trial. Lancet Oncol.
14:1278–1286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Glabbeke MV, van
Oosterom AT, Christian MC, et al: New guidelines to evaluate the
response to treatment in solid tumors. European Organization for
Research and Treatment of Cancer, National Cancer Institute of the
United States, National Cancer Center Institute of Canada. J Natl
Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimoyama M: The Japanese edition of the
National Cancer Institute-common toxicity criteria. Jpn J Cancer
Chemother. 26:1084–1144. 1999.(In Japanese).
|
|
10
|
Cassidy J, Tabernero J, Twelves C, Brunet
R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N, et
al: XELOX (capecitabine plus oxaliplatin): Active first-line
therapy for patients with metastatic colorectal cancer. J Clin
Oncol. 22:2084–2091. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
deGramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, CortesFunes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000.PubMed/NCBI
|
|
12
|
Giacchetti S, Perpoint B, Zidani R, Le
Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y,
Coudert B, et al: Phase III multicenter randomized trial of
oxaliplatin added to chronomodulated fluorouracil-leucovorin as
first-line treatment of metastatic colorectal cancer. J Clin Oncol.
18:136–147. 2000.PubMed/NCBI
|
|
13
|
Douillard JY, Cunningham D, Roth AD,
Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J,
Alakl M, et al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: A multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meyerhardt JA and Mayer RJ: Systemic
therapy for colorectal cancer. N Engl J Med. 352:476–487. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohtsu A, Baba H, Sakata Y, Mitachi Y,
Horikoshi N, Sugimachi K and Taguchi T: Phase II study of S-1, a
novel oral fluoropyrimidine derivative, in patients with metastatic
colorectal carcinoma. S-1 Cooperative Colorectal Carcinoma Study
Group. Br J Cancer. 83:141–145. 2000.PubMed/NCBI
|
|
16
|
Van den Brande J, Schöffski P, Schellens
JH, Roth AD, Duffaud F, Weigang-Köhler K, Reinke F, Wanders J, de
Boer RF, Vermorken JB, et al: EORTC Early Clinical Studies Group
early phase II trial of S-1 in patients with advanced or metastatic
colorectal cancer. Br J Cancer. 88:648–653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shirao K, Ohtsu A, Takada H, Mitachi Y,
Hirakawa K, Horikoshi N, Okamura T, Hirata K, Saitoh S, Isomoto H,
et al: Phase II study of oral S-1 for treatment of metastatic
colorectal carcinoma. Cancer. 100:2355–2361. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zang DY, Lee BH, Park HC, Song HH, Kim HJ,
Jung JY, Kim JH, Kim HY, Kwon JH, Hwang SW, et al: Phase II study
with oxaliplatin and S-1 for patients with metastatic colorectal
cancer. Ann Oncol. 20:892–896. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamada Y, Tahara M, Miya T, Satoh T,
Shirao K, Shimada Y, Ohtsu A, Sasaki Y and Tanigawara Y: Phase I/II
study of oxaliplatin with oral S-1 as FIRST-line therapy for
patients with metastatic colorectal cancer. Br J Cancer.
98:1034–1038. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Overman MJ, Maru DM, Charnsangavej C,
Loyer EM, Wang H, Pathak P, Eng C, Hoff PM, Vauthey JN, Wolff RA,
et al: Oxaliplatin-mediated increase in spleen size as a biomarker
for the development of hepatic sinusoidal injury. J Clin Oncol.
28:2549–2555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
RubbiaBrandt L, Audard V, Sartoretti P,
Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane
O, Chaussade S, et al: Severe hepatic sinusoidal obstruction
associated with oxaliplatin-based chemotherapy in patients with
metastatic colorectal cancer. Ann Oncol. 15:460–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tournigand C, Cervantes A, Figer A, Lledo
G, Flesch M, Buyse M, Mineur L, Carola E, Etienne PL, Rivera F, et
al: OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with
oxaliplatin in a stop-and-go fashion in advanced colorectal canceR
- A GERCOR study. J Clin Oncol. 24:394–400. 2006. View Article : Google Scholar : PubMed/NCBI
|