Introduction
Non-Hodgkin lymphoma (NHL) accounts for ~85% of all
malignant lymphomas and consists of a complex group of cancers
arising mainly from B lymphocytes, and occasionally from T
lymphocytes. NHL is heterogeneous regarding its clinical,
immunophenotypic and genetic characteristics. With the accelerated
process of industrialization and environmental pollution, the
incidence of NHL is increasing annually. In China, the incidence of
lymphoma is 1.39/100,000 in men and 0.84/100,000 in women. Lymphoma
ranks from eleventh to thirteenth in overall cancer mortality
(1.5/100,000) (1). NHL ranks twelfth
in overall cancer morbidity and tenth in overall cancer mortality,
with a cumulative NHL risk of 0.54 and a cumulative mortality risk
of 0.26 (2). NHL severely affects the
physical and mental health of the patients. NHL is characterized by
an onset with distinct regional differences, and the etiology is
unknown. There are several difficulties in current cancer research,
including diversity of clinical status and complex pathological
type, whereas the molecular pathogenesis has not been fully
elucidated.
Uncontrolled cell proliferation is the main
characteristic of tumors. Disorders of the cell cycle and an
unbalance between cell proliferation and death due to various
causes play a crucial role in tumorigenesis and tumor progression.
p27Kip1, a negative cell cycle regulator, is a universal
cyclin-dependent kinase (CDK) inhibitor (CKI) that belongs to the
Cip/Kip group of CDK inhibitors. p27Kip1 shares a
sequence homology with p21 and p57 (3) and it may bind to and inhibit the
activity of cyclin-CDK complexes. Due to the inhibition of
p27Kip1, cyclin-CDK cannot effectively phosphorylate the
retinoblastoma protein; thus, E2F transcription factors cannot be
released, downstream genes cannot be transcribed and the cell cycle
process is blocked (4).
p27Kip1 inhibits the G1-S phase transition in the cell
cycle, resulting in cell cycle arrest in the G1 phase and cessation
of cell proliferation (5). Low p27
expression is associated with higher tumor grade (6). Therefore, p27Kip1 is
considered to be a tumor suppressor.
The human Jun activation domain-binding protein
1/COP9 signalosome subunit 5 (Jab1/CSN5) was initially identified
as a coactivator of the gene regulatory activator protein (AP-1),
which is involved in the control of cell proliferation (7). Jab1 is also referred to as the fifth
component of the COP9 signalosome (CSN) complex. CSN is a
multiprotein complex involved in modulating signal transduction,
gene transcription and protein stability (8,9). Jab1 is a
nuclear export protein that targets p27Kip1 for
transportation from the nucleus to the cytoplasm and promotes its
subsequent degradation (10).
Jab1/CSN5 interacts with a number of proteins and regulates their
function, and is involved in different signal transduction
pathways, including degradation of target proteins by regulating
gene transcription and cell cycle through phosphorylation (8). Jab1 regulates cell proliferation through
p27 (11). These findings indicate
that Jab1 may play a significant role in oncogenesis. Jab1
expression is inversely correlated with p27Kip1 protein
expression, and is significantly associated with adverse
clinicopathological characteristics. Recent research indicates that
Jab1 participates in the nuclear export of the p27Kip1
protein (10). Jab1 overexpression
may induce p27 downregulation by nuclear export (12). Some scholars investigated the
expression of Jab1 in pancreatic (13) and ovarian cancer (14), and found that the increase of Jab1
expression level is correlated with a decrease of
p27Kip1 levels and poor prognosis.
F-box protein S-phase kinase-interacting protein-2
(Skp2), the substrate recognition subunit of the Skp1-Cul1-F-box
protein (SCF) ubiquitin protein ligase complex, targets substrates
such as p27, p21, p57, or p130 for degradation (15). Skp2, as an important cell cycle
regulatory factor, is able to identify phosphorylated substrates
specifically and mediate ubiquitin degradation. It has been
demonstrated that Skp2 is able to specifically recognize
pThr187p27Kip1, then mediate the ubiquitination and
subsequent proteolysis of p27Kip1 (16). Due to the important role of Skp2,
scholars have investigated it and found Skp2 protein overexpression
in gastric carcinoma (17),
small-cell lung cancer (18) and oral
squamous cell carcinoma (19), which
is associated with the degree of differentiation and prognosis. The
role of Skp2 in controlling p27Kip1 levels has been
reported in several types of cancer, including colon, breast,
prostate and oral squamous cell carcinoma (20–23).
Jab1 and Skp2 dysfunction in NHL may cause a
decrease in the level of p27Kip1 and disrupt its
function, leading to the occurrence of this malignancy. To the best
of our knowledge, an investigation of both Skp2 and Jab1 has not
been reported in NHL to date. Thus, the aim of the present study
was to concurrently evaluate the abnormal expression of Jab1 and
Skp2 by immunohistochemistry, with a comparative analysis of p27
expression and proliferative activity in NHL.
Materials and methods
Patients
Fresh surgical specimens from 50 patients with NHL
were provided by the Department of Pathology of the Affiliated
Hospital of Nantong University (collected from 2005 to 2009,
following an Institutional Review Board-approved human subjects
study protocol. Informed consent was obtained from all patients (34
men and 16 women; age range, 10–90 years; mean age, 55.6 years).
The histology of the disease was determined based on hematoxylin
and eosin-stained preparations, according to the criteria of the
World Health Organization (24).
Immunohistochemistry
Paraffin sections (5 µm) from the samples were
deparaffinized in 100% xylene and rehydrated in descending
ethanol-water ratio solutions according to the standard protocol.
The sections were treated with 10 mmol/l citrate buffer (pH 6.0)
and heated to 121°C for 20 min to enhance the accessibility of the
antigens. The slides were incubated at 4°C overnight with anti-Jab1
(monoclonal, mouse anti-human, dilution 1:100; Santa Cruz
Biotechnology, Dallas, TX, USA; sc-13157), anti-Skp2 (polyclonal,
rabbit anti-human, dilution 1:50; Santa Cruz Biotechnology,
sc-7164), anti-p27 (polyclonal, rabbit anti-human, dilution 1:50;
Santa Cruz Biotechnology, sc-528), or anti-Ki-67 (polyclonal,
rabbit anti-human, dilution 1:150; ZSGB-Bio, Beijing, China;
ZM-0165). After washing, the sections were treated with rabbit
anti-mouse/rabbit immunoglobulin for 30 min at room temperature.
Staining for Jab1, Skp2, p27 and Ki-67 were completed by using the
streptavidin-biotin-peroxidase complex method with diaminobenzidine
(DAB) as a chromogen. Counterstaining was performed using
haematoxylin. The stained sections were examined under a light
microscope. At least 10 high-power fields were randomly selected
and at least 300 cells/field were counted per section. Jab1, Skp2,
p27 and Ki-67 indices were scored as the percentage of positive
cells for each antigen. The staining results were scored
semiquantitatively. Intensity was estimated compared with the
control and scored as follows: 0, negative staining; 1, weak
staining; 2, moderate staining; and 3, strong staining. Scores
representing the percentage of tumor cells that stained positive
were as follows: 0, <1%; 1, 1–10%; 2, 10–50%; 3, 50–75%; and 4,
>75% positive tumor cells. A final score was calculated by
adding the scores for percentage and intensity, resulting in scores
of 0 and 2–7. A score of 0 was considered as negative; 2–3 was
considered weak; 4–5 was considered moderate; and 6–7 was
considered strong. For statistical analysis, scores 0–3 were
considered as low expression, while scores 4–7 were considered as
overexpression (25). In half of the
samples, staining was repeated twice to avoid technical errors, but
similar results were obtained in these samples.
Statistical analysis
The correlations among clinicopathological factors
and the expression levels of Jab1, p27, Skp2 and Ki-67 were
analyzed using the Chi-square test, the Mann-Whitney U test and
logistic regression analysis. Survival analysis was performed by
the Kaplan-Meier method and survival curves were compared with the
log-rank test. The Cox proportional hazard model with a forward
stepwise procedure was used in the multivariate analysis to
determine independently significant prognostic factors. Data are
expressed as mean ± standard error. P-values <0.05 were
considered to indicate statistically significant differences. All
the statistical analyses were performed with SPSS 13.0 statistical
software (SPSS Inc., Chicago, IL, USA).
Results
Expression of p27Kip1,
Jab1, Skp2 and Ki-67 and their correlation in NHL
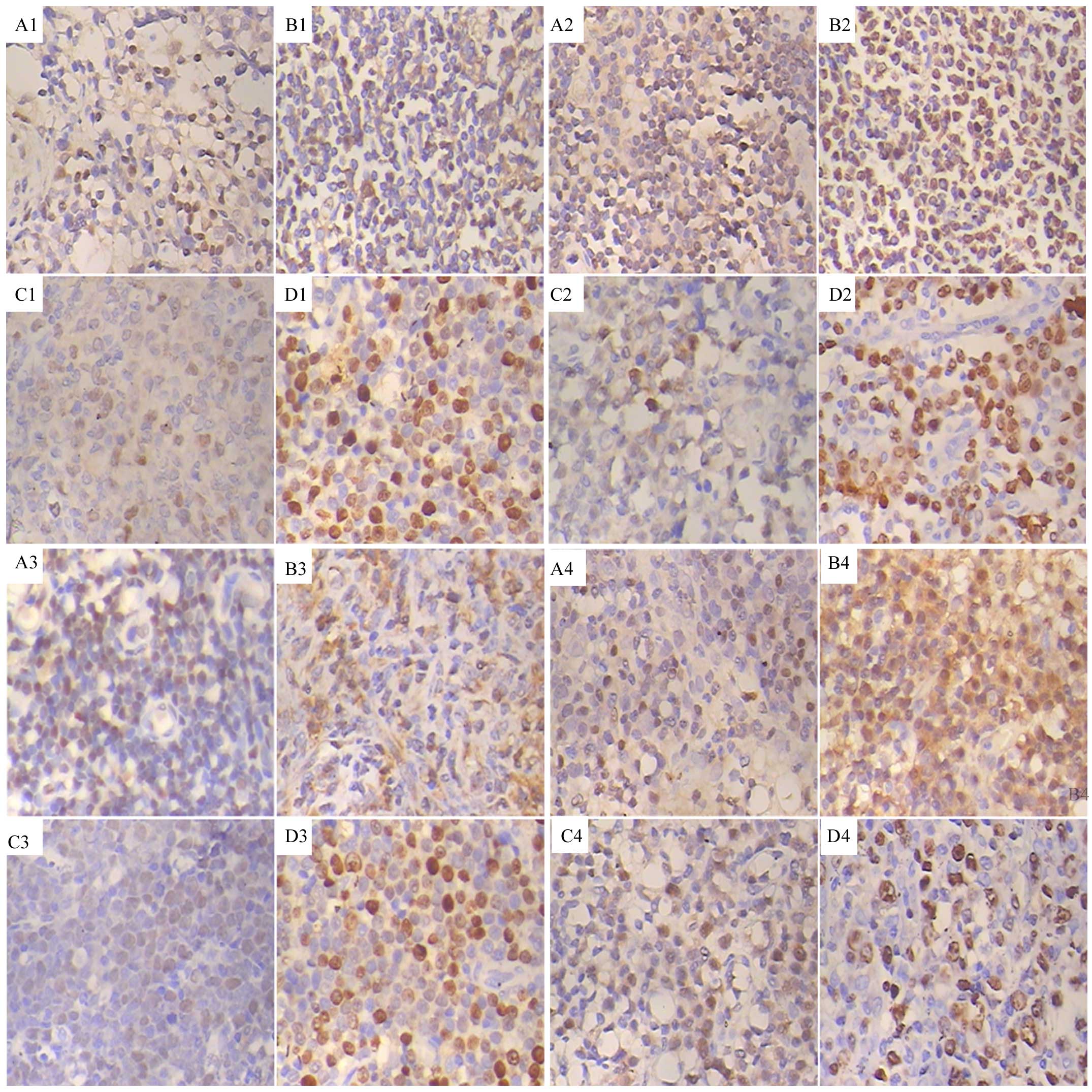
Immunohistochemical analysis revealed that the tumor
cells expressed p27Kip1, Jab1 and Skp2. The pattern of
p27Kip1, Jab1 and Skp2 expression varied in the same
sample as follows:
p27+/Jab1−/Skp2−;
p27+/Jab1+/Skp2−;
p27+/Jab1−/Skp2+;
p27+/Jab1+/Skp2+ (Fig. 1);
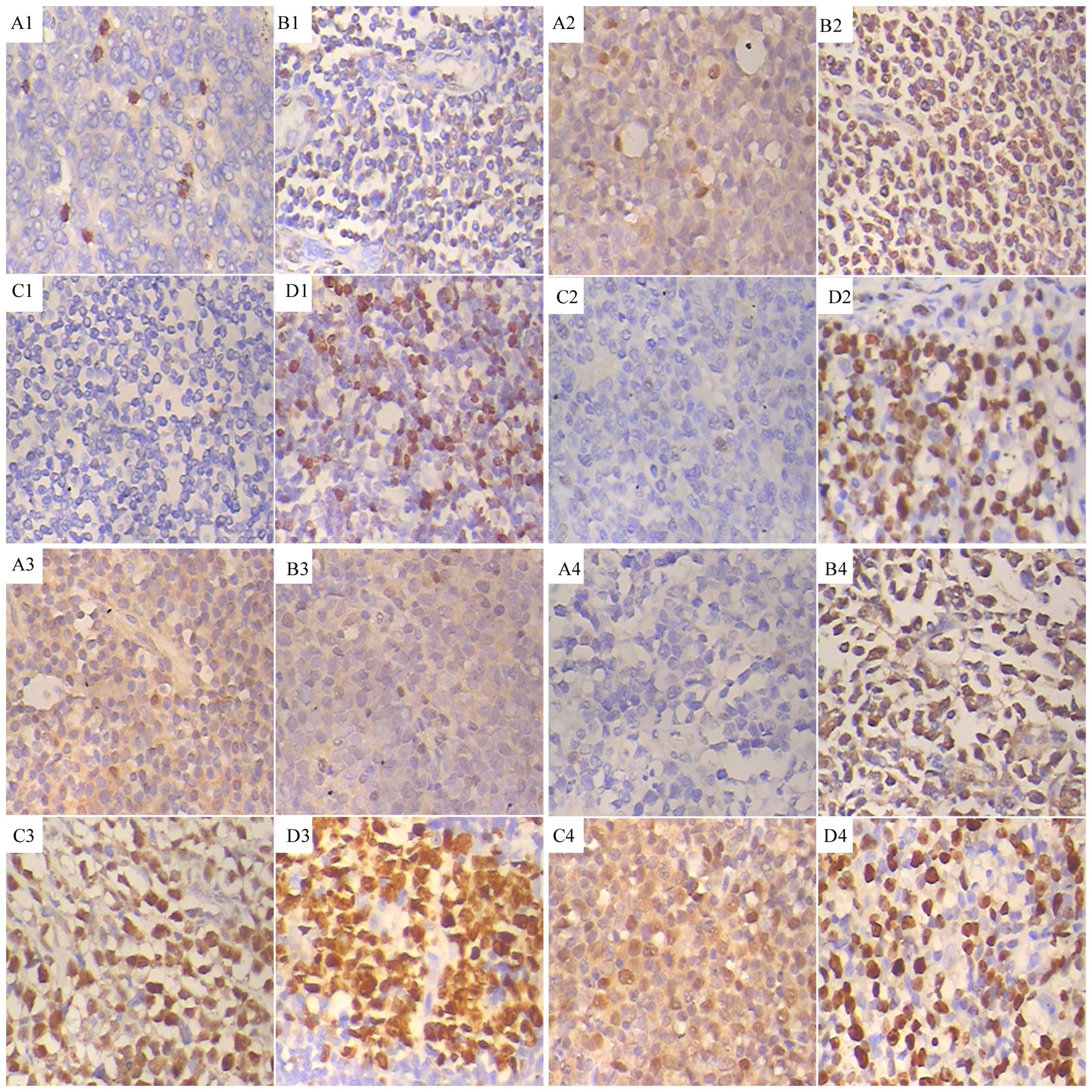
p27−/Jab1−/Skp2−;
p27−/Jab1+/Skp2−;
p27−/Jab1−/Skp2+; and
p27−/Jab1+/Skp2+ (Fig. 2). The positivity ratio of
p27Kip1, Jab1 and Skp2 was 38, 70 and 32%, respectively.
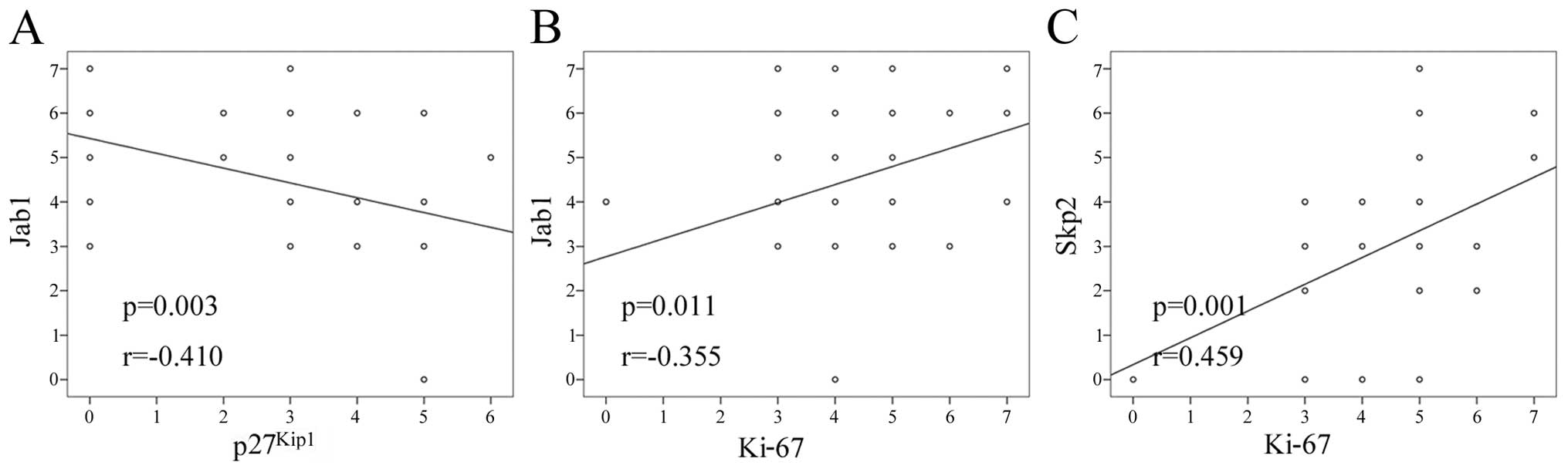
The correlation among the expressions of p27Kip1, Jab1,
Skp2 and Ki-67 was investigated by Spearman's rank correlation
(Fig. 3). A negative correlation
between Jab1 and p27Kip1 expression was identified
(Table I, r=−0.410, P=0.003). The
result was consistent with previous findings (26). The expressions of Jab1 (r=0.355,
P=0.011) and Skp2 (r=0.459, P=0.001) were positively correlated
with Ki-67 expression. The expressions of Jab1 and Skp2 exhibited a
trend for positive correlation (r=0.237, P=0.097). There was no
correlation between the expressions of Skp2 and p27 (Table I, r=0.177, P=0.218).
 | Table I.Correlations among
p27Kip1, Jab1, Skp2 and Ki-67. |
Table I.
Correlations among
p27Kip1, Jab1, Skp2 and Ki-67.
|
|
p27Kip1 | Jab1 | Skp2 | Ki-67 |
|---|
|
|
|
|
|
|
|---|
| Marker
expression | P-value | r | P-value | r | P-value | r | P-value | r |
|---|
|
p27Kip1 | – | – |
0.003a | −0.410 | 0.28 | 0.177 | 0.614 | −0.073 |
| Jab1 |
0.003a | −0.410 | – | – |
0.097 | 0.237 | 0.011a |
0.355 |
| Skp2 | 0.28 |
0.177 | 0.097 | 0.237 | – | – | 0.001a |
0.459 |
| Ki67 |
0.614 | −0.073 |
0.011a | 0.355 |
0.001a | 0.459 | – | – |
Correlation of p27Kip1,
Jab1 and Skp2 with clinicopathological parameters
The correlation of the expressions of
p27Kip1, Jab1 and Skp2 with clinicopathological
parameters, such as age, gender, tumor size, metastasis and
surgery, is summarized in Table II.
In this study, we found that decreased expression of p27 was
associated with age (P=0.018), and increased expression of Jab1 was
significantly associated with tumor size (P=0.033). In addition,
increased expression of Skp2 was significantly associated with
metastasis (P=0.008). Finally, the expressions of p27, Jab1, Skp2
and Ki-67 were all associated with IPI. Other clinicopathological
parameters exhibited no association with p27, Jab1 or Skp2.
 | Table II.Correlations of p27, Jab1 and Skp2
expression with clinicopathological parameters in NHL. |
Table II.
Correlations of p27, Jab1 and Skp2
expression with clinicopathological parameters in NHL.
|
|
|
p27Kip1 |
| Jab1 |
| Skp2 |
| Ki-67 |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Variables | Patients, n
(%) | High | Low | P-value | High | Low | P-value | High | Low | P-value | High | Low | P-value |
|---|
| Age, years |
|
|
| 0.018a |
|
| 1.0 |
|
| 0.126 |
|
| 0.095 |
|
≥60 | 22 (44) | 4 | 18 |
| 19 | 3 |
| 10 | 12 |
| 18 | 4 |
|
|
<60 | 28 (56) | 15 | 13 |
| 16 | 12 |
| 6 | 22 |
| 17 | 11 |
|
| Gender |
|
|
| 1.0 |
|
| 1.0 |
|
| 0.746 |
|
| 0.572 |
|
Male | 34 (68) | 13 | 21 |
| 24 | 10 |
| 10 | 24 |
| 24 | 10 |
|
|
Female | 16 (32) | 6 | 10 |
| 11 | 5 |
| 6 | 10 |
| 11 | 5 |
|
| Tumor size, cm |
|
|
| 1.0 |
|
| 0.033a |
|
| 0.076 |
|
| 0.287 |
| ≥2 | 22 (44) | 8 | 14 |
| 11 | 10 |
| 4 | 18 |
| 14 | 8 |
|
|
<2 | 28 (56) | 11 | 17 |
| 23 | 5 |
| 12 | 16 |
| 21 | 7 |
|
| Metastasis |
|
|
| 0.284 |
|
| 0.298 |
|
| 0.008a |
|
| 0.227 |
|
Positive | 4 (8) | 0 | 4 |
| 4 | 0 |
| 4 | 0 |
| 4 | 0 |
|
|
Negative | 46 (92) | 19 | 27 |
| 30 | 15 |
| 12 | 34 |
| 31 | 15 |
|
| Surgery |
|
|
| 1.0 |
|
| 0.470 |
|
| 0.256 |
|
| 0.018 |
|
Yes | 40 (80) | 15 | 25 |
| 28 | 11 |
| 11 | 29 |
| 25 | 15 |
|
| No | 10 (20) | 4 | 6 |
| 6 | 4 |
| 5 | 5 |
| 10 | 0 |
|
| IPI score |
|
|
| 0.000a |
|
|
0.001b |
|
| 0.001a |
|
| 0.002a |
| 0 or
1 | 19 (38) | 14 | 5 |
| 9 | 10 |
| 2 | 17 |
| 10 | 9 |
|
| 2 | 8 (16) | 3 | 5 |
| 4 | 4 |
| 0 | 8 |
| 3 | 5 |
|
| 3 | 3 (6) | 0 | 3 |
| 3 | 0 |
| 2 | 1 |
| 3 | 0 |
|
| 4 or
5 | 20 (40) | 2 | 18 |
| 19 | 1 |
| 12 | 8 |
| 19 | 1 |
|
Prognostic value of
p27Kip1, Jab1 and Skp2 expression
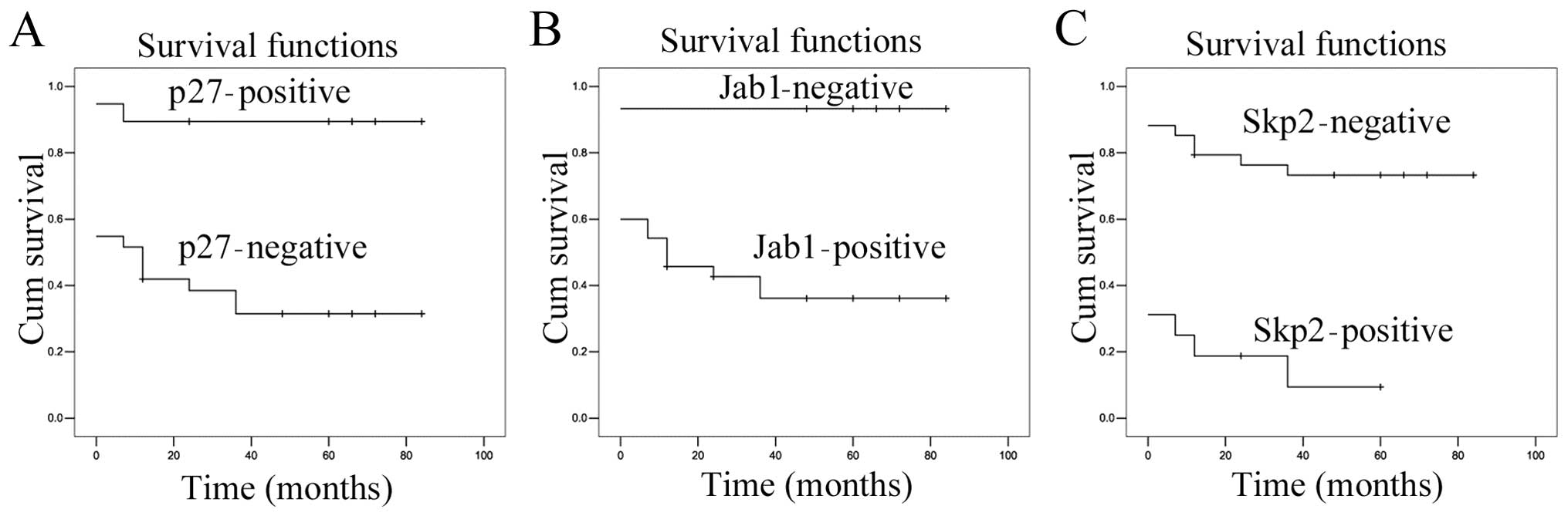
The Kaplan-Meier survival analysis demonstrated that
Jab1 (P=0.000) and Skp2 (P=0.000) overexpression had a significant
adverse effect on overall survival (Fig.
4B and C). This result was consistent with previous findings
(27). However, p27Kip1
expression exerted a beneficial effect on overall survival
(Fig. 4A, P=0.000). We also observed
that age was associated with survival rate (Table III, P=0.020). In present study,
other parameters exhibited no association with survival.
 | Table III.Correlation between
clinicopathological variables and survival rate. |
Table III.
Correlation between
clinicopathological variables and survival rate.
|
|
| Patients, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Variables | Patients, n
(%) | Survival | Mortality | Survival rate
(%) | P-value |
|---|
| Age (y) |
|
|
|
| 0.020a |
|
≥60 | 22 (44) | 5 | 17 | 22.7 |
|
|
<60 | 28 (56) | 22 | 6 | 78.6 |
|
| Gender |
|
|
|
| 0.288 |
|
Male | 34 (68) | 27 | 17 | 79.4 |
|
|
Female | 16 (32) | 10 | 6 | 62.5 |
|
| Tumor size |
|
|
|
| 0.219 |
| ≥2
cm | 22 (44) | 14 | 8 | 63.6 |
|
| <2
cm | 28 (56) | 13 | 15 | 46.4 |
|
| Metastasis |
|
|
|
| 0.617 |
|
Positive | 4 (8) | 0 | 4 | 0 |
|
|
Negative | 46 (92) | 27 | 19 | 58.7 |
|
| Surgery |
|
|
|
| 0.091 |
|
Yes | 40 (80) | 24 | 16 | 60 |
|
| No | 10 (20) | 3 | 7 | 30 |
|
| IPI |
|
|
|
| 0.000a |
| 0 or
1 | 19 (38) | 19 | 0 | 100 |
|
| 2 | 8 (16) | 8 | 0 | 100 |
|
| 3 | 3 (6) | 0 | 3 | 0 |
|
| 4 or
5 | 20 (40) | 0 | 20 | 0 |
|
|
p27Kip1 | 19 (38) |
|
|
| 0.176 |
|
Positive | 19 (38) | 17 | 2 | 89.5 |
|
|
Negative | 31 (62) | 9 | 22 | 29 |
|
| Jab1 |
|
|
|
| 0.052 |
|
Positive | 35 (70) | 13 | 22 | 37.1 |
|
|
Negative | 15 (30) | 14 | 1 | 93.3 |
|
| Skp2 |
|
|
|
| 0.022a |
|
Positive | 16 (32) | 2 | 14 | 12.5 |
|
|
Negative | 34 (68) | 25 | 9 | 73.5 |
|
| Ki-67 |
|
|
|
| 0.052 |
|
Positive | 35 (70) | 13 | 22 | 37.1 |
|
|
Negative | 15 (30) | 14 | 1 | 93.3 |
|
The Cox proportional hazard regression analysis
demonstrated that age [P=0.020, hazard ratio (HR)=1.038, 95%
confidence interval (CI): 1.006–1.072], Skp2 (P=0.022, HR=2.893,
95% CI: 1.162–7.203) and IPI (P=0.000, HR=6.000, 95% CI:
2.576–13.971) were independent prognostic factors (Table IV).
 | Table IV.Multivariate analysis with Cox
regression model. |
Table IV.
Multivariate analysis with Cox
regression model.
| Variable | Hazard ratio | 95% CI | P-value |
|---|
| Age | 1.038 | 1.006–1.072 |
0.020a |
| Gender | 1.676 | 0.647–4.342 | 0.288 |
| Tumor size | 0.524 | 0.186–1.471 | 0.219 |
| Metastasis | 0.712 | 0.188–2.697 | 0.617 |
| Surgery | 0.438 | 0.168–1.140 | 0.091 |
| IPI | 6.000 |
2.576–13.971 |
0.000a |
|
p27Kip1 | 0.340 | 0.071–1.624 | 0.176 |
| Jab1 | 7.638 |
0.987–59.135 | 0.052 |
| Skp2 | 2.893 | 1.162–7.203 |
0.022a |
| Ki-67 | 1.573 |
0.158–14.363 | 0.722 |
Discussion
The cell cycle is the basic process in cell life and
uncontrolled cell proliferation is the main characteristic of
tumors. The unbalance between cell proliferation and death and
disorders of the cell cycle play an important role in tumorigenesis
and tumor progression. p27Kip1 is a universal CKI that
is able to bind to and inhibit the activity of cyclin-CDK
complexes. p27Kip1 inhibits the G1-S phase transition in
the cell cycle resulting in cell cycle arrest in the G1-phase and
cessation of cell proliferation (5).
p27Kip1 protein levels are increased in quiescent cells
and rapidly decrease after cells are stimulated with mitogens
(28). Cellular abundance of the
p27Kip1 protein is regulated by various mechanisms, the
most important of which is the ubiquitin-proteasome pathway.
Studies have demonstrated that improving the level of Jab1
expression may induce a decrease of CDK specifically; furthermore,
p27Kip1 is also degraded (29). Skp2 may mediate the degradation of
p27Kip1 in liver cancer (30). There is an inverse correlation between
p27Kip1 and Skp2 expression in intrahepatic
cholangiocarcinomas (31). The
expression of Skp2 was significantly negatively correlated with the
expression of p27 in gastrointestinal stromal tumors (23). Our study demonstrated that low
expression of the p27Kip1 protein is associated with
poor prognosis (P<0.01), which is consistent with a previous
report that reduced expression of p27 is associated with poor
prognosis (32).
Jab1 interacts with a wide range of proteins,
regulates their function, and plays a role in different signal
transduction pathways, including degrading target proteins by
regulating gene transcription and cell cycle by phosphorylation
(8). Jab1 may lead to cell
proliferation and regulate the cell cycle; it also interacts with
p53 inducing phosphorylation mediated by CSN and subsequent
degradation (33). These results
indicate that Jab1 may play a significant role in oncogenesis. Jab1
overexpression may induce p27 downregulation (12). In addition, the chromosome region
maintenance 1 protein homolog and 26S proteasome-dependent
proteolysis is accelerated (34). The
Jab1 expression level is correlated with a decrease of the
p27Kip1 level and poor prognosis. Our
immunohistochemical staining results demonstrated that Jab1
expression was inversely correlated to p27Kip1 protein
expression (P<0.01), which is consistent with previous results
demonstrating that Jab1 negatively regulates p27 in nasopharyngeal
carcinoma (35). The expression of
Jab1 was positively correlated with Ki-67 expression (P=0.011), a
proliferating cell marker, expressed specifically in the cell
nucleus from the late G1 to the S phase. Overexpression of Jab1 is
associated with poor prognosis (P<0.01). These results suggest
that Jab1 may play an important role in the development and
progression of NHL and controlling Jab1 expression may be a novel
therapeutic target in NHL.
Skp2, as an important cell cycle regulatory factor,
is able to identify phosphorylated substrates specifically and
mediate ubiquitin degradation. Skp2 may mediate ubiquitination and
subsequent proteolysis of p27Kip1 (16). Carrano et al (29) demonstrated that the rate-limiting
factor of p27Kip1 degradation is SCF ubiquitin ligase
complex, including Skp2 as the special substrate recognition sites.
It was previously demonstrated that Skp2 protein overexpression
decreased p27Kip1 expression level in mantle cell
lymphoma, whereas inhibition of Skp2 by small interfering RNA,
increased the p27Kip1 and p21WAF1 levels
(36). In the present study, we
observed that Skp2 was associated with poor prognosis (P=0.000).
However, there was no inverse correlation between
p27Kip1 and Skp2 expression (r=0.177, P=0.218). The role
of Skp2 in controlling p27Kip1 level has been reported
in a number of cancers, including colon, breast, prostate and oral
squamous cell carcinoma (20–23). However, the p27 level was not found to
be inversely correlated with increasing Skp2 expression in
carcinoma of the uterine cervix, and our result is consistent with
that study (37). The different
association between p27 and Skp2 may be elucidated by the
difference in the tumor types, the patients' selection and the
cut-off values.
Jab1 is associated with degradation of
p27Kip1, which is the key protein in cell cycle
regulation. It is possible that Jab1 dysfunction causes a decrease
in the level of p27Kip1 and/or loss of function, thereby
leading to the occurrence of NHL. In conclusion, the overexpression
of Jab1 and Skp2 and the low expression of p27Kip1 are
associated with oncogenesis and poor prognosis. Jab1 expression was
found to be inversely correlated with p27Kip1 protein
expression. Thus, the expression of p27Kip1, Jab1 and
Skp2 may provide a clinical reference for the treatment of NHL.
Acknowledgements
The present study was supported by the Six Talent
Peaks foundation (WSN-061), the Post-doctoral Program of Jiangsu
Province (1201028C), the National Natural Scientific Foundation of
China (31370803), the Science and Technology Program of Nantong
City (MS22015071), the Scientific Research Program of Jiangsu
Province Health Department (H201423), and the Doctoral Program of
Nantong University (14B44).
References
|
1
|
Wu M and Zhu J: Changes in nutrition
metabolism of lymphoma after treatment and the nutritional
supports. Acta Academiae Medicinae Sinicae. 36:446–449. 2014.(In
Chinese). PubMed/NCBI
|
|
2
|
Fu ZY, Zhu J, Song YQ, Liu WP, Ji XQ and
Zhan SY: Prognostic analysis of 525 Chinese patients with diffuse
large B-cell lymphoma. J Peking Univ (Health Sci). 46:405–411.
2014.(In Chinese).
|
|
3
|
Zhao H, Bauzon F, Bi E, Yu JJ, Fu H, Lu Z,
Cui J, Jeon H, Zang X, Ye BH and Zhu L: Substituting threonine 187
with alanine in p27Kip1 prevents pituitary tumorigenesis
by two-hit loss of Rb1 and enhances humoral immunity in old age. J
Biol Chem. 290:5797–5809. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ha SY, Lee CH, Chang HK, Chang S, Kwon KY,
Lee EH, Roh MS and Seo B: Differential expression of forkhead box
M1 and its downstream cyclin-dependent kinase inhibitors p27(kip1)
and p21(waf1/cip1) in the diagnosis of pulmonary neuroendocrine
tumours. Histopathology. 60:731–739. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dahinden C, Ingold B, Wild P, Boysen G,
Luu VD, Montani M, Kristiansen G, Sulser T, Bühlmann P, Moch H and
Schraml P: Mining tissue microarray data to uncover combinations of
biomarker expression patterns that improve intermediate staging and
grading of clear cell renal cell cancer. Clin Cancer Res. 16:88–98.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Claret FX, Hibi M, Dhut S, Toda T and
Karin M: A new group of conserved coactivators that increase the
specificity of AP-1 transcription factors. Nature. 383:453–457.
1996. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chamovitz DA and Segal D: JAB1/CSN5 and
the COP9 signalosome. A complex situation. EMBO Rep. 2:96–101.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwechheimer C and Deng XW: COP9
signalosome revisited: A novel mediator of protein degradation.
Trends Cell Biol. 11:420–426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sankar U and Means AR: Gfer is a critical
regulator of HSC proliferation. Cell Cycle. 10:2263–2268. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Porrello E, Rivellini C, Dina G, Triolo D,
Del Carro U, Ungaro D, Panattoni M, Feltri ML, Wrabetz L, Pardi R,
et al: Jab1 regulates Schwann cell proliferation and axonal sorting
through p27. J Exp Med. 211:29–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tomoda K, Kubota Y, Arata Y, Mori S, Maeda
M, Tanaka T, Yoshida M, Yoneda-Kato N and Kato JY: The cytoplasmic
shuttling and subsequent degradation of p27Kip1 mediated
by Jab1/CSN5 and the COP9 signalosome complex. J Biol Chem.
277:2302–2310. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Wang Y, Yang C, Wang P, Oelschlager
DK, Zheng Y, Tian DA, Grizzle WE, Buchsbaum DJ and Wan M:
Polyethylene glycosylated curcumin conjugate inhibits pancreatic
cancer cell growth through inactivation of Jab1. Mol Pharmacol.
76:81–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sui L, Dong Y, Watanabe Y, Yamaguchi F,
Sugimoto K and Tokuda M: Clinical significance of Skp2 expression,
alone and combined with Jab1 and p27 in epithelial ovarian tumors.
Oncol Rep. 15:765–771. 2006.PubMed/NCBI
|
|
15
|
Kitagawa K, Kotake Y and Kitagawa M:
Ubiquitin-mediated control of oncogene and tumor suppressor gene
products. Cancer Sci. 100:1374–1381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Serres MP, Zlotek-Zlotkiewicz E, Concha C,
Gurian-West M, Daburon V, Roberts JM and Besson A: Cytoplasmic p27
is oncogenic and cooperates with Ras both in vivo and in vitro.
Oncogene. 30:2846–2858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JH, Go HY, Jin DH, Kim HP, Hong MH,
Chung WY, Park JH, Jang JB, Jung H, Shin YC, et al: Inhibition of
the PI3K-Akt/PKB survival pathway enhanced an ethanol extract of
Rhus verniciflua Stokes-induced apoptosis via a mitochondrial
pathway in AGS gastric cancer cell lines. Cancer Lett. 265:197–205.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hung WC, Tseng WL, Shiea J and Chang HC:
Skp2 overexpression increases the expression of MMP-2 and MMP-9 and
invasion of lung cancer cells. Cancer Lett. 288:156–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tosco P, La Terra Maggiore GM, Forni P,
Berrone S, Chiusa L and Garzino-Demo P: Correlation between Skp2
expression and nodal metastasis in stage I and II oral squamous
cell carcinomas. Oral Dis. 17:102–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Signoretti S, Di Marcotullio L, Richardson
A, Ramaswamy S, Isaac B, Rue M, Monti F, Loda M and Pagano M:
Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast
cancer. J Clin Invest. 110:633–641. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hershko D, Bornstein G, Ben-Izhak O,
Carrano A, Pagano M, Krausz MM and Hershko A: Inverse relation
between levels of p27(Kip1) and of its ubiquitin ligase subunit
Skp2 in colorectal carcinomas. Cancer. 91:1745–1751. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gstaiger M, Jordan R, Lim M, Catzavelos C,
Mestan J, Slingerland J and Krek W: Skp2 is oncogenic and
overexpressed in human cancers. Proc Natl Acad Sci USA.
98:5043–5048. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Di Vizio D, Demichelis F, Simonetti S,
Pettinato G, Terracciano L, Tornillo L, Freeman MR and Insabato L:
Skp2 expression is associated with high risk and elevated Ki67
expression in gastrointestinal stromal tumours. BMC Cancer.
8:1342008. View Article : Google Scholar
|
|
24
|
Rassidakis GZ, Claret FX, Lai R, et al:
Expression of p27(Kip1) and c-Jun activation binding protein 1 are
inversely correlated in systemic an aplastic cell lymphoma. Clin
Cancer Res. 9:1121–1128. 2003.PubMed/NCBI
|
|
25
|
Xie F, Liu H, Zhu YH, Qin YR, Dai Y, Zeng
T, Chen L, Nie C, Tang H, Li Y, et al: Overexpression of GPR39
contributes to malignant development of human esophageal squamous
cell carcinoma. BMC Cancer. 11:862011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahn J, Hong SA, Lee SE, Kim J, Oh YS, Park
SJ and Chung YJ: Cytoplasmic localization of Jab1 and
p27Kip1 might be associated with invasiveness of
papillary thyroid carcinoma. Endocr J. 56:707–713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seki R, Ohshima K, Fujisaki T, Uike N,
Kawano F, Gondo H, Makino S, Eto T, Moriuchi Y, Taguchi F, et al:
Prognostic significance of S-phase kinase-associated protein 2 and
p27Kip1 in patients with diffuse large B-cell lymphoma:
Effects of rituximab. Ann Oncol. 21:833–841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Polyak K, Lee MH, Erdjument-Bromage H,
Koff A, Roberts JM, Tempst P and Massagué J: Cloning of
p27Kip1, a cyclin-dependent kinase inhibitor and a
potential mediator of extracellular antimitogenic signals. Cell.
78:59–66. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carrano AC, Eytan E, Hershko A and Pagano
M: SKP2 is required for ubiquitin-mediated degradation of the CDK
inhibitor p27. Nat Cell Biol. 1:193–199. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qi M, Liu D, Zhang S, Hu P and Sang T:
Inhibition of S-phase kinase-associated protein 2-mediated p27
degradation suppresses tumorigenesis and the progression of
hepatocellular carcinoma. Mol Med Rep. 11:3934–3940.
2015.PubMed/NCBI
|
|
31
|
Hashimoto N, Yachida S, Okano K,
Wakabayashi H, Imaida K, Kurokohchi K, Masaki T, Kinoshita H,
Tominaga M, Ajiki T, et al: Immunohistochemically detected
expression of p27(Kip1) and Skp2 predicts survival in patients with
intrahepatic cholangiocarcinomas. Ann Surg Oncol. 16:395–403. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kouvaraki MA, Korapati AL, Rassidakis GZ,
Tian L, Zhang Q, Chiao P, Ho L, Evans DB and Claret FX: Potential
role of Jun activation domain-binding protein 1 as a negative
regulator of p27Kip1 in pancreatic adenocarcinoma.
Cancer Res. 66:8581–8589. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bech-Otschir D, Kraft R, Huang X, Henklein
P, Kapelari B, Pollmann C and Dubiel W: COP9 signalosome-specific
phosphorylation targets p53 to degradation by the ubiquitin system.
Embo J. 20:1630–1639. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Naumann M, Bech-Otschir D, Huang X,
Ferrell K and Dubiel W: COP9 signalosome-directed c-Jun
activation/stabilization is independent of JNK. J Biol Chem.
274:35297–35300. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pan Y, Zhang Q, Tian L, Wang X, Fan X,
Zhang H, Claret FX and Yang H: Jab1/CSN5 negatively regulates p27
and plays a role in the pathogenesis of nasopharyngeal carcinoma.
Cancer Res. 72:1890–1900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lwin T, Hazlehurst LA, Dessureault S, Lai
R, Bai W, Sotomayor E, Moscinski LC, Dalton WS and Tao J: Cell
adhesion induces p27Kip1-associated cell-cycle arrest
through down-regulation of the SCFSkp2 ubiquitin ligase pathway in
mantle-cell and other non-Hodgkin B-cell lymphomas. Blood.
110:1631–1638. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dowen SE, Scott A, Mukherjee G and Stanley
MA: Overexpression of Skp2 in carcinoma of the cervix does not
correlate inversely with p27 expression. Int J Cancer. 105:326–330.
2003. View Article : Google Scholar : PubMed/NCBI
|