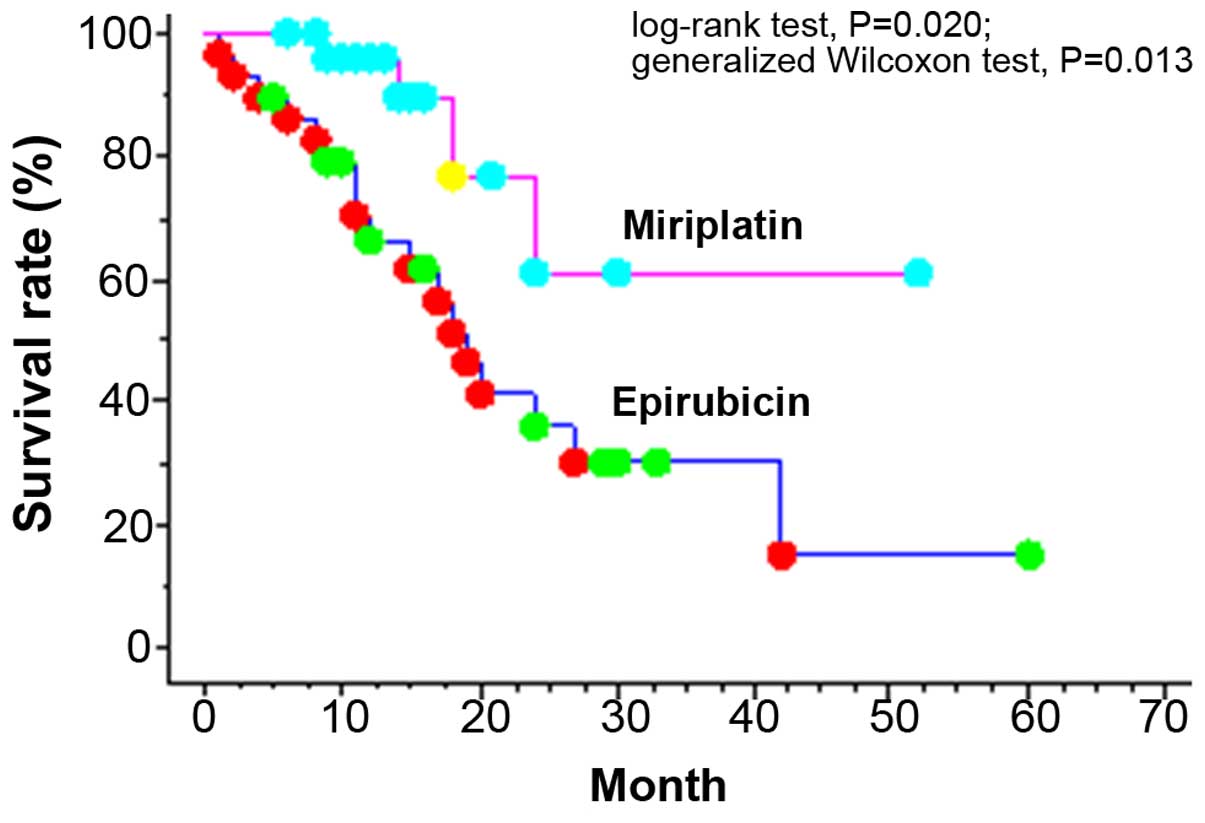
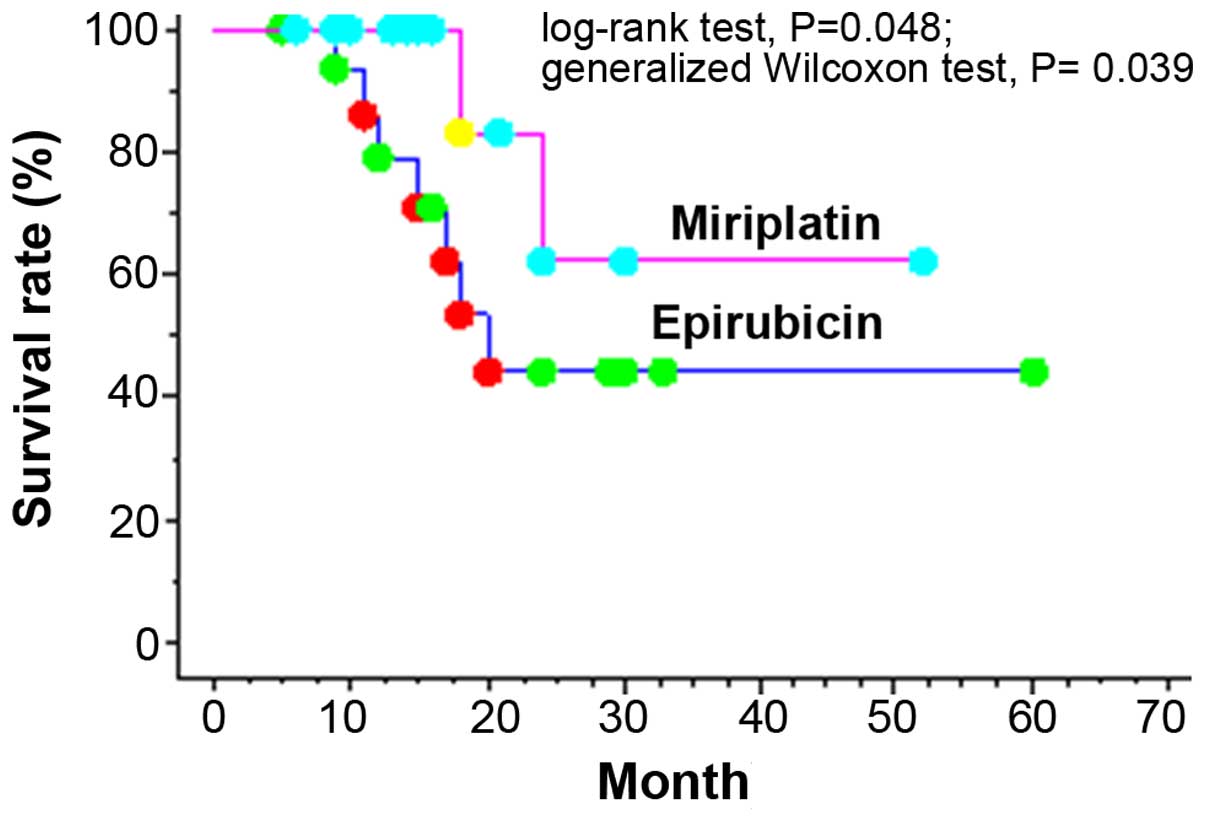
|
1
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tommasini M, Colombo M, Sangiovanni A,
Orefice S, Bignami P, Doci R and Gennari L: Intrahepatic
doxorubicin in unresectable hepatocellular carcinoma. The
unfavorable role of cirrhosis. Am J Clin Oncol. 9:8–11. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ichida T, Kato M, Hayakawa A, Watanabe M,
Igarashi K, Hata K, Doya Y, Miura M, Sato H and Asakura H:
Treatment of hepatocellular carcinoma with a
CDDP-epirubicin-lipiodol suspension: A pilot
clinico-pharmacological study. Cancer Chemother Pharmacol.
31:(Suppl). S51–S54. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aoyama K, Tsukishiro T, Okada K, Tsuchida
T, Aiba N, Nambu S, Miyabayashi C, Yasuyama T, Higuchi K and
Watanabe A: Evaluation of transcatheter arterial embolization with
epirubicin-lipiodol emulsion for hepatocellular carcinoma. Cancer
Chemother Pharmacol. 31:(Suppl). S55–S59. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colleoni M, Gaion F, Liessi G,
Mastropasqua G and Nelli P: Arterial chemoembolization with
epirubicin in unresectable hepatocellular carcinoma in cirrhosis.
Oncol Rep. 1:1171–1175. 1994.PubMed/NCBI
|
|
7
|
Ohnishi K, Tsuchiya S, Nakayama T, Hiyama
Y, Iwama S, Goto N, Takashi M, Ohtsuki T, Kono K, Nakajima Y, et
al: Arterial chemoembolization of hepatocellular carcinoma with
mitomycin C microcapsules. Radiology. 152:51–55. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sasaki Y, Imaoka S, Kasugai H, Fujita M,
Kawamoto S, Ishiguro S, Kojima J, Ishikawa O, Ohigashi H, Furukawa
H, et al: A new approach to chemoembolization therapy for hepatoma
using ethiodized oil, cisplatin, and gelatin sponge. Cancer.
60:1194–1203. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishikawa T, Higuchi K, Kubota T, Seki K,
Honma T, Yoshida T and Kamimura T: Prevention of intrahepatic
distant recurrence by transcatheter arterial infusion chemotherapy
with platinum agents for stage I/II hepatocellular carcinoma.
Cancer. 117:4018–4025. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishikawa T, Kubota T, Abe S, Watanabe Y,
Sugano T, Inoue R, Iwanaga A, Seki K, Honma T and Yoshida T:
Hepatic arterial infusion chemotherapy with cisplatin before
radical local treatment of early hepatocellular carcinoma (JIS
score 0/1) improves survival. Ann Oncol. 25:1379–1384. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamada R, Sato M, Kawabata M, Nakatsuka H,
Nakamura K and Takashima S: Hepatic artery embolization in 120
patients with unresectable hepatoma. Radiology. 148:397–401. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsui O, Kadoya M, Yoshikawa J, Gabata T,
Arai K, Demachi H, Miyayama S, Takashima T, Unoura M and Kogayashi
K: Small hepatocellular carcinoma: Treatment with subsegmental
transcatheter arterial embolization. Radiology. 188:79–83. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HC, Lee JH, Chung JW, Kang B, Yoon JH,
Kim YJ, Lee HS, Jae HJ and Park JH: Transarterial chemoembolization
with additional cisplatin infusion for hepatocellular carcinoma
invading the hepatic vein. J Vasc Interv Radiol. 24:274–283. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanaka M, Yoshida S and Kimura K:
Mechanism of inhibition of DNA polymerases by 4′-epiadriamycin and
4′-O-tetrahydropyranyladriamycin. Gan. 74:829–836. 1983.PubMed/NCBI
|
|
15
|
Nagasue N, Yukaya H, Okamura J, Kuroda C,
Kubo Y, Hirai K, Tanikawa K, Okita K, Ando K and Tamura K:
Intra-arterial administration of epirubicin in the treatment of
non-resectable hepatocellular carcinoma. Epirubicin study group for
hepatocellular carcinoma. Cancer & chemotherapy. 13:2786–2792.
1986.(In Japanese).
|
|
16
|
Yoshikawa M, Saisho H, Ebara M, Iijima T,
Iwama S, Endo F, Kimura M, Shimamura Y, Suzuki Y, Nakano T, et al:
A randomized trial of intrahepatic arterial infusion of
4′-epidoxorubicin with Lipiodol versus 4′-epidoxorubicin alone in
the treatment of hepatocellular carcinoma. Cancer Chemother
Pharmacol. 33:(Suppl). S149–S152. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maeda M, Uchida NA and Sasaki T:
Liposoluble platinum (II) complexes with antitumor activity. Jpn J
Cancer Res. 77:523–525. 1986.PubMed/NCBI
|
|
18
|
Kishimoto S, Ohtani A, Fukuda H, Fukushima
S and Takeuchi Y: Relation between intracellular accumulation and
cytotoxic activity of cis-[((1R, 2R)-1,2-cyclohexanediamine-N,
N')bis(myristato)]platinum(II) suspended in Lipiodol. Biol Pharm
Bull. 26:683–686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanada M, Baba A, Tsutsumishita Y, Noguchi
T, Yamaoka T, Chiba N and Nishikaku F: Intra-hepatic arterial
administration with miriplatin suspended in an oily lymphographic
agent inhibits the growth of tumors implanted in rat livers by
inducing platinum-DNA adducts to form and massive apoptosis. Cancer
Chemother Pharmacol. 64:473–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanada M, Baba A, Tsutsumishita Y, Noguchi
T and Yamaoka T: Intra-hepatic arterial administration with
miriplatin suspended in an oily lymphographic agent inhibits the
growth of human hepatoma cells orthotopically implanted in nude
rats. Cancer Sci. 100:189–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okusaka T, Okada S, Nakanishi T, Fujiyama
S and Kubo Y: Phase II trial of intra-arterial chemotherapy using a
novel lipophilic platinum derivative (SM-11355) in patients with
hepatocellular carcinoma. Invest New Drugs. 22:169–176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyayama S, Yamashiro M, Shibata Y,
Hashimoto M, Yoshida M, Tsuji K, Toshima F and Matsui O: Comparison
of local control effects of superselective transcatheter arterial
chemoembolization using epirubicin plus mitomycin C and miriplatin
for hepatocellular carcinoma. Jpn J Radiol. 30:263–270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Handa T, Imai Y, Sugawara K, Chikayama T,
Nakazawa M, Ando S, Hamaoka K, Inao M, Nakayama N and Mochida S:
Transcatheter arterial chemoembolization for hepatocellular
carcinoma: Comparison of the therapeutic efficacies between
miriplatin and epirubicin. Hepatol Res. 44:1072–1080. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamimura K, Suda T, Tamura Y, Takamura M,
Yokoo T, Igarashi M, Kawai H, Yamagiwa S, Nomoto M and Aoyagi Y:
Phase I study of miriplatin combined with transarterial
chemotherapy using CDDP powder in patients with hepatocellular
carcinoma. BMC Gastroenterol. 12:1272012. View Article : Google Scholar : PubMed/NCBI
|