Introduction
Brain metastases (BMs), the most common neurological
complication, develop in 20–40% of patients with cancer (1). In 40–50% of these patients, the
dominant primary tumor was lung cancer (LC) (2). Patients with LC who presented BMs at
their initial primary tumor diagnosis had a poor prognosis in
untreated patients (3,4). Surgery, whole brain radiation therapy
(WBRT) and stereotactic radiosurgery (SRS) were frequently applied
to the treatment of BMs. A limited number (predominantly 1–3 and in
certain cases >3) of BMs can be effectively intervened by
surgical resection or SRS (5,6).
However, a considerable proportion of patients, being unsuitable
for surgery or SRS, were controlled using a palliative approach,
including WBRT, to improve neurological symptoms and local lesions.
Unfortunately, radiotherapy can only prolong the median survival
time to 6 months for LC patients with BMs (7). Therefore, establishing a multimodality
therapy, including targeted agents or chemotherapy plus
radiotherapy, is urgently required to maximize the therapeutic
effect. Accordingly, certain relevant trials (8,9)
combining antitumor agents with radiotherapy have been performed in
recent decades. Some of these treatments demonstrated favorable
efficacy and safety of antitumor agents plus radiotherapy in
dealing with BMs (3,10–17),
while other trials failed to confirm this (18–21). The
role of antitumor agents plus radiotherapy for managing BMs remains
controversial. Therefore, the present study performed a
meta-analysis to investigate the efficacy and safety of antitumor
agents plus radiotherapy compared with radiotherapy alone for BMs
from lung cancer, to optimize the therapeutic strategy.
Materials and methods
Data sources and search
A thorough search in PubMed was performed without
language restriction from inception until October 2015 using the
following keywords and Mesh terms: [‘anti-tumor agents’ (all
fields) OR ‘anti-cancer drugs’ (all fields)] AND [‘EGFR tyrosine
kinase inhibitors’ (all fields) OR ‘epidermal growth factor
receptor’ (all fields) OR ‘targeted agents’ (all fields)] AND
[‘chemotherapy’ (all fields) AND ‘irradiation’ (all fields) OR
‘radiation therapy’ (all fields) OR ‘radiotherapy’ (all fields) OR
‘radiotherapeutics’ (all fields)] AND [‘lung cancer’ (Mesh) OR
‘lung neoplasms’ (Mesh) OR ‘lung carcinoma’ (Mesh)] AND [‘brain
metastases’ (Mesh) OR ‘intracranial metastases’ (Mesh) OR
‘intracranial metastatic tumor’ (Mesh) OR ‘brain neoplasms’
(Mesh)’]. All reviews, and preclinical and animal trials were
excluded.
Study selection
Eligible trials were those that met the following
criteria: i) Randomized controlled trials (RCTs) or clinical
controlled trails with voluntarily enrolled patients; ii) Patients
suffered from histologically or cytologically confirmed lung cancer
and had been diagnosed with brain metastases using computed
tomography (CT) or magnetic resonance imaging (MRI); iii) The
trials were anti-tumor agents plus radiotherapy (WBRT/SRS or in
combination) which were considered as combination group vs.
radiotherapy alone group; iv) Trials excluded patients with double
or multiple primary cancer or presence of unstable systemic
disease; v) The analyses included objective response rate (ORR),
overall survival (OS), progression-free survival (PFS), time to
central nervous system/neurological progression
(CNS-TTP)/neurological PFS/progression-free survival of
intracranial disease/local progression-free survival/interval to
neurologic progression (all were assigned to CNS-TTP in the present
study), severe adverse events (AEs; Grade ≥3); vi) Response rate
was determined using the Response Evaluation Criteria in Solid
Tumors (RECIST 1.0 or 1.1 standards) or World Health Organization
(WHO) criteria (11–13,15,17,18,21);
vii) Adverse events were evaluated according to the National Cancer
Institute Common Terminology Criteria for Adverse Events (CTCAE 2.0
or 3.0) or Radiation Therapy Oncology Group (RTOG) adverse events
grading criteria (3,10–12,14,15,17–21).
Data extraction and quality
assessment
For each selected publication, the following
information was extracted: First author, year of publication,
country of original trial, type of trial, trial phase, number of
patients, median ages, interventions and outcomes. To assess the
quality and applicability of each previous study, checklists from
The Cochrane Handbook for Systematic Reviews of intervention
(version 5.1.0) were used, based on the following criteria: i)
Random sequence generation; ii) Allocation concealment; iii)
Blinding of participants and personnel; iv) Blinding of outcome
assessment; v) Incomplete outcome data; vi) Selective reporting;
vii) Other bias. Each trial for bias based on the criteria listed
above was marked as low, high or unclear risk. The quality of the
trails were defined as following: A rating, meeting all criteria of
low risk; B rating, meeting one or more criteria of unclear risk
without high risk; C rating, meeting one or more criteria of high
risk.
Statistical analysis
Statistical analysis was performed using RevMan 5.3
software (Cochrane Collaboration's, which is a non-profit and
non-governmental organization, Information Management System).
Analysis of the data comprised of the pooled risk ratio (RR) for
dichotomous endpoints (e.g., ORR, severe AEs), using the
Mantel-Haenszel method (22). The
events and total number of patients from the combination group and
radiotherapy alone group in the trials for ORR and severe AEs were
extracted from the trials (10–15,
17–21). OS, PFS and CNS-TTP were calculated
using effect variables and expressed as the hazard ratio (HR). HRs
with 95% confidence intervals (CIs) were extracted from trials or
from the survival curves using the methods described by Tierney
et al (23) for OS, PFS and
CNS-TTP when HRs were unavailable. The 95% CIs were calculated and
presented in forest plots. Statistical heterogeneity of different
trials was evaluated using the χ2 and I2
tests (24); no heterogeneity
existed when P>0.1 and I2<50% and a fixed-effect
model was applied to pool the study results. Significant
heterogeneity was found if P<0.1 or I2>50%, and a
random-effects statistical model was used (25). The risk of publication bias was
evaluated via visual appraisal of funnel plots.
Results
Study characteristics
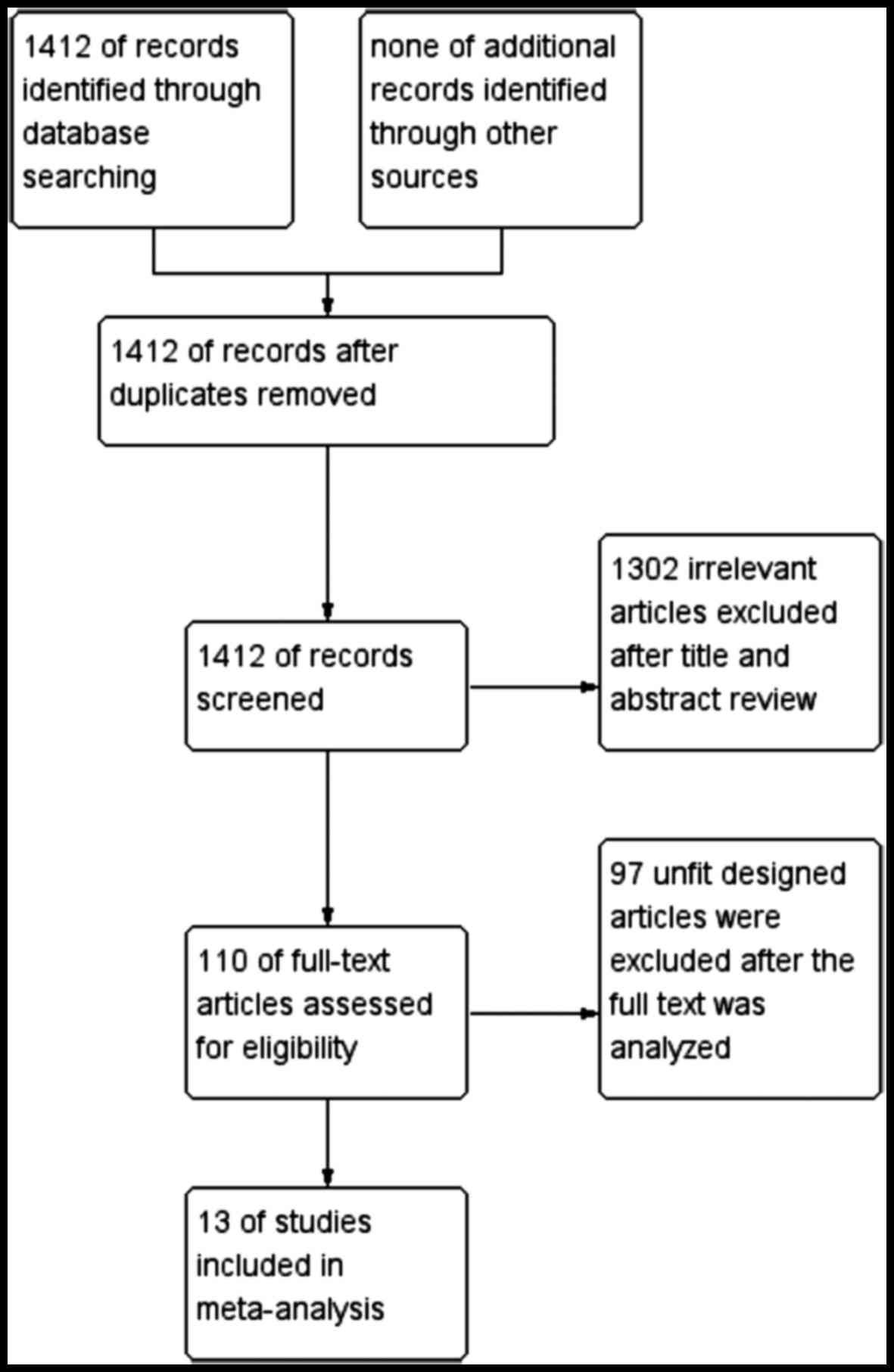
A total of 1,412 previous studies met the selection
criteria after searching the relevant databases. By verifying
related terms in the titles and abstracts, 1,302 irrelevant
articles and another 97 articles with an unfit design were excluded
after the full text was analyzed. Finally, 13 clinical controlled
trials (3,10–21) were
included in the present meta-analysis. A flowchart depicting
inclusion is shown in Fig. 1.
In these 13 controlled trials, 1,783 patients with
BMs were observed, including 853 patients with antitumor agents
plus radiotherapy and 930 patients with radiotherapy alone. These
results are summarized in Table I.
Among these 13 trials, 5 were phase III clinical trials (3,10,11,20,21),
6 phase II trials (12,14,15,17–19) and
2 studies failed to mention a trial phase (13,16). Of
these trails, 11 involved an antitumor agents plus WBRT, compared
with WBRT alone (3,10–15,17–19,21), and
the others included antitumor agents combined with WBRT plus SRS,
compared with WBRT plus SRS (16,20).
Outcomes included ORR, OS, PFS, CNS-TTP and severe AEs.
 | Table I.Characteristics of the trials
included in the metaanalysis. |
Table I.
Characteristics of the trials
included in the metaanalysis.
|
|
|
|
|
| Interventions | Outcomes |
|---|
|
|
|
|
|
|
|
|
|---|
| Author, year | Country | Trial phase | No patients
(C/R) | Ages (C/R,
years) | Combination
group | Radiotherapy alone
group | ORR | OS | CNS- TTP | PFS | AEs (grade ≥3) | Refs. |
|---|
| Mehta et al,
2003 | America | III | 123/128 | N | MGd + WBRT | WBRT | N | N | Y | N | Y | (3) |
| Mehta et al,
2009 | America | III | 279/275 | 59/59 | MGd + WBRT | WBRT | N | N | Y | N | Y | (10) |
| Neuhaus et
al, 2009 | Germany | III | 47/49 | 58/59 | Topotecan +
WBRT | WBRT | Y | Y | N | Y | Y | (11) |
| Ge et al,
2013 | China | II | 38/38 | 59/60.5 | Topotecan +
WBRT | WBRT | Y | Y | Y | Y | Y | (12) |
| Ushio et al,
1991 | Japan | N | 29/25 | 58/63 | CENU + tegafur +
WBRT | WBRT | Y | Y | N | N | N | (13) |
| Lee et al,
2014 | UK | II | 40/40 | 61.3/62.2 | Erlotinib +
WBRT | Placebo + WBRT | N | Y | Y | N | Y | (14) |
| Jiang et al,
2014 | China | II | 40/40 | 67/64 | Endostatin +
WBRT | WBRT | Y | Y | N | N | Y | (15) |
| Cai et al,
2014 | China | N | 104/178 | 65/65 | TKI+
WBRT/SRS/S | WBRT/SRS/S | N | Y | Y | N | N | (16) |
| Zhuang et
al, 2013 | China | II | 23/31 | 60/63 | Erlotinib +
WBRT | WBRT | Y | Y | Y | Y | Y | (17) |
| Hassler et
al, 2013 | Austria | II | 22/13 | 69/64 | TMZ + WBRT | WBRT | Y | Y | N | Y | Y | (18) |
| Chua et al,
2010 | China | II | 47/48 | 59/62 | TMZ + WBRT | WBRT | N | Y | Y | N | Y | (19) |
| Sperduto et
al, 2013 | America | III | 40/44 | 63/64 | TMZ + WBRT +
SRS | WBRT+ SRS | N | Y | N | N | Y | (20) |
| Guerrieri et
al, 2004 | Australia | III | 21/21 | 60/63 | Carboplatin +
WBRT | WBRT | Y | Y | N | N | Y | (21) |
Data for all characteristics are summarized in
Table II. Gender, Karnofsky
performance score, number of BMs, extracranial metastases,
histology, epidermal growth factor receptor mutation status and
recursive partitioning analysis were available for 11, 4, 3, 8,7, 2
and 5 of the 13 trials, respectively.
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
|
Characteristics | Cgroup (%)
n=853 | Rgroup (%)
n=930 |
|---|
| Gender |
|
|
|
Male | 414 (49) | 454 (49) |
|
Female | 276 (32) | 304 (33) |
|
Unknown | 163 (19) | 172 (18) |
| KPS |
|
|
|
≥70 | 389 (46) | 394 (42) |
|
<70 | 0 (0) | 0 (0) |
|
Unknown | 464 (54) | 536 (58) |
| No. of BMs |
|
|
| ≤3 | 82 (10) | 87 (9) |
|
>3 | 36 (4) | 35 (4) |
|
Unknown | 735 (86) | 808 (87) |
| Extracranial
metastases |
|
|
|
Yes | 331 (39) | 393 (42) |
| No | 287 (34) | 310 (33) |
|
Unknown | 235 (27) | 227 (25) |
| Histology |
|
|
|
Adenocarcinoma | 348 (41) | 403 (43) |
|
Squamous | 64 (7) | 72 (8) |
|
Other | 92 (11) | 88 (9) |
|
Unknown | 349 (41) | 367 (40) |
| EGFR mutation |
|
|
|
Positive | 40 (5) | 26 (3) |
|
Negative | 33 (4) | 33 (3) |
|
Unknown | 780 (91) | 871 (94) |
| RPA |
|
|
| I | 57 (7) | 55 (6) |
| II | 347 (41) | 348 (37) |
|
Unknown | 449 (52) | 527 (57) |
Methodological quality
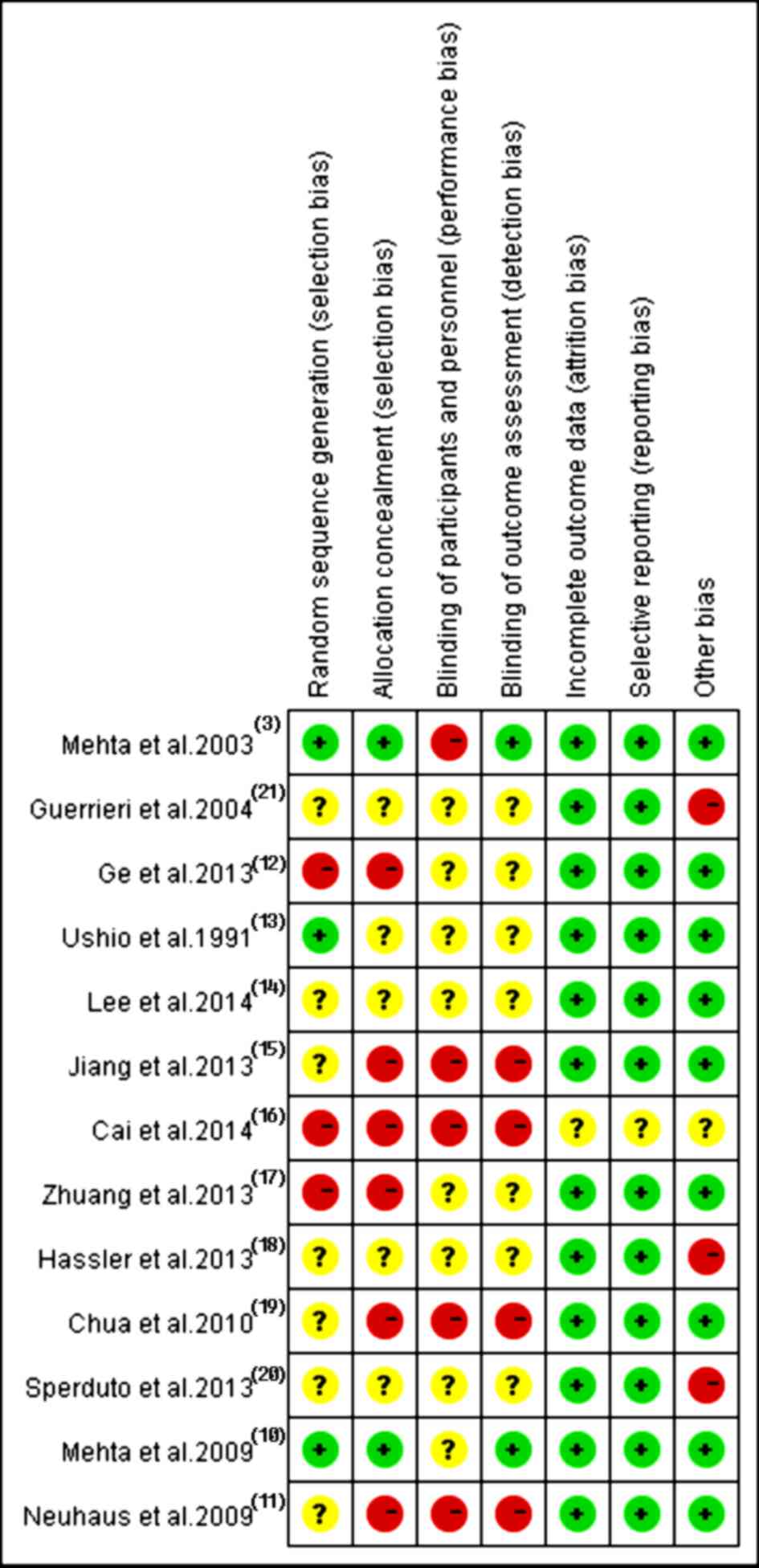
In accordance with the recommendations of the
Cochrane Handbook for Systematic Reviews, the present study
assessed the eligible trials using the seven aspects mentioned
above. Among these 13 recruited trials, ten (3,10–11,13–15,18–21)
referred to the use of random allocation, three discussed the
methods (3,10,13), two
(3,10) performed or reported their blinding
methods and two (18,19) reported their allocation concealment.
All trials applied the intent-to-treat analysis and underwent
quality assessment. Eventually, three (10,13,14)
received B quality scores and 10 (3,11,12,15–21)
received C quality scores, as shown in Fig. 2.
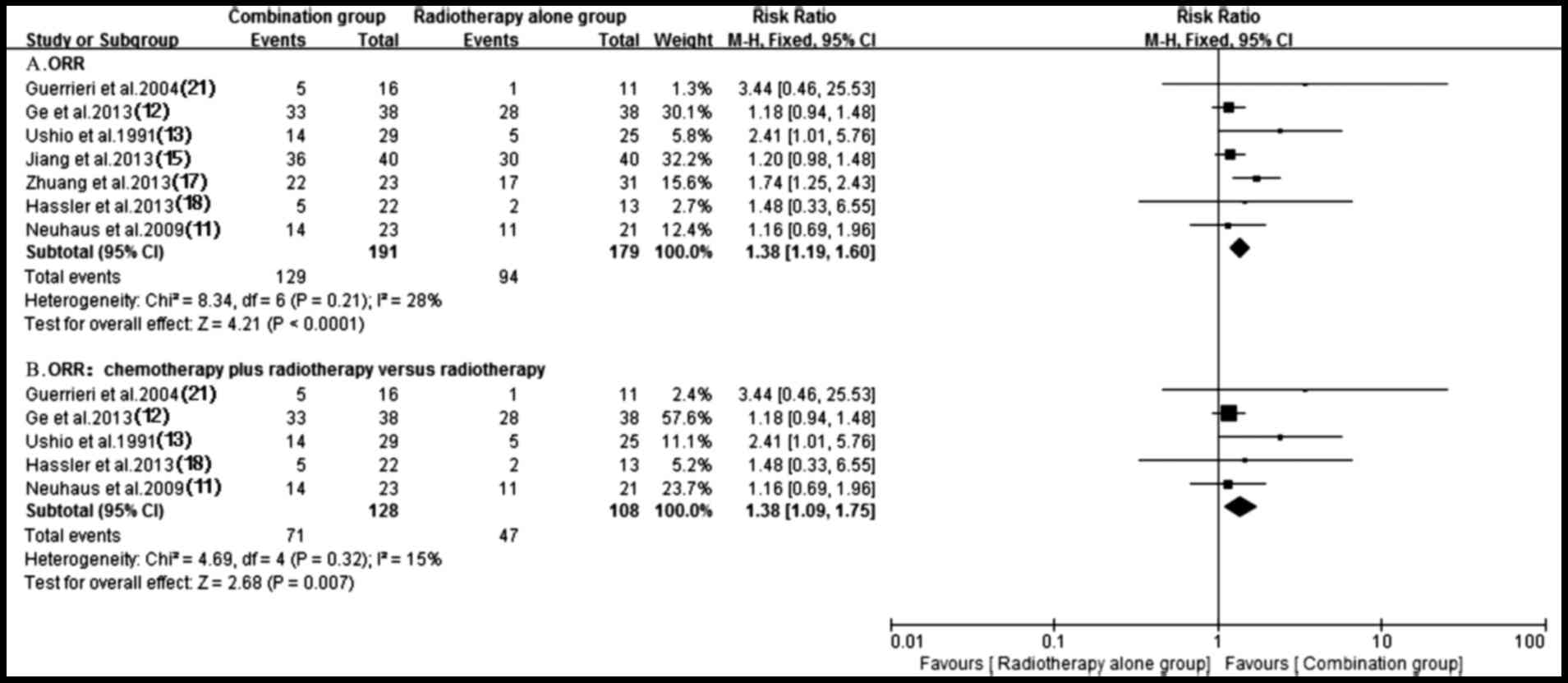
Local response rate
A total of seven trials (11–13,15,17,18,21)
testing response rate of treatment using antitumor agents plus
radiotherapy compared with radiotherapy alone were identified. A
fixed-effect model was utilized for the meta-analysis since
heterogeneity did not exist (P=0.21; I2=28%). Notably,
pooled data from the study results revealed a significant
difference in terms of ORR between the groups of patients who were
treated with antitumor agents plus radiotherapy and those who had
received radiotherapy alone (RR, 1.38; 95% CI, 1.19–1.60;
P<0.0001) (Fig. 3). Subgroup
analysis of radiochemotherapy vs. radiotherapy alone demonstrated a
significant ORR benefit for the radiochemotherapy arm (RR, 1.38;
95% CI, 1.09–1.75; P=0.007) (Fig.
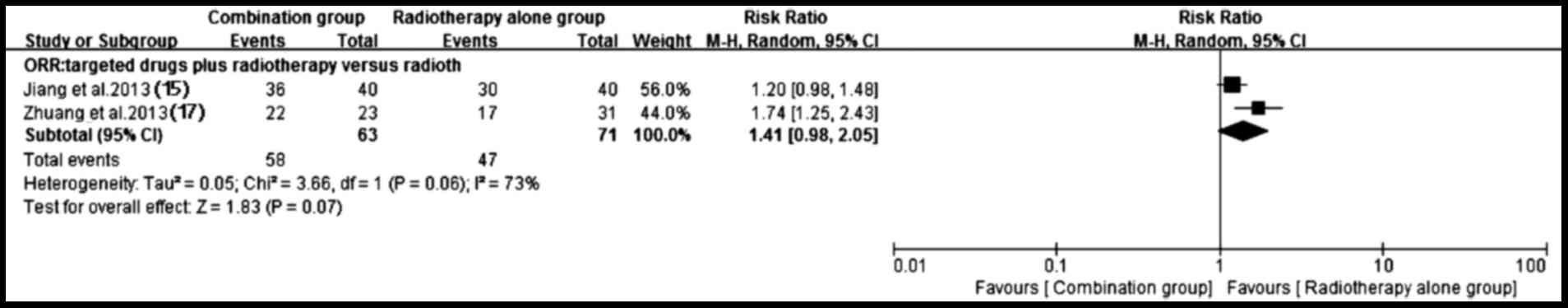
3). By contrast, a higher ORR (P=0.07) trend favoring targeted
drugs plus radiotherapy group was observed (15,17)
despite the existence of heterogeneity (P=0.06; I2=73%)
(Fig. 4).
Time to central nervous system
progression
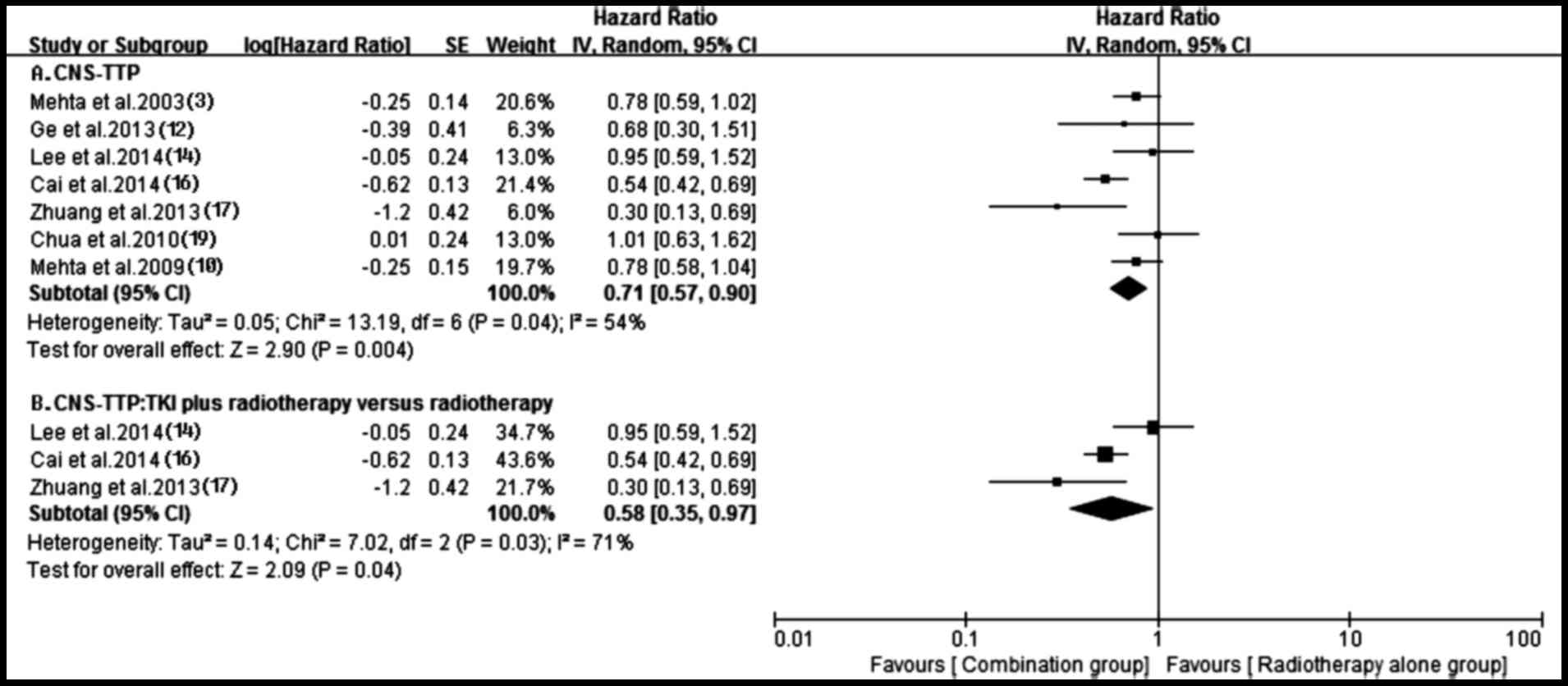
A total of seven trials (3,10,12,14,16,17,19)
reported time to central nervous system progression (CNS-TTP) in
both groups. A random-effects model was applied based on the
heterogeneity values (P=0.04, I2=54%). The outcome
suggested that compared with radiotherapy alone, antitumor agents
plus radiotherapy possessed a superior CNS-TTP for patients (HR,
0.71; 95% CI, 0.57–0.90; P=0.004) (Fig.
5). The subgroup analysis obtained a similar superior CNS-TTP
in the tyrosine kinase inhibitor (TKI) plus radiotherapy group,
compared with the radiotherapy alone group (HR, 0.58; 95% CI,
0.35–0.97; P=0.04), although heterogeneity existed among them
(P=0.03, I2=71%) (Fig.
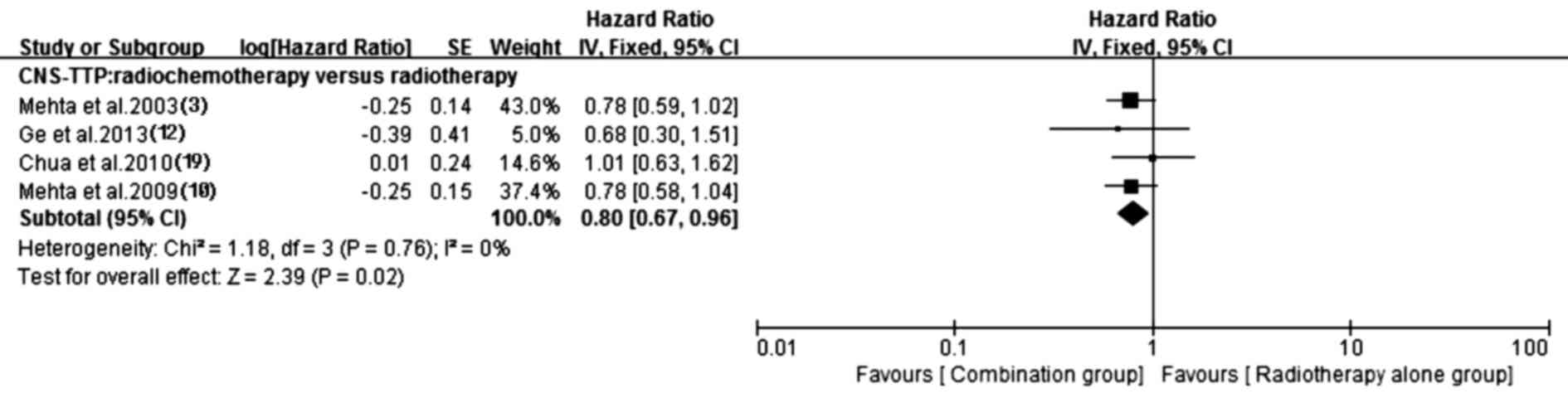
5). Additionally, the prolonged CNS-TTP also appeared in the
chemotherapy plus radiotherapy group (HR, 0.80; 95% CI, 0.67–0.96;
P=0.02) without heterogeneity (P=0.76, I2=0%). Details
of this pooled analysis are shown in Fig. 6.
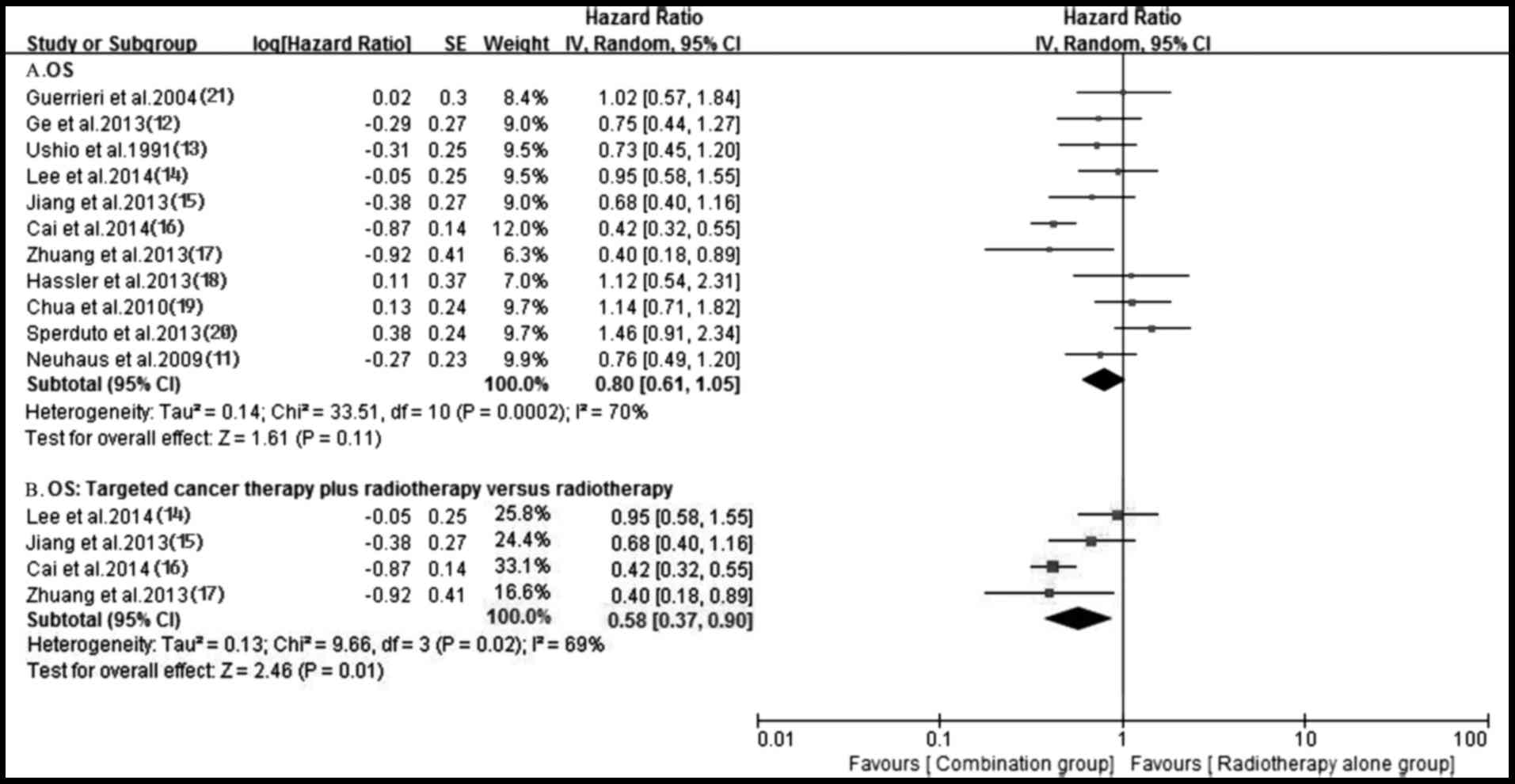
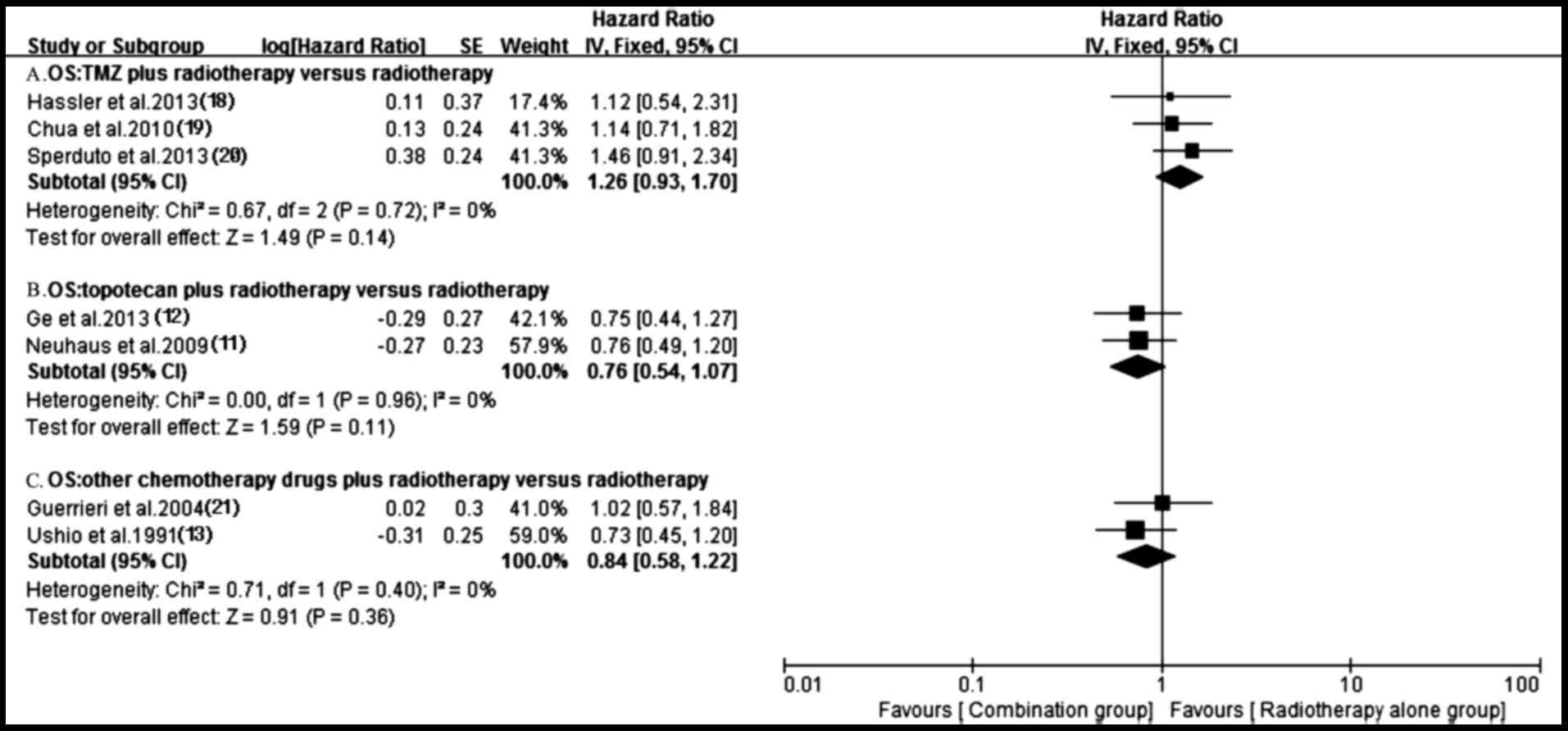
Survival
A total of 11 trials (11–21)
reported OS in both groups. Owing to the heterogeneity values
(P=0.0002, I2=70%), a random-effects model was employed
to analyze OS and no significantly longer OS was observed in the
antitumor agents plus radiotherapy group compared with that in
radiotherapy alone group (HR, 0.80; 95% CI, 0.61–1.05; P=0.11)
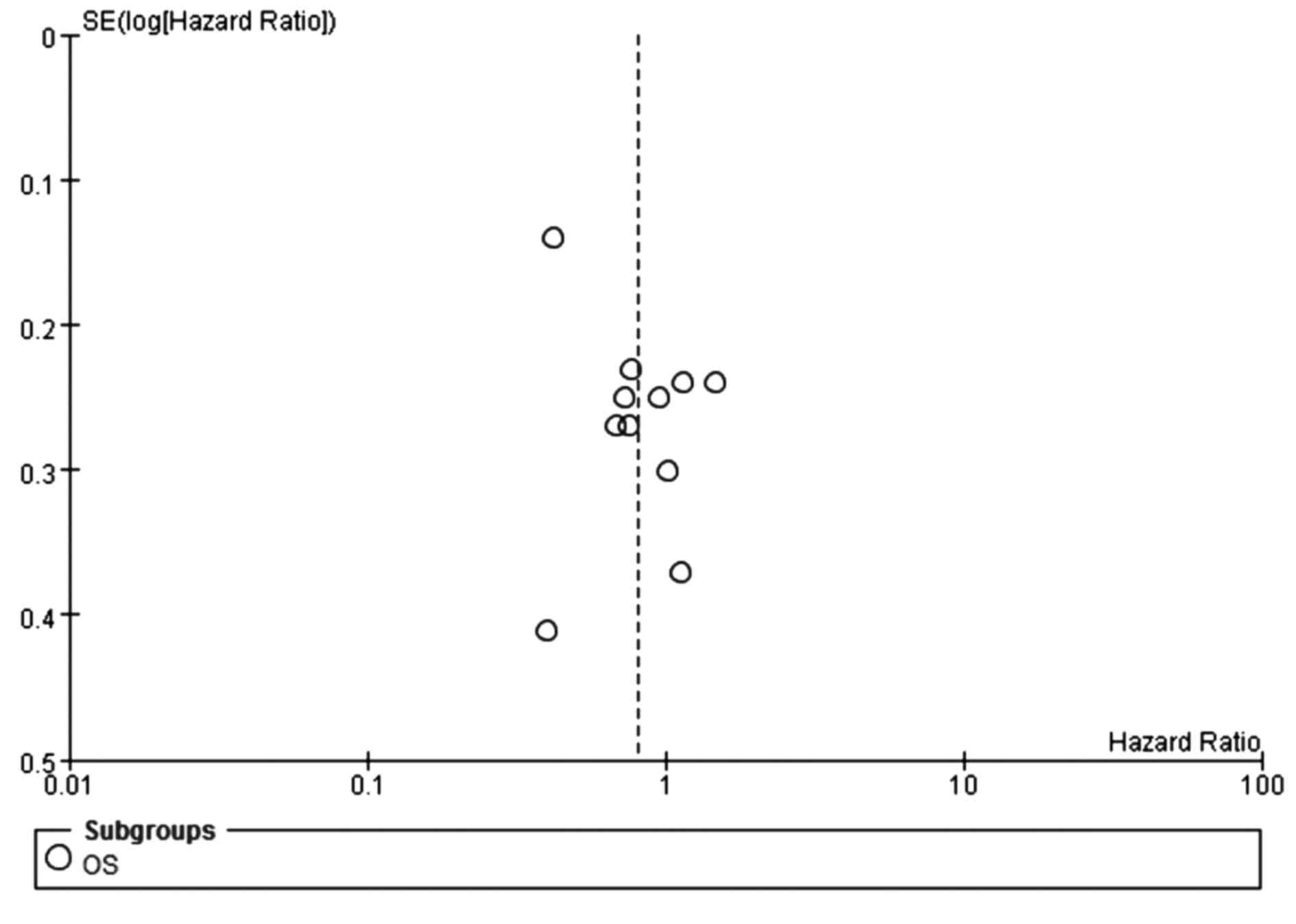
(Fig. 7). The funnel plot indicated
no significant publication bias for OS (Fig. 8). Additionally, no prolonged OS was
observed in the chemotherapy plus radiotherapy group compared with
the radiotherapy alone group (HR, 0.96; 95% CI, 0.79–1.17; P=0.68)
(Fig. 9). Notably, the OS of the
targeted agents plus radiotherapy was significantly superior to
radiotherapy alone (HR, 0.58; 95% CI, 0.37–0.90; P=0.01) with an
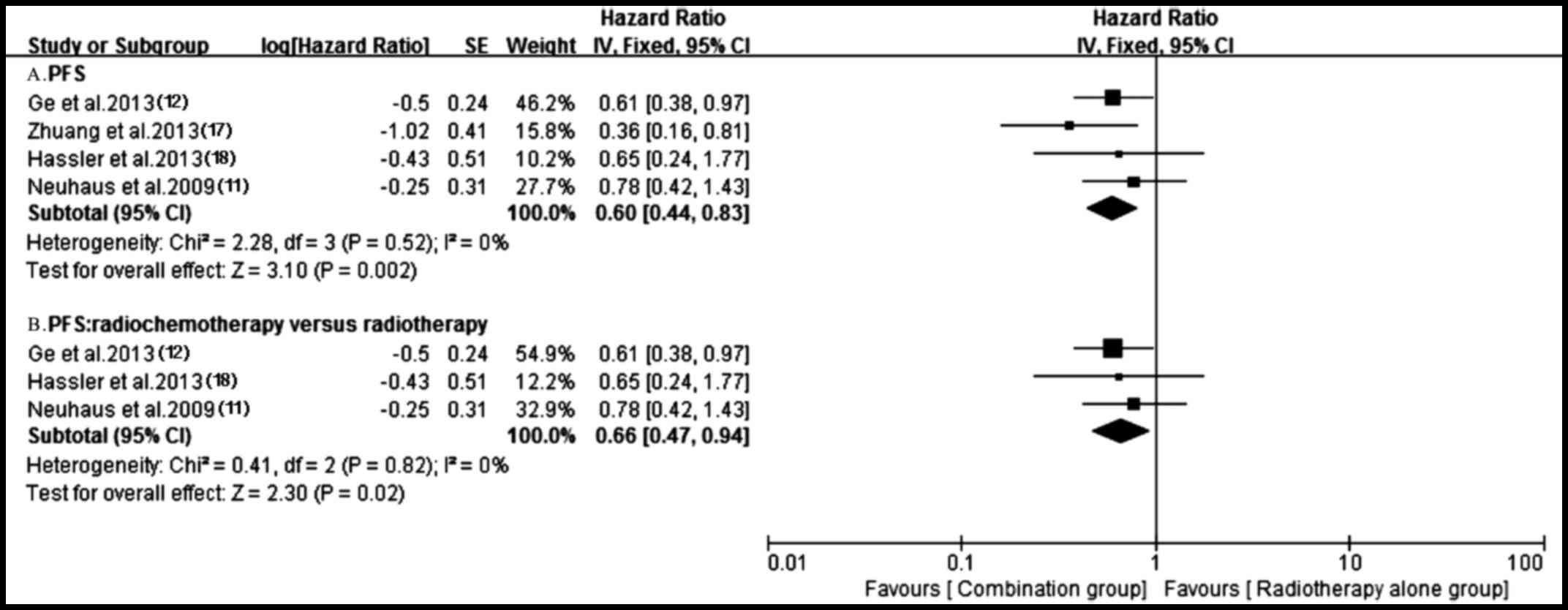
important heterogeneity (P=0.02, I2=69%) (Fig. 7). A total of four trials (11,12,17,18)
reporting PFS were analyzed applying a fixed-effect model based on
the heterogeneity values (P=0.52, I2=0%) and revealed
that, compared with radiotherapy alone, antitumor agents plus
radiotherapy significantly prolonged PFS (HR, 0.60; 95% CI,
0.44–0.83; P=0.002) (Fig. 10).
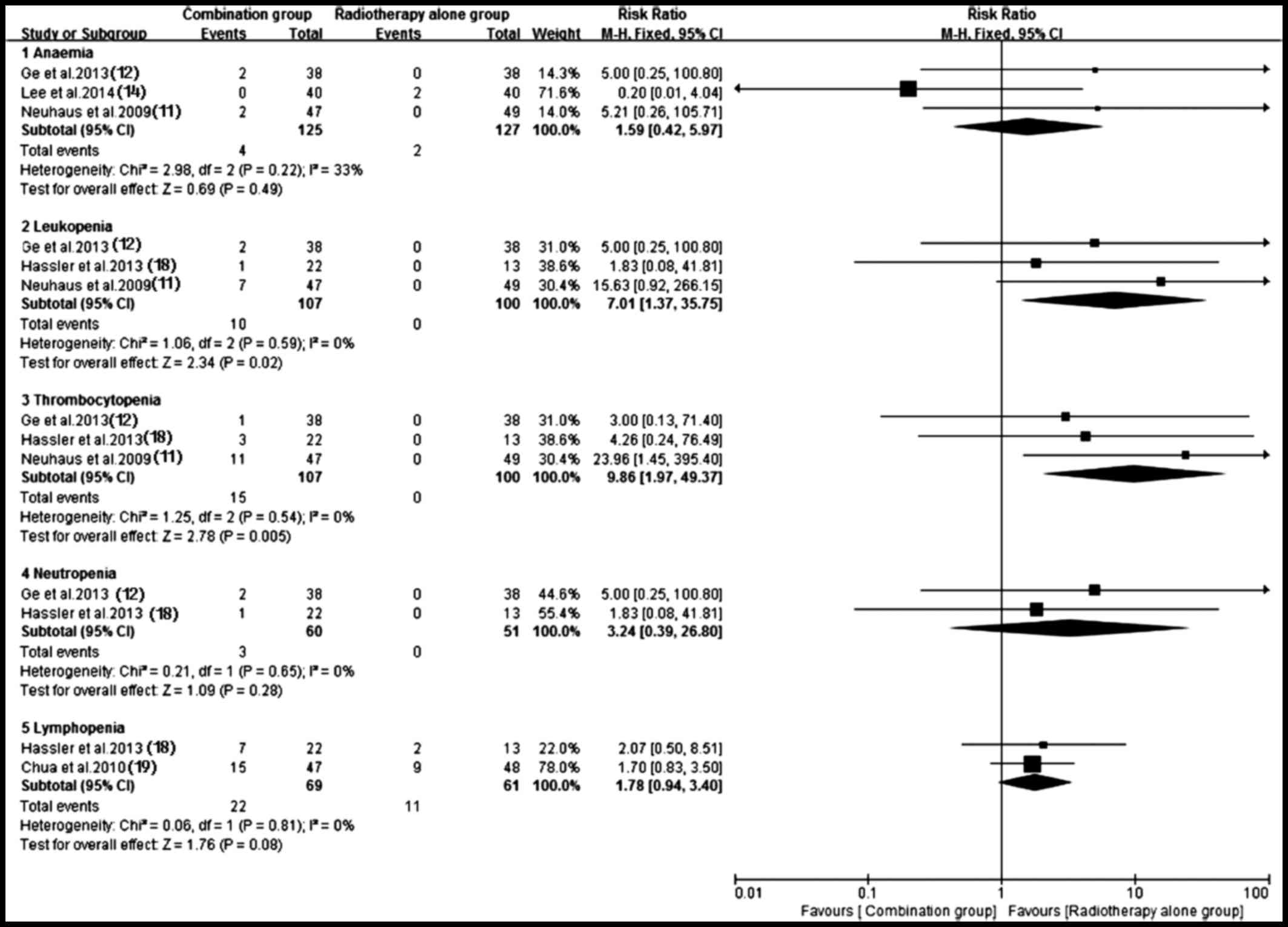
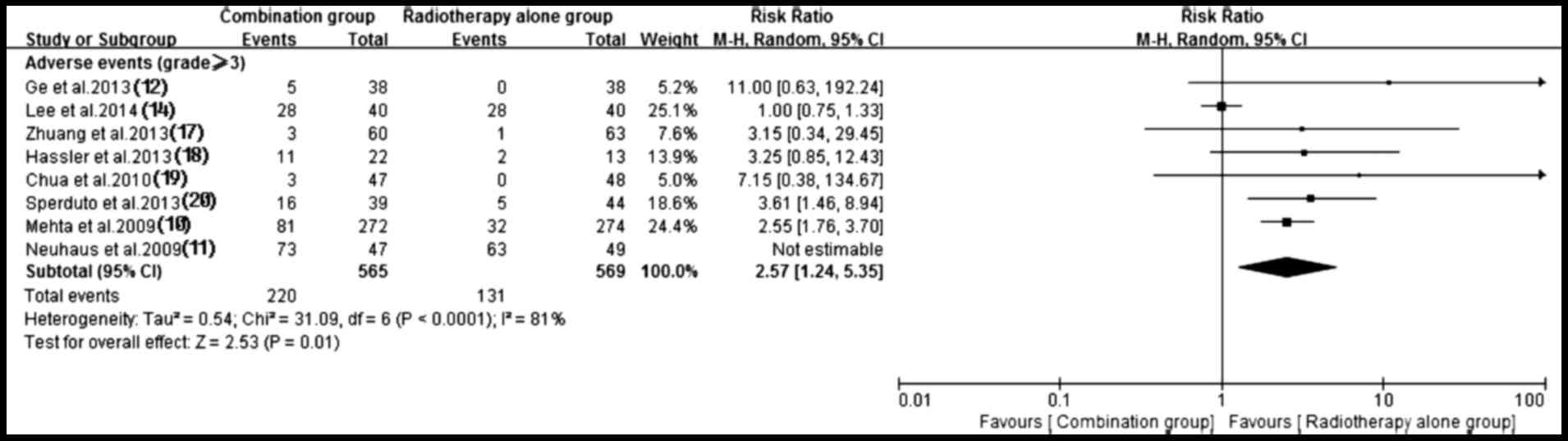
Adverse events
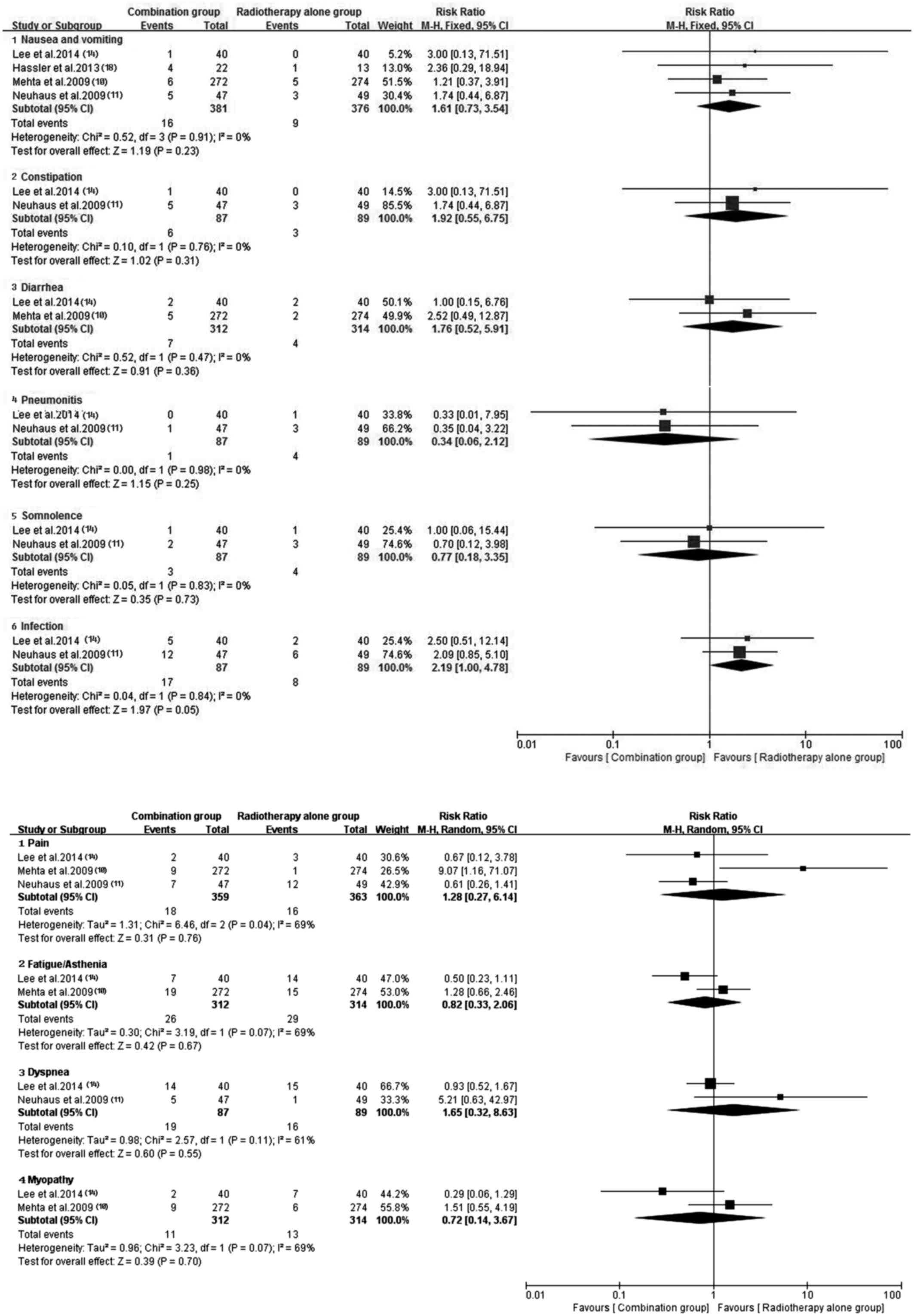
Of the included trials, eight (10–12,14,17–20) with
sufficient data of treatment-related toxicity and severe AEs
grading were applied to analyze AEs. A random-effects model was
used to analyze overall severe AEs based on the heterogeneity
values (P<0.0001, I2=81%). The results indicated that
antitumor agents plus radiotherapy elevated the incidence of
overall severe AEs (RR, 2.57; 95% CI, 1.24–5.35; P=0.01) (Fig. 11). Consequently, the present study
performed a subgroup analysis for the severe AEs (Figs. 12 and 13). Hematotoxicity was the most common AE
in the antitumor agents plus radiotherapy group. A notably higher
incidence of grade III–IV leukopenia (RR, 7.01; 95% CI, 1.37–35.75;
P=0.02) and thrombocytopenia (RR, 9.86; 95% CI, 1.97–49.37;
P=0.005) was observed in the antitumor agents plus radiotherapy
group. However, the most common non-hematological AEs, which were
largely mild and tolerable without significant difference between
antitumor agents plus radiotherapy and radiotherapy alone,
including fatigue/asthenia (RR, 0.82; 95% CI, 0.33–2.06; P=0.67),
dyspnea (RR, 1.65; 95% CI, 0.32–8.63; P=0.55), pain (RR, 1.28; 95%
CI, 0.27–6.14; P=0.76), myopathy (RR, 0.72; 95% CI, 0.14–3.67;
P=0.70), nausea/vomiting (RR, 1.61; 95% CI, 0.73–3.54; P=0.23) and
infection (RR, 2.19; 95% CI, 1.00–4.78; P=0.05) (Fig. 13). Additionally, no severe
hematotoxicity was observed in both the targeted agents plus
radiotherapy group and the radiotherapy alone group.
Discussion
Since radiation therapy only acquires limited local
control of BMs, it is reasonable to make an attempt to combine
antitumor agents with radiotherapy to maximize efficacy.
Theoretically, due to the effect of the blood-brain barrier (BBB),
the majority of chemotherapeutic agents were unable to reach
sufficient concentration in BMs lesions (26), and LC patients with BMs may not gain
benefit from these agents. However, previous clinical studies
demonstrated that temozolomide, tegafur, chloroethyl nitrosoureas
(methyl-CCNU or ACNU) and topotecan were effective in dealing with
BMs, which partly derived from the distinctive property of the
drugs, including their high capacity of penetrating the BBB and
unique antitumor mechanisms (27–33).
These interesting findings have inspired oncologists to design
modality of combining antitumor agents with radiotherapy.
The present study demonstrated that antitumor agents
plus radiotherapy possessed a significant benefit in terms of ORR,
CNS-TTP and PFS, which may have a potential application value for
LC patients with BMs. In addition, subgroup analysis also revealed
superior ORR and CNS-TTP in the chemoradiotherapy group to
radiotherapy alone group. The clinical outcomes may be elaborated
by the following mechanisms: i) Radiotherapy, immature tumor
angiogenesis and edema may amplify the destruction of the BBB and
result in increasing permeability of the BBB (34–36); ii)
certain antitumor agents own radiosensitizing effects (37,38);
iii) the tumor growth may disrupt the BBB (39).
Furthermore, the present study demonstrated that
targeted agents plus radiotherapy prolonged the OS without severe
hematotoxicity. The mechanisms may be as follows: i) Smaller
molecular targeted agents possess a high capacity of penetrating
the BBB (40,41); ii) targeted agents have synergistic
effect with radiotherapy in managing BMs (42); iii) unlike chemotherapy agents,
targeted agents own a high selectivity against cancer cells, which
partly accounts for the mild and low toxicity of this regimen
(43).
Undoubtedly, cytotoxic agents inhibited bone marrow
cells, which accounted for the hematotoxicity observed in patients
treated with chemotherapy, while EGFR-TKI therapy frequently
resulted in mild and reversible acne rash and diarrhea (44). Compared with standard chemotherapy,
the milder the toxicity profile was produced by EGFR-TKI, the less
quality of life was intervened (45). Additionally, the higher risk of
treatment interruption due to severe AEs in radiochemotherapy may
partly explain its unfavorable OS.
In conclusion, the present study demonstrated that
targeted agents plus radiotherapy yielded desirable effects with
mild AEs. Therefore, it is advisable to combine targeted agents
with radiotherapy to deal with inoperable BMs from LC. Secondary to
best, chemoradiotherapy is an alternative option for patients
without suitable molecular targets.
References
|
1
|
Soffietti R, Rudà R and Trevisan E: Brain
metastases: Current management and new developments. Curr Opin
Oncol. 20:676–684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Delattre JY, Krol G, Thaler HT and Posner
JB: Distribution of brain metastases. Arch Neurol. 45:741–744.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehta MP, Rodrigus P, Terhaard CH, Rao A,
Suh J, Roa W, Souhami L, Bezjak A, Leibenhaut M, Komaki R, et al:
Survival and neurologic outcomes in a randomized trial of motexafin
gadolinium and whole-brain radiation therapy in brain metastases. J
Clin Oncol. 21:2529–2536. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sundstörm JT, Minn H, Lertola KK and
Nordman E: Prognosis of patients treated for intracranial
metastases with whole-brain irradiation. Ann Med. 30:296–299. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patchell RA, Tibbs PA, Walsh JW, Dempsey
RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS and Young
B: A randomized trial of surgery in the treatment of single
metastases of the brain. N Engl J Med. 322:494–500. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho KH, Hall WA, Gerbi BJ, Higgins PD,
Bohen M and Clark HB: Patient selection criteria for the treatment
of brain metastases with stereotactic radiosurgery. J Neurooncol.
40:73–86. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morris PG, Correa DD, Yahalom J, Raizer
JJ, Schiff D, Grant B, Grimm S, Lai RK, Reiner AS, Panageas K, et
al: Rituximab, methotrexate, procarbazine, and vincristine followed
by consolidation reduced-dose whole-brain radiotherapy and
cytarabine in newly diagnosed primary CNS lymphoma: Final results
and long-term outcome. J Clin Oncol. 31:397l–3979. 2013. View Article : Google Scholar
|
|
8
|
Cortot AB, Gerinière L, Robinet G, Breton
JL, Corre R, Falchero L, Berard H, Gimenez C, Chavaillon JM, Perol
M, et al: Phase II trial of temozolomide and cisplatin followed by
whole brain radiotherapy in non-small-cell lung cancer patients
with brain metastases: A GLOT-GFPC study. Ann Oncol. 17:1412–1417.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lukas RV, Nicholas MK, Villaflor V,
Hoffman PC and Salgia R: Temozolomide and/or erlotinib in the
treatment of lung cancer patients with progressive central nervous
system metastases. J Neurol Res. 2:1–9. 2012.PubMed/NCBI
|
|
10
|
Mehta MP, Shapiro WR, Phan SC, Gervais R,
Carrie C, Chabot P, Patchell RA, Glantz MJ, Recht L, Langer C, et
al: Motexafin gadolinium combined with prompt whole brain
radiotherapy prolongs time to neurologic progression in
non-small-cell lung cancer patients with brain metastases: Results
of a phase III trial. Int J Radiat Oncol Biol Phys. 73:1069–1076.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neuhaus T, Ko Y, Muller RP, Grabenbauer
GG, Hedde JP, Schueller H, Kocher M, Stier S and Fietkau R: A phase
III trial of topotecan and whole brain radiation therapy for
patients with CNS-metastases due to lung cancer. Br J Cancer.
100:291–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ge XH, Lin Q, Ren XC, Liu YE, Chen XJ,
Wang DY, Wang YQ, Cao B, Li ZG and Liu ML: Phase II clinical trial
of whole-brain irradiation plus three-dimensional conformal boost
with concurrent topotecan for brain metastases from lung cancer.
Radiat Oncol. 8:2382013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ushio Y, Arita N, Hayakawa T, Mogami H,
Hasegawa H, Bitoh S, Oku Y, Ikeda H, Kanai N and Kanoh M:
Chemotherapy of brain metastases from lung carcinoma: A controlled
randomized study. Neurosurgery. 28:201–205. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SM, Lewanski CR, Counsell N,
Ottensmeier C, Bates A, Patel N, Wadsworth C, Ngai Y, Hackshaw A
and Faivre-Finn C: Randomized trial of erlotinib plus whole-brain
radiotherapy for NSCLC patients with multiple brain metastases. J
Natl Cancer Inst. 106:pii: dju151. 2014. View Article : Google Scholar
|
|
15
|
Jiang X, Ding M, Qiao Y, Liu Y and Liu L:
Recombinant human endostatin combined with radiotherapy in the
treatment of brain metastases of non-small cell lung cancer. Clin
Transl Oncol. 16:630–636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai L, Zhu JF, Zhang XW, Lin SX, Su XD,
Lin P, Chen K and Zhang LJ: A comparative analysis of EGFR mutation
status in association with the efficacy of TKI in combination with
WBRT/SRS/surgery plus chemotherapy in brain metastasis from
non-small cell lung cancer. J Neurooncol. 120:423–430. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhuang H, Yuan Z, Wang J, Zhao L, Pang Q
and Wang P: Phase II study of whole brain radiotherapy with or
without erlotinib in patients with multiple brain metastases from
lung adenocarcinoma. Drug Des Devel Ther. 7:1179–1186. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hassler MR, Pfeifer W, Knocke-Abulesz TH,
Geissler K, Altorjai G, Dieckmann K and Marosi C: Temozolomide
added to whole brain radiotherapy in patients with multiple brain
metastases of non-small-cell lung cancer: A multicentric Austrian
phase II study. Wien Klin Wochenschr. 125:481–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chua D, Krzakowski M, Chouaid C, Pallotta
MG, Martinez JI, Gottfried M, Curran W and Throuvalas N:
Whole-brain radiation therapy plus concomitant temozolomide for the
treatment of brain metastases from non-small-cell lung cancer: A
randomized, open-label phase II study. Clin Lung Cancer.
11:176–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sperduto PW, Wang M, Robins HI, Schell MC,
Werner-Wasik M, Komaki R, Souhami L, Buyyounouski MK, Khuntia D,
Demas W, et al: A phase 3 trial of whole brain radiation therapy
and stereotactic radiosurgery alone versus WBRT and SRS with
temozolomide or erlotinib for non-small cell lung cancer and 1 to 3
brain metastases: Radiation therapy oncology group 0320. Int J
Radiat Oncol Biol Phys. 85:1312–1318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guerrieri M, Wong K, Ryan G, Millward M,
Quong G and Ball DL: A randomized phase III study of palliative
radiation with concomitant carboplatin for brain metastases from
non-small cell carcinoma of the lung. Lung Cancer. 46:107–111.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
23
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ford AC, Forman D, Hunt RH, Yuan Y and
Moayyedi P: Helicobacter pylori eradication therapy to prevent
gastric cancer in healthy asymptomatic infected individuals:
Systematic review and meta-analysis of randomized controlled
trials. BMJ. 348:g31742014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Vulpen M, Kal HB, Taphoorn MJ and El
Sharouni S: Changes in blood-brain barrier permeability induced by
radiotherapy: Implications for timing of chemotherapy? (Review).
Oncol Rep. 9:683–688. 2002.PubMed/NCBI
|
|
27
|
Middlemas DS, Stewart CF, Kirstein MN,
Poquette C, Friedman HS, Houghton PJ and Brent TP: Biochemical
correlates of temozolomide sensitivity in pediatric solid tumor
xenograft models. Clin Cancer Res. 6:998–1007. 2000.PubMed/NCBI
|
|
28
|
Raymond E, Izbicka E, Soda H, Gerson SL,
Dugan M and Von Hoff DD: Activity of temozolomide against human
tumor colony-forming units. Clin Cancer Res. 3:1769–1074.
1997.PubMed/NCBI
|
|
29
|
Srivenugopal KS, Shou J, Mullapudi SR,
Lang FF Jr, Rao JS and Ali-Osman F: Enforced expression of
wild-type p53 curtails the transcription of the
O(6)-methylguanine-DNA methyltransferase gene in human tumor cells
and enhances their sensitivity to alkylating agents. Clin Cancer
Res. 7:1398–4009. 2001.PubMed/NCBI
|
|
30
|
Kohno T, Shitara N, Takakura K and Fujita
H: Role of FT-207 in the treatment of metastatic brain tumors.
Cancer Chemother (Tokyo). 3:729–734. 1976.
|
|
31
|
Ushio Y, Posner JB and Shapiro WR:
Chemotherapy of experimental meningeal carcinomatosis. Cancer Res.
37:1232–1237. 1977.PubMed/NCBI
|
|
32
|
Sung C, Blaney SM, Cole DE, Balis FM and
Dedrick RL: A pharmacokinetic model of topotecan clearance from
plasma and cerebrospinal fluid. Cancer Res. 54:5118–5122.
1994.PubMed/NCBI
|
|
33
|
Baker SD, Heideman RL, Crom WR, Kuttesch
JF, Gajjar A and Stewart CF: Cerebrospinal fluid pharmacokinetics
and penetration of continuous infusion topotecan in children with
central nervous system tumors. Cancer Chemother Pharmacol.
37:195–202. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
O'Connor MM and Mayberg MR: Effects of
radiation on cerebral vasculature: A review. Neurosurgery.
46:138–151. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hutchinson F: Molecular biology of
mutagenesis of mammalian cells by ionizing radiation. Semin Cancer
Biol. 4:85–92. 1993.PubMed/NCBI
|
|
36
|
Harms-Ringdahl M, Nicotera P and Radford
IR: Radiation induced apoptosis. Mutat Res. 366:171–179. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Groen H, Sleijfer S, Meijer C, Kampinga
HH, Konings AW, De Vries EG and Mulder NH: Carboplatin- and
cisplatin-induced potentiation of moderate-dose radiation
cytotoxicity in human lung cancer cell lines. Br J Cancer.
72:1406–1411. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu S, Zakian K, Thaler H, Matei C, Alfieri
A, Chen Y and Koutcher JA: Effects of motexafin gadolinium on tumor
metabolism and radiation sensitivity. Int J Radiat Oncol Biol Phys.
49:1381–1390. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grimm SA: Treatment of brain metastases:
Chemotherapy. Curr Oncol Rep. 14:85–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weber B, Winterdahl M, Memon A, Sorensen
BS, Keiding S, Sorensen L, Nexo E and Meldgaard P: Erlotinib
accumulation in brain metastases from non-small cell lung cancer:
Visualization by positron emission tomography in a patient
harbouring a mutation in the epidermal growth factor receptor. J
Thorac Oncol. 6:1287–1289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
McKillop D, Hutchison M, Partridge EA,
Bushby N, Cooper CM, Clarkson-Jones JA, Herron W and Swaisland HC:
Metabolic disposition of Gefitinib, an epidermal growth factor
receptor tyrosine kinase inhibitor, in rat, dog and man.
Xenobiotica. 34:917–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chinnaiyan P, Huang S, Vallabhaneni G,
Armstrong E, Varambally S, Tomlins SA, Chinnaiyan AM and Harari PM:
Mechanisms of enhanced radiation response following epidermal
growth factor receptor signaling inhibition by erlotinib (Tarceva).
Cancer Res. 65:3328–3335. 2005.PubMed/NCBI
|
|
43
|
Chen X, Pan Y, Zhang S, Chen D, Yang S, Li
X and Ma S: Rechallenge with gefitinib following severe
drug-induced hepatotoxicity in a patient with advanced non-small
cell lung cancer: A case report and literature review. Oncol Lett.
7:878–880. 2014.PubMed/NCBI
|
|
44
|
Hirsh V: Managing treatment-related
adverse events associated with egfr tyrosine kinase inhibitors in
advanced non-small cell lung cancer. Curr Oncol. 18:126–138. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fukuoka M, Wu YL, Thongprasert S,
Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu
DT, Saijo N, et al: Biomarker analyses and final overall survival
results from a phase III, randomized, open-label, first-line study
of gefitinib versus carboplatin/paclitaxel in clinically selected
patients with advanced non-small-cell lung cancer in Asia (IPASS).
J Clin Oncol. 29:2866–2874. 2011. View Article : Google Scholar : PubMed/NCBI
|