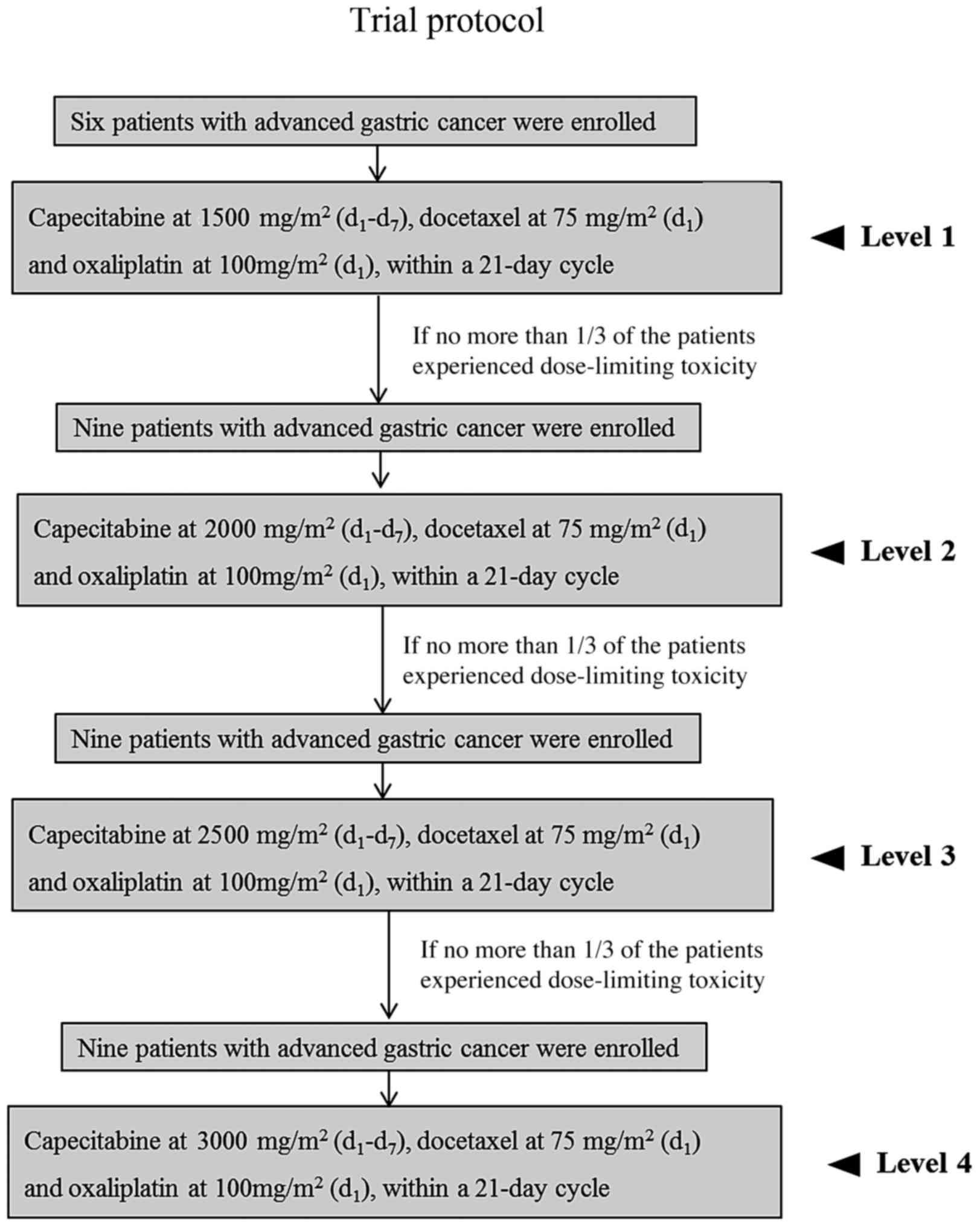
|
1
|
van Cutsem E, Moiseyenko VM, Tjulandin S,
Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi
E, et al: Phase III study of docetaxel and cisplatin plus
fluorouracil compared with cisplatin and fluorouracil as first-line
therapy for advanced gastric cancer: A report of the V325 study
group. J Clin Oncol. 24:4991–4997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil, and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Herpen CM, Mauer ME, Mesia R, Degardin
M, Jelic S, Coens C, Betka J, Bernier J, Remenar E, Stewart JS, et
al: Short-term health-related quality of life and symptom control
with docetaxel, cisplatin, 5-fluorouracil and cisplatin (TPF),
5-fluorouracil (PF) for induction in unresectable locoregionally
advanced head and neck cancer patients (EORTC 24971/TAX 323). Br J
Cancer. 103:1173–1181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Awada A, Gil T, Whenham N, Van Hamme J,
Besse-Hammer T, Brendel E, Delesen H, Joosten MC, Lathia CD, Loembé
BA, et al: Safety and pharmacokinetics of sorafenib combined with
capecitabine in patients with advanced solid tumors: Results of a
phase 1 trial. J Clin Pharmacol. 51:1674–1684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haller DG, Tabernero J, Maroun J, de Braud
F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K and
Schmoll HJ: Capecitabine plus oxaliplatin compared with
fluorouracil and folinic acid as adjuvant therapy for stage III
colon cancer. J Clin Oncol. 29:1465–1471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montagnani F, Turrisi G, Marinozzi C,
Aliberti C and Fiorentini G: Effectiveness and safety of
oxaliplatin compared to cisplatin for advanced, unresectable
gastric cancer: A systematic review and meta-analysis. Gastric
Cancer. 14:50–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hameed H and Cassidy J: Use of
capecitabine in management of early colon cancer. Cancer Manag Res.
3:295–299. 2011.PubMed/NCBI
|
|
8
|
Chen AP, Setser A, Anadkat MJ, Cotliar J,
Olsen EA, Garden BC and Lacouture ME: Grading dermatologic adverse
events of cancer treatments: The Common Terminology Criteria for
Adverse Events Version 4.0. J Am Acad Dermatol. 67:1025–1039. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roth AD, Fazio N, Stupp R, et al:
Docetaxel, Cisplatin, and Fluorouracil; docetaxel and cisplatin;
and epirubicin, cisplatin, and fluorouracil as systemic treatment
for advanced gastric carcinoma: A randomized phase II trial of the
Swiss group for clinical cancer research. J Clin Oncol.
25:3217–3223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu J, Yamamoto H, Lu X, Ngan CY, Tsujino
T, Konishi K, Takemasa I, Ikeda M, Nagata H, Hashimoto S, et al:
Low-dose oxaliplatin enhances the antitumor efficacy of paclitaxel
in human gastric cancer cell lines. Digestion. 74:19–27. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gamelin E, Gamelin L, Bossi L and
Quasthoff S: Clinical aspects and molecular basis of oxaliplatin
neurotoxicity: Current management and development of preventive
measures. Semin Oncol. 29 5 Suppl 15:S21–S33. 2002. View Article : Google Scholar
|
|
13
|
Piccart MJ and Di Leo A: Future
perspectives of docetaxel (Taxotere) in front-line therapy. Semin
Oncol. 24 4 Suppl 10:S10-S27–S10-S33. 1997.
|
|
14
|
Shepherd FA, Dancey J, Ramlau R, Mattson
K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R,
et al: Prospective randomized trial of docetaxel versus best
supportive care in patients with non-small-cell lung cancer
previously treated with platinum-based chemotherapy. J Clin Oncol.
18:2095–2103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okines AF, Norman AR, McCloud P, Kang YK
and Cunningham D: Meta-analysis of the REAL-2 and ML17032 trials:
Evaluating capecitabine-based combination chemotherapy and infused
5-fluorouracil-based combination chemotherapy for the treatment of
advanced oesophago-gastric cancer. Ann Oncol. 20:1529–1534. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sawada N, Ishikawa T, Fukase Y, Nishida M,
Yoshikubo T and Ishitsuka H: Induction of thymidine phosphorylase
activity and enhancement of capecitabine efficacy by taxol/taxotere
in human cancer xenografts. Clin Cancer Res. 4:1013–1019.
1998.PubMed/NCBI
|
|
17
|
Cassidy J, Tabernero J, Twelves C, Brunet
R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N, et
al: XELOX (capecitabine plus oxaliplatin): Active first-line
therapy for patients with metastatic colorectal cancer. J Clin
Oncol. 22:2084–2091. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Traina TA, Dugan U, Higgins B, Kolinsky K,
Theodoulou M, Hudis CA and Norton L: Optimizing chemotherapy dose
and schedule by Norton-Simon mathematical modeling. Breast Dis.
31:7–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amarantidis K, Xenidis N, Chelis L,
Chamalidou E, Dimopoulos P, Michailidis P, Tentes A, Deftereos S,
Karanikas M, Karayiannakis A and Kakolyris S: Docetaxel plus
oxaliplatin in combination with capecitabine as first-line
treatment for advanced gastric cancer. Oncology. 80:359–365. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hennessy BT, Gauthier AM, Michaud LB,
Hortobagyi G and Valero V: Lower dose capecitabine has a more
favorable therapeutic index in metastatic breast cancer:
Retrospective analysis of patients treated at M. D. Anderson cancer
center and a review of capecitabine toxicity in the literature. Ann
Oncol. 16:1289–1296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goel G, Jauhri M, Negi A and Aggarwal S:
Feasibility study of docetaxel, oxaliplatin and capecitabine
combination regimen in advanced gastric or gastroesophageal
adenocarcinoma. Hematol Oncol Stem Cell Ther. 3:55–59. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Di Lauro L, Vici P, Belli F, Tomao S,
Fattoruso SI, Arena MG, Pizzuti L, Giannarelli D, Paoletti G, Barba
M, et al: Docetaxel, oxaliplatin and capecitabine combination
chemotherapy for metastatic gastric cancer. Gastric Cancer.
17:718–724. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Cutsem E, Boni C, Tabernero J, Massuti
B, Middleton G, Dane F, Reichardt P, Pimentel FL, Cohn A, Follana
P, et al: Docetaxel plus oxaliplatin with or without fluorouracil
or capecitabine in metastatic or locally recurrent gastric cancer:
A randomized phase II study. Ann Oncol. 26:149–156. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stein A, Arnold D, Thuss-Patience PC,
Moehler M, Grothe W, Seufferlein T, Reinacher-Schick A, Geissler M,
Hofheinz RD and Schmoll HJ: Docetaxel, oxaliplatin and capecitabine
(TEX regimen) in patients with metastatic gastric or
gastro-esophageal cancer: Results of a multicenter phase I/II
study. Acta Oncol. 53:392–398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sym SJ, Ryu MH, Kang HJ, Lee SS, Chang HM,
Lee JL, Kim TW, Yook JH, Oh ST, Kim BS and Kang YK: Phase I study
of 3-weekly docetaxel, capecitabine and oxaliplatin combination
chemotherapy in patients with previously untreated advanced gastric
cancer. Cancer Chemother Pharmacol. 66:373–380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rivera F, Massutí B, Salcedo M, Sastre J,
Martínez Galán J, Valladares-Ayerbes M, Serrano R, García de
Paredes ML, Manzano JL, Galán M, et al: Phase II trial of miniDOX
(reduced dose docetaxel-oxaliplatin-capecitabine) in ‘suboptimal’
patients with advanced gastric cancer (AGC). TTD 08–02. Cancer
Chemother Pharmacol. 75:319–324. 2015. View Article : Google Scholar : PubMed/NCBI
|