Introduction
Von Hippel-Lindau (VHL) disease is a systemic
neoplastic syndrome with autosomal-dominant transmission, complete
penetrance, and variable expression that is caused by mutations in
the VHL gene (1). The disease has a
prevalence of 2–3 per 100,000, and an estimated incidence of
between 1 in 36,000 and 1 in 52,000 live births (1,2). All the
clinical features generally appear by the age of 65 (3), and involve different organs: The
central nervous system (CNS) and retinal hemangioblastomas,
endolymphatic sac tumors, clear-cell renal carcinoma and renal
cysts, pheocromocytomas, pancreatic cysts and pancreatic
neuroendrocrine tumors (pNETs) (4,5).
Currently, there is no single protocol to be followed for the
treatment of VHL disease when multiple organs are involved. In
these cases, the treatment criteria take into consideration the
number and size of the tumor(s), their location, the type of
resection (which may vary according to the more or less
conservative options that are possible, from tumor enucleation to
total resection), and the possibility of performing simultaneous or
staged surgery (6). The present case
study reports the experiences of the present authors in using a
single-stage laparoscopic approach for the treatment of adrenal and
pancreatic manifestations of VHL disease.
Case report
The patient was a 67-year-old woman, who came to our
department (the Department of Surgery, IRCCS - Azienda Ospedaliera
Universitaria San Martino-IST, University of Genoa, Italy) with
symptoms of epigastric pain and dyspeptic symptoms. The patient had
a significant history of hypertension. An initial abdominal
examination was negative. However, on subsequently performing an
abdominal ultrasound, there was evidence of a well-vascularized
solid lesion in the left adrenal gland (38 mm in diameter). A
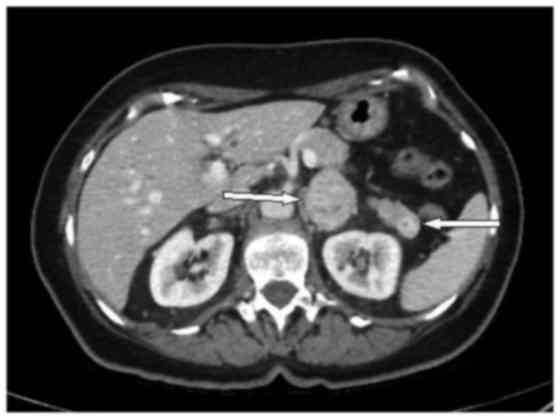
computed tomography scan revealed a solid lesion of ~4 cm in
diameter, with heterogeneous enhancement in the left adrenal gland
and a hypervascular, homogenous and well-circumscribed 17-mm lesion
in the pancreatic tail (Fig. 1).
Somatostatin receptor scintigraphy revealed
increased signal intensity in the region of the pancreatic tail,
and a mild increase in the specific vector in the left adrenal
gland. A blood test revealed abnormal levels of chromogranin A
(973.8 ng/ml) and a high catecholamine concentration in the 24 h
urine collection.
Even though the patient's family history was
negative for genetic diseases, a program including molecular
genetic analysis of the VHL gene and clinical screening, which
featured retinoscopy and magnetic resonance imaging of the CNS, was
started. No alterations in the CNS were detected, but molecular
genetic investigation of a blood sample revealed a large deletion
of exon 3 of the VHL gene.
A surgical approach for both the pancreatic lesion
and the left adrenal gland tumor was decided upon. The procedure
was performed laparoscopically, with one umbilical and three
subcostal ports. After sectioning of the splenocolic ligament, the
left colic flexure was mobilized, and Gerota's fascia was exposed.
The adrenal lesion was progressively isolated with a radiofrequency
device (Covidien Italia, Segrate, Italy). Following separation from
the intact adrenal cortex, excision of the lesion was achieved
following the division of the adrenal artery and vein.
Progressive isolation of the pancreatic body-tail
revealed the lesion, protruding from the pancreatic anterior
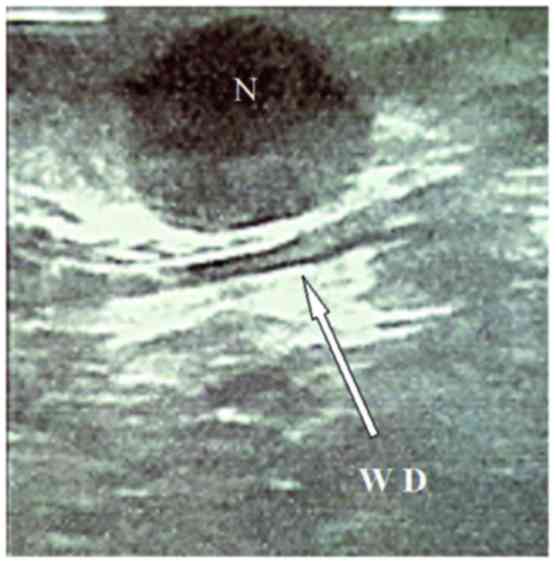
aspect. An intraoperative ultrasonography performed using a
laparoscopic probe (Esaote Biomedica, Genoa, Italy) confirmed the
hypoechoic lesion, well-demarcated and located very close to the
Wirsung duct (Fig. 2).
After having completed the enucleation of the
lesion, the two specimens were inserted into a sterile bag and
removed through a port site in the left upper abdomen. The
operating time was 240 min, and the estimated blood loss was 100
ml. Upon histolopathological analysis, the adrenal lesion was
determined to be a pheocromocytoma with a potentially aggressive
biological behavior [pheochromocytoma of the adrenal gland scaled
score (PASS), 6/20] (7).
The pancreatic tumor was a well-differentiated grade
1 pNET, according to the World Health Organization (WHO), 2010 and
European Neuroendocrine Tumor Society (ENETS) classification
systems, and it stained positively for pancreatic polypeptide. The
growth fraction measured with the proliferation marker, MIB-1
(Ki-67), was 2%.
The patient developed a grade C postoperative
pancreatic fistula with subsequent fluid collection formation,
necessitating treatment of the percutaneous abdominal drainage.
After the pancreatic function had been completely recovered and
complete oral intake was resumed, the patient was discharged, to be
followed as an outpatient. Removal of the abdominal drainage was
made after a further 8 weeks.
Discussion
In ~20% of adrenal masses, it is possible to perform
surgery, particularly when the masses are >4 cm, have hormonal
activity, and there is a suspicion of malignancy at the
radiological examination stage (8).
In the present case study, adrenalectomy was indicated for the
preoperative diagnosis of pheochromocytoma with clinically relevant
symptoms. Adrenal surgery in the past was understood as a total
adrenal resection, creating problems and important side-effects
with resulting adrenal insufficiency and/or the need of using
hormone therapy for the remainder of the patient's life,
particularly when the resection involved both glands (8). Consequently, the idea of partial
adrenalectomy was introduced, trying to achieve a cortex-sparing
surgery (9). In the present case
study, it was possible to perform adrenalectomy since the lesion
was well demarcated, and a part of the normal gland remained at its
upper pole.
Several studies have explored the safety and
feasibility of non-operative management for asymptomatic sporadic
non-functioning pNETs ≤2 cm, particularly when a major pancreatic
resection is required. A conservative approach would appear to be
safe to assume, as previous studies have shown that the majority of
the observed tumors did not exhibit any significant changes during
follow-up (10,11). However, tumor size correlates with
malignancy, and the majority of studies have reported a risk of
lymph node metastasis in pNETs <2 cm of ~10–15% (12,13).
Therefore, surgical resection is the gold standard
when the following criteria are present: Tumors are >30 mm in
diameter (or >20 mm, if located in the pancreatic head),
abdominal surgery is under way for other VHL-associated resections,
or the tumors are symptomatic (14).
For our patient, the options available for treating the pancreatic
lesion were limited due to its size, but, given the proximity to
the adrenal lesion, the possibility of a resection was taken into
account. Pancreatic enuclation is the gold standard for lesions
located close to the surface of the head or body of the pancreas
and far (>2 mm) away from the Wirsung duct, and when the lesions
are multiple (13). Song et
al (15) limited the
laparoscopic approach to superficial and anterior lesions that are
located in the left side of the superior mesenteric vein, similarly
to the present case study.
When the possibility of a laparoscopic approach is
properly set out, laparoscopic enucleation, compared with ‘open’
enucleation, has a shorter operating time, lower estimated blood
loss, and faster recovery times, the pancreatic function being
preserved in the two approaches (16). The final anatomical limitation for
enucleation is the distance between the tumors and the main
pancreatic duct, which should be, even if not yet evidence-based,
>2 mm (13). Indeed, one of the
major risks associated with enucleating large lesions is a major
pancreatic duct injury, leading to high output pancreatic fistula,
as occurred in the present case study. This distance is better
assessed intraoperatively using ultrasonography. In combining
parenchyma-sparing surgery with a laparoscopic single-stage
approach, an optimal, minimally invasive therapeutical option may
be realized (6). In the present case
study, an adrenal cortex-sparing procedure and a pancreatic
enucleation were performed with the same amount of trocars that
should have been used for a single procedure. Furthermore, a
single-stage procedure for multiple organ tumors has the following
advantages: It limits the requirement for further surgery at a
later stage, particularly in view of the likelihood of recurrence
or in case of new onset tumors; it avoids adhesions and scarring at
every new procedural stage; and it avoids delays in definitive
therapy for certain tumors that may potentially metastatize
(17,18).
In conclusion, a single-stage surgical approach for
multiple-organ intra-abdominal tumors is a viable option for
patients with VHL disease. With careful patient selection and
surgical planning, combined procedures may be safely performed in
one operative setting via a laparoscopic approach, thus reducing
surgical trauma and preserving organ function.
References
|
1
|
Maher ER, Iselius L, Yates JR, Littler M,
Benjamin C, Harris R, Sampson J, Williams A, Ferguson-Smith MA and
Morton N: Von Hippel-Lindau disease: A genetic study. J Med Genet.
28:443–447. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neumann HP and Wiestler OD: Clustering of
features of von Hippel-Lindau syndrome: Evidence for a complex
genetic locus. Lancet. 337:1052–1054. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maher ER, Yates JR, Harries R, Benjamin C,
Harris R, Moore AT and Ferguson-Smith MA: Clinical features and
natural history of von Hippel-Lindau disease. Q J Med.
77:1151–1163. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maher ER and Kaelin WG Jr: von
Hippel-Lindau disease. Medicine (Baltimore). 76:381–391. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clifford SC and Maher ER: Von
Hippel-Lindau disease: Clinical and molecular perspectives. Adv
Cancer Res. 82:85–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hwang JJ, Uchio EM, Pavlovich CP, Pautler
SE, Libutti SK, Linehan WM and Walther MM: Surgical management of
multi-organ visceral tumors in patients with von Hippel-Lindau
disease: A single stage approach. J Urol. 169:895–898. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thompson LD: Pheochromocytoma of the
Adrenal gland Scaled Score (PASS) to separate benign from malignant
neoplasms: A clinicopathologic and immunophenotypic study of 100
cases. Am J Surg Pathol. 26:551–566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kutikov A, Crispen PL and Uzzo RG:
Pathophysiology, evaluation, and medical management of adrenal
disordersCampbell's Urology. Wein AJ A.J..Kavoussi LR L.R..Novick
AC A.C..Partin AW A.W..Peters CA C.A.: Elsevier Saunders; pp.
1685–1736. 2012, View Article : Google Scholar
|
|
9
|
Esen T, Acar O, Tefekli A, Musaoğlu A,
Rozanes I and Emre A: Adrenal cortex-sparing surgery for bilateral
multiple pheochromocytomas in a patient with von hippel-lindau
disease. Case Rep Med. 2012:6591042012.PubMed/NCBI
|
|
10
|
Crippa S, Partelli S, Zamboni G, Scarpa A,
Tamburrino D, Bassi C, Pederzoli P and Falconi M: Incidental
diagnosis as prognostic factor in different tumor stages of
nonfunctioning pancreatic endocrine tumors. Surgery. 155:145–153.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheema A, Weber J and Strosberg JR:
Incidental detection of pancreatic neuroendocrine tumors: An
analysis of incidence and outcomes. Ann Surg Oncol. 19:2932–2936.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukhopadhyay D, Knebelmann B, Cohen HT,
Ananth S and Sukhatme VP: The von Hippel-Lindau tumor suppressor
gene product interacts with Sp1 to repress vascular endothelial
growth factor promoter activity. Mol Cell Biol. 17:5629–5639. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cherif R, Gaujoux S, Couvelard A, Dokmak
S, Vuillerme MP, Ruszniewski P, Belghiti J and Sauvanet A:
Parenchyma-sparing resections for pancreatic neuroendocrine tumors.
J Gastrointest Surg. 16:2045–2055. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Libutti SK, Choyke PL, Bartlett DL, Vargas
H, Walther M, Lubensky I, Glenn G, Linehan WM and Alexander HR:
Pancreatic neuroendocrine tumors associated with von Hippel Lindau
disease: Diagnostic and management recommendations. Surgery.
124:1153–1159. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song KB, Kim SC, Hwang DW, Lee JH, Lee DJ,
Lee JW, Jun ES, Sin SH, Kim HE, Park KM, et al: Enucleation for
benign or low-grade malignant lesions of the pancreas:
Single-center experience with 65 consecutive patients. Surgery.
158:1203–1210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang RC, Zhou YC, Mou YP, Huang CJ, Jin
WW, Yan JF, Wang YX and Liao Y: Laparoscopic versus open
enucleation for pancreatic neoplasms: Clinical outcomes and
pancreatic function analysis. Surg Endosc. 30:2657–2665. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Mestier L, Gaujoux S, Cros J, Hentic O,
Vullierme MP, Couvelard A, Cadiot G, Sauvanet A, Ruszniewski P,
Richard S, et al: Long-term prognosis of resected pancreatic
neuroendocrine tumors in von Hippel-Lindau disease is favorable and
not influenced by small tumors left in place. Ann Surg.
262:384–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weisbrod AB, Kitano M, Thomas F, Williams
D, Gulati N, Gesuwan K, Liu Y, Venzon D, Turkbey I, Choyke P, et
al: Assessment of tumor growth in pancreatic neuroendocrine tumors
in von Hippel Lindau syndrome. J Am Coll Surg. 218:163–169. 2014.
View Article : Google Scholar : PubMed/NCBI
|