Introduction
Dural metastasis from extraneural malignancies is
rare, having been detected during autopsy in 10% of the cases of
extraneural malignancies (1). Breast
cancer is one of the tumors that frequently metastasizes to the
dura (2), whereas dural metastasis
with subdural hematoma is infrequently reported (3–5). By
contrast, Nayak et al reported that 25% of dural metastases,
in which the most common primary site was breast cancer, exhibited
diffuse dural involvement (6).
However, only few reports detail large en plaque subdural tumor
without hematoma in breast cancer (7). We herein report the case of a patient
with breast cancer with a widespread en plaque subdural tumor,
which mimicked subdural hematoma and caused rapid loss of
consciousness and cerebral herniation.
Case report
A 49-year-old woman was admitted to the National
Hospital Organization Osaka National Hospital (Osaka, Japan) with
nausea and headache. The patient had previously undergone
resection, radiotherapy and chemotherapy for bilateral breast
cancer. Local control of the primary lesion was maintained, but
multiple systemic metastasis to the bones and lymph nodes
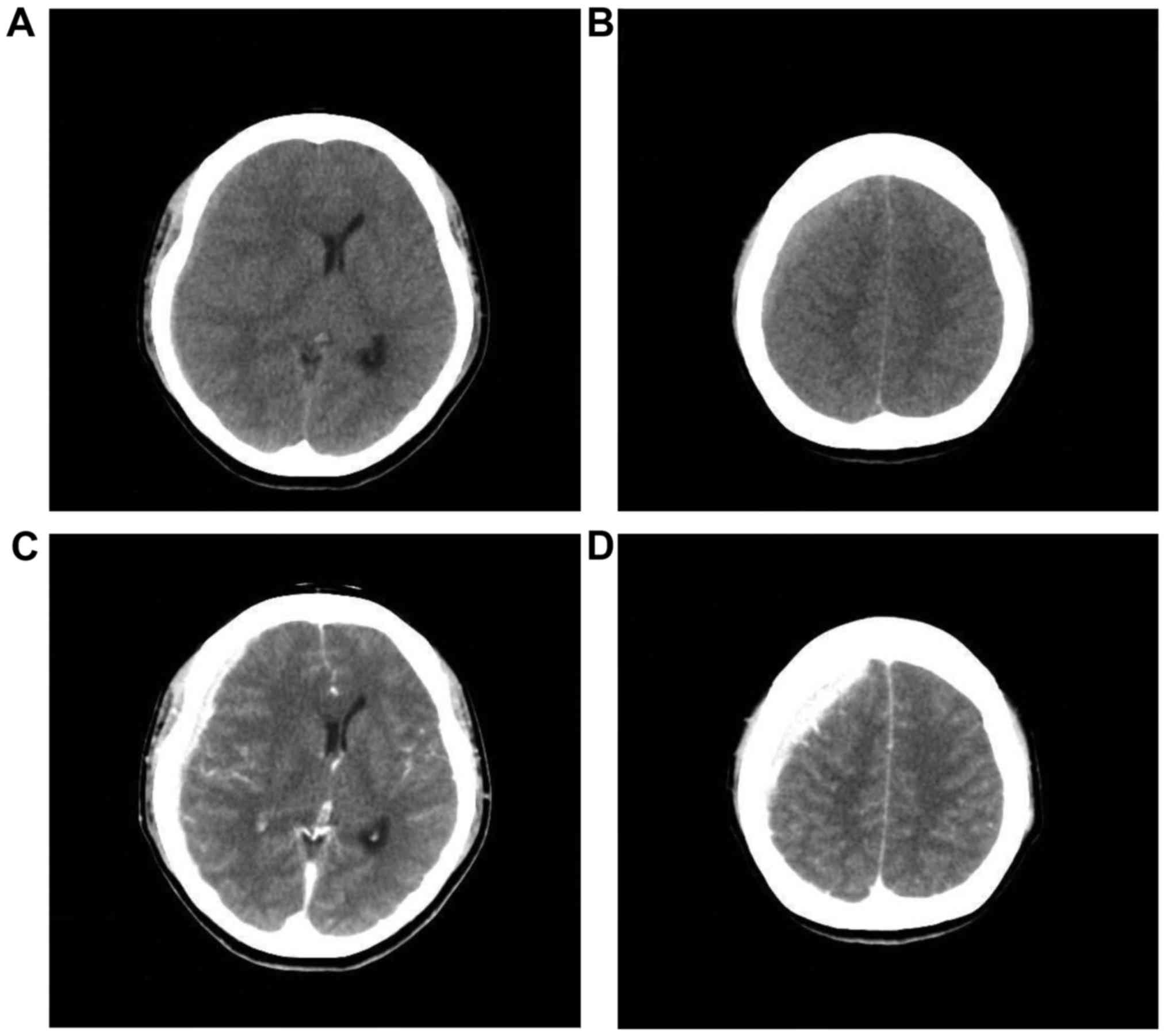
developed. Computed tomography (CT) revealed a high-density lesion
in the right subdural space, suggesting hematoma. Midline shift to
the left was observed (Fig. 1A and
B). Enhanced CT showed no parenchymal brain lesions. A
high-density lesion in the right subdural space was homogeneously
enhanced (Fig. 1C and D). As a
tissue expander had been implanted in the patient's breast,
magnetic resonance imaging (MRI) could not be performed.
A right frontal burr hole was drilled for subdural
hematoma drainage. Upon opening the dura mater, a yellowish-white
subdural tumor was identified, without evidence of old or fresh
hemorrhage. A biopsy was performed and the histopathological
examination of the tissue revealed metastatic breast cancer. The
patient received 40 mg/day prednisolone and the neurological
symptoms gradually subsided. However, 12 days after the first
operation, the clinical course was complicated by vomiting and
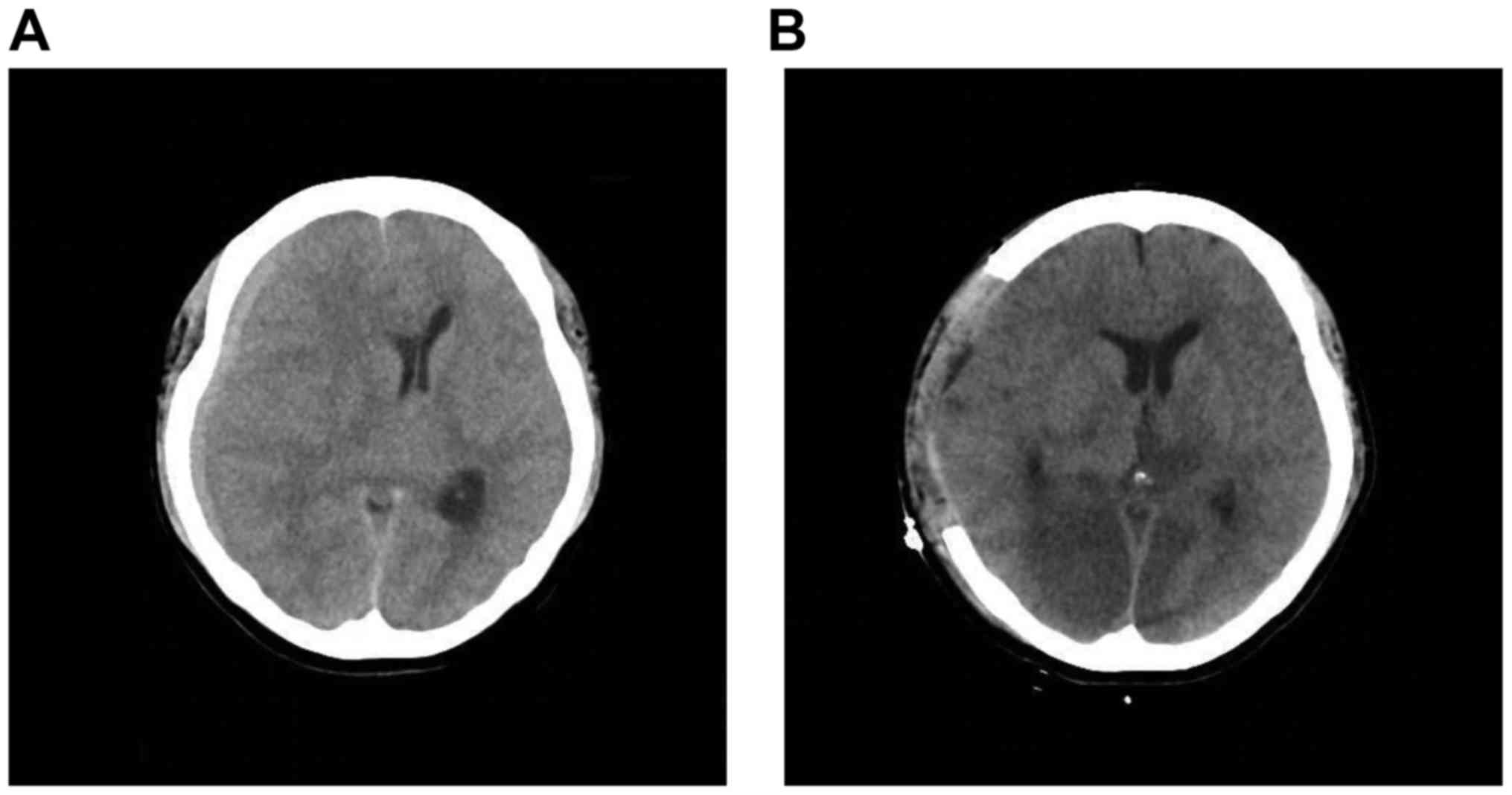
rapid loss of consciousness. An emergency CT examination revealed
that the subdural tumor had enlarged and the midline shift to the
left was exacerbated. The subdural tumor also caused uncal
herniation, with upper brainstem compression (Fig. 2A). A fronto-temporo-parietal
craniotomy for decompression was performed as life-saving surgery.
Direct inspection indicated that the outer membrane of the dura and
the inner side of the skull were not breached by the tumor. The
dura was opened and an en plaque fronto-temporo-parietal subdural
yellowish-white tumor was identified in the subdural space, with no
cortical involvement, but with involvement of the adjacent
dura.
Unfortunately, even post-surgery, the patient's
consciousness failed to improve and a CT revealed bilateral
posterior cerebral artery infarct due to the cerebral herniation
(Fig. 2B). The patient's condition
progressively deteriorated and she finally succumbed to the disease
2 months after the second operation.
Discussion
Dural metastasis is now more frequently diagnosed
owing to advanced radiological imaging techniques. Dural metastasis
from extraneural malignancies has been detected in ~10% of autopsy
cases (1). In a series of 198 cases
of dural metastasis, the tumors most commonly metastasizing to the
dura mater were cancer of the prostate (19.5%), breast (16.5%),
lung (11%) and stomach (7.5%) (2).
Tsukada et al reported that the more common clinically
diagnosed central nervous system metastatic patterns in breast
cancer were metastasis to the brain alone (25.0%), a combination of
brain and cranial dura metastasis (15.6%), and metastasis to the
cranial dura alone (13.5%) (8).
Nzokou et al identified three image patterns
in dural metastasis: i) A nodule in a subdural hematoma, ii)
multinodular metastasis surrounded by a subdural hematoma and iii)
an extensive en plaque subdural tumor (9). In cases of extensive en plaque subdural
tumors, where unexpectedly no blood was found, a larger craniotomy
revealed an extensive tumor not amenable to surgical treatment
(9). The characteristics of our case
conformed to the latter type, namely a large en plaque subdural
tumor. In a previous report, 25% of dural metastases, in which the
most common primary site of was breast cancer, exhibited diffuse
dural involvement (6). However, only
few reports have detailed widespread en plaque subdural tumors in
breast cancer, and the prognosis in the reported cases was poor
(7). Neurological symptoms of dural
metastasis are due to irritation of the dura by the tumor,
intracranial edema and cerebral herniation (10). As the brain is enclosed in a rigid
container, within which there is little room for expansion, a
widespread en plaque subdural tumor may cause rapid loss of
consciousness and cerebral herniation, which was the case in our
patient. Consequently, life-saving decompression was warranted.
Dural metastasis has been reported to mimic subdural
hematoma (7,11–13). The
operative method appropriate for metastatic subdural tumor with
midline shift may differ completely from that for chronic subdural
hematoma. In cases of en plaque subdural tumors, the preoperatively
planned burr hole or craniotomy must be converted into a larger
craniotomy (9). If preoperative
diagnosis is difficult, the operative procedures must be adjusted.
Contrast-enhanced CT is appropriate for an emergency operation and
should reveal a homogeneously enhanced, thickened dura in cases of
subdural metastasis (11). In the
present case, contrast-enhanced CT revealed a homogeneously
enhanced lesion in the dura, representing a metastasis. Therefore,
clinicians should bear in mind the possibility of not only brain
parenchymal, but also dural metastasis from primary breast
cancer.
Acknowledgements
The present study was supported by Grant-in-Aid for
Scientific Research from the Ministry of Education, Science and
Culture of Japan (no. 16K20033 to Y.O.).
References
|
1
|
Meyer PC and Reah TG: Secondary neoplasms
of the central nervous system and meninges. Br J Cancer. 7:438–448.
1953. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laigle-Donadey F, Taillibert S, Mokhtari
K, Hildebrand J and Delattre JY: Dural metastases. J Neurooncol.
75:57–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kunii N, Morita A, Yoshikawa G and Kirino
T: Subdural hematoma associated with dural metastasis-case report.
Neurol Med Chir (Tokyo). 45:519–522. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Otsuka A, Asakura K, Takahashi K, Tasaki
T, Okada K and Suzuki Y: Nontraumatic chronic subdural hematoma due
to dural metastases of breast cancer. Case report. No Shinkei Geka.
13:999–1004. 1985.(In Japanese). PubMed/NCBI
|
|
5
|
Tseng SH, Liao CC, Lin SM, Chen Y and Shun
CT: Dural metastasis in patients with malignant neoplasm and
chronic subdural hematoma. Acta Neurol Scand. 108:43–46. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nayak L, Abrey LE and Iwamoto FM:
Intracranial dural metastases. Cancer. 115:1947–1953. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Villano JL and Ryan CW: Patients
presenting with CNS lesions. Case 2. Subdural presentation of
recurrent breast cancer. J Clin Oncol. 21:4060–4062. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsukada Y, Fouad A, Pickren JW and Lane
WW: Central nervous system metastasis from breast carcinoma.
Autopsy study. Cancer. 52:2349–2354. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nzokou A, Magro E, Guilbert F, Fournier JY
and Bojanowski MW: Subdural metastasis of prostate cancer. J Neurol
Surg Rep. 76:e123–e127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Posner JB and Chernik NL: Intracranial
metastases from systemic cancer. Adv Neurol. 19:579–592.
1978.PubMed/NCBI
|
|
11
|
Cheng YK, Wang TC, Yang JT, Lee MH and Su
CH: Dural metastasis from prostatic adenocarcinoma mimicking
chronic subdural hematoma. J Clin Neurosci. 16:1084–1086. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patil S, Veron A, Hosseini P, Bates R,
Brown B, Guthikonda B and DeSouza R: Metastatic prostate cancer
mimicking chronic subdural hematoma: A case report and review of
the literature. J La State Med Soc. 162:203–205. 2010.PubMed/NCBI
|
|
13
|
Tomlin JM and Alleyne CH: Transdural
metastasis from adenocarcinoma of the prostate mimicking subdural
hematoma: Case report. Surg Neurol. 58:329–331. 2002. View Article : Google Scholar : PubMed/NCBI
|