Introduction
Gastrointestinal stromal tumors (GISTs) are a type
of sarcoma, and the most common mesenchymal tumor of the
gastrointestinal tract. They arise from the intestinal cells of
Cajal, typically present in older individuals, and are most
commonly identified in the stomach, followed by the small intestine
(1). In Japan, GISTs arise from the
stomach most frequently in 1–2 per 100,000 people (2). Most GISTs express the protein product
of the KIT proto-oncogene, a transmembrane receptor tyrosine
kinase, the activity of which would normally be regulated by the
binding of its ligand. KIT is positive in ~95% of GISTs, and so, if
a tumor is positive for KIT on performing immunohistochemistry and
the cells appear as GIST morphologically on hematoxylin-eosin
staining, it may be diagnosed as GIST (3). CD34 is positive in 70–80% of the cases
of GIST, and certain CD34-positive tumors may be diagnosed as GIST,
unless KIT is negative (4).
More than 40% of GISTs are clinically malignant and
thought to be metastatic (1,5–7), so
there are a large number of GIST cases for which systemic
chemotherapy is indicated. Imatinib mesylate is an oral
multitargeted receptor tyrosine kinase inhibitor that is effective
as adjuvant chemotherapy for primary high-risk cases (8,9), and as
palliative chemotherapy for unresectable or metastatic cases
(10,11).
For unresectable or metastatic cases of
imatinib-resistant GIST, second-line chemotherapy is recommended.
Sunitinib mesylate is an oral multitargeted receptor tyrosine
kinase inhibitor whose effects are associated with blockade of
receptor tyrosine kinase signaling by KIT, platelet-derived growth
factor receptors (PDGFRs), three isoforms of the vascular
endothelial growth factor receptors (VEGFR-1, VEGFR-2 and VEGFR-3),
and Fms-like tyrosine kinase-3 receptor (FLT3). In cases of GIST,
sunitinib exhibited significant longer median progression-free
survival (mPFS) and higher response rates (RRs) compared with a
placebo (12). Continuous daily
dosing of sunitinib was also reported to be effective for disease
control (13). However, to the best
of our knowledge, there have been no cases showing a complete
response (CR) in the prospective clinical trials examining the
effects of sunitinib for GIST, and in CR case reports
worldwide.
Therefore, a very rare case of a patient with
metastatic GIST who achieved CR with sunitinib as second-line
chemotherapy is reported in the present study. This is a very rare
case report of CR with sunitinib as second-line chemotherapy for
GIST worldwide.
Case presentation
A 54-year-old woman presented with persistent upper
abdominal pain and anorexia, and she visited a local doctor in
March 2015. The patient had a history of operations for a myoma of
the uterus, and she had no history of smoking or drinking. Her
family history included no cancers. Abdominal ultrasound revealed a
subcutaneous mass of the upper abdomen, and an upper
gastrointestinal endoscopy revealed a submucosal tumor of the
posterior wall of the stomach. Since GIST of the stomach was
suspected, the patient was referred to the Department of Surgery of
Miyazaki Prefectural Miyazaki Hospital (Miyazaki, Japan) in April
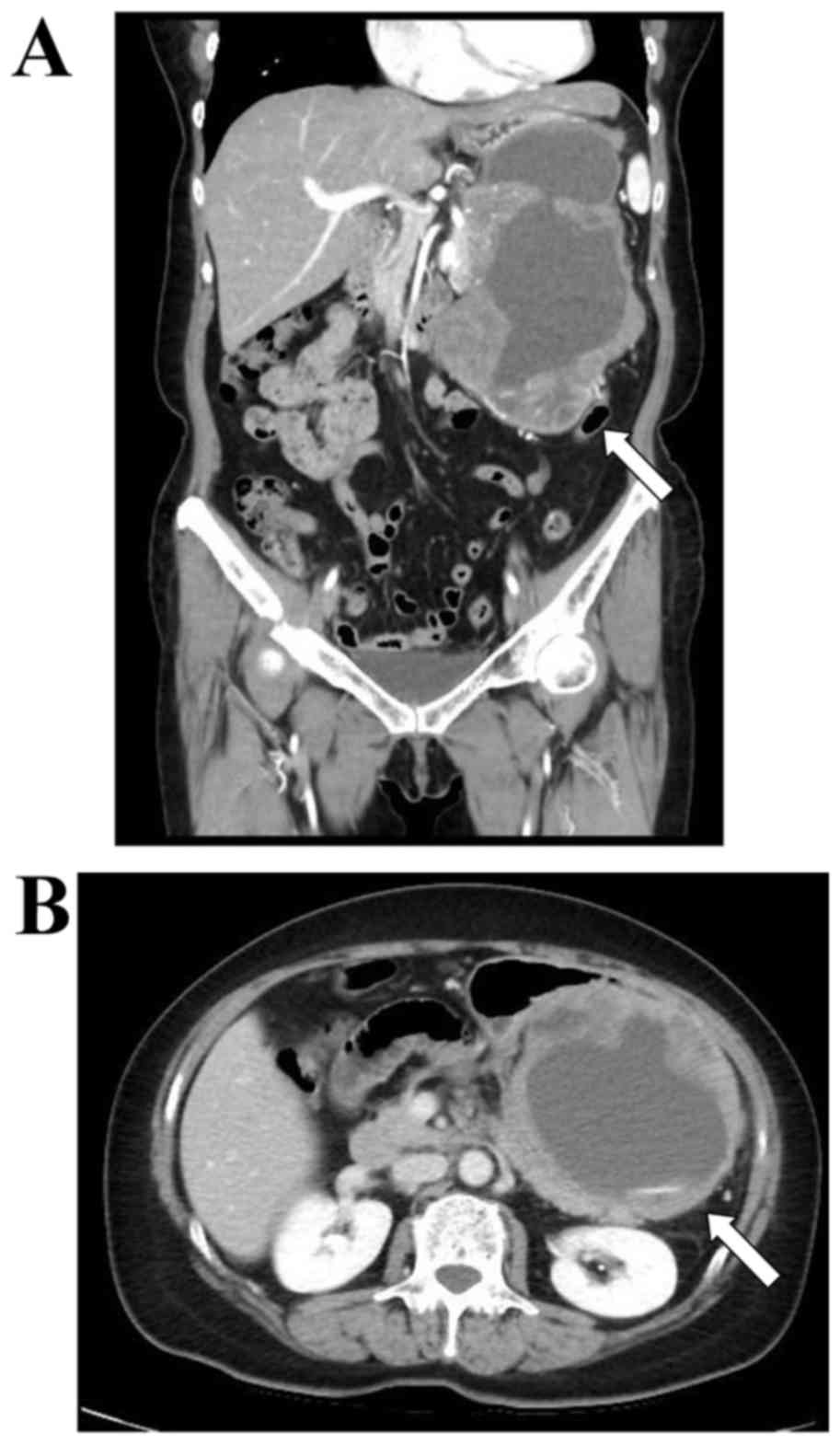
2015. An abdominal ultrasound revealed free space in the tumor.
Since the patient's systolic blood pressure was 80 mmHg and her
hemoglobin level was 8.0 g/dl, she was diagnosed with hypovolemic
shock due to tumor hemorrhage. Since no distant metastases were
observed on computed tomography (CT) (Fig. 1), the patient underwent total
gastorectomy and distal pancreatectomy with splenectomy for
advanced invasive tumor. Histological examination of the resected
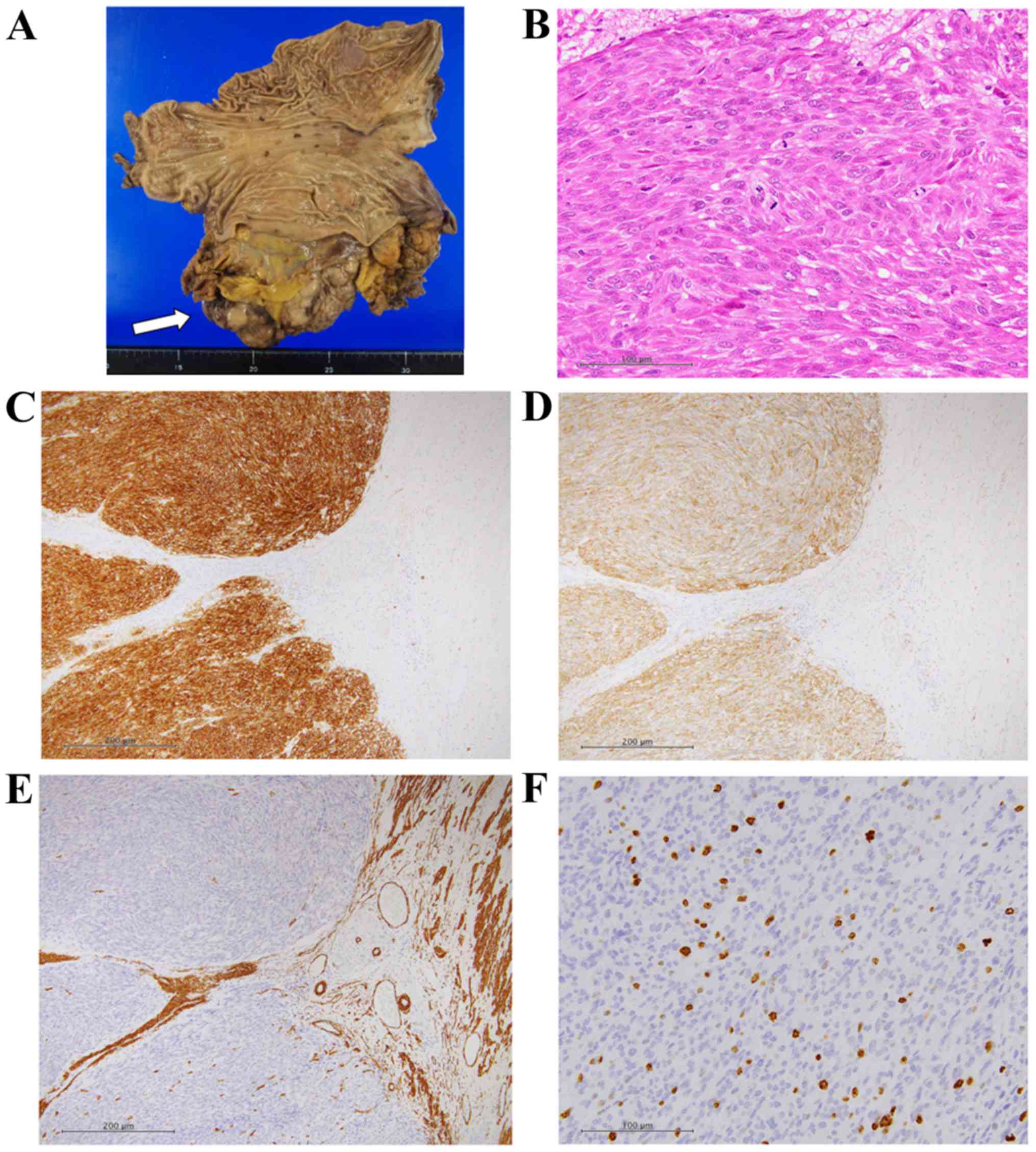
specimen demonstrated rupture and hemorrhage of the tumor, the
maximum diameter of which was >15 cm (Fig. 2A). The tumor consisted of spindle or
polygonal-shaped cells having larger nuclei. Immunohistochemically,
the cells were positive for KIT and CD34, and partly positive for
smooth muscle actin and p53 (Fig.
2B-E). They were negative for S100 and desmin, and the MIB-1
positive rate was 10.07% (288/2859 cells) (Fig. 2F). The tumor was completely resected.
Since the patient was diagnosed as high-risk GIST using the
modified-Fletcher classification (rupture, size >10 cm, mitoses
>10/50 per high-power field), adjuvant chemotherapy with
imatinib for 3 years was indicated.
The patient was referred to our department in May
2015. Her general condition was good, and organ functions were well
preserved. Therefore, systemic chemotherapy was started with 400
mg/body/day of imatinib. The dose of imatinib was reduced to 300
mg/body/day due to Common Terminology Criteria for Adverse Events
Version 4.0 (CTCAEv4.0) grade 2 diarrhea in June 2015. However,
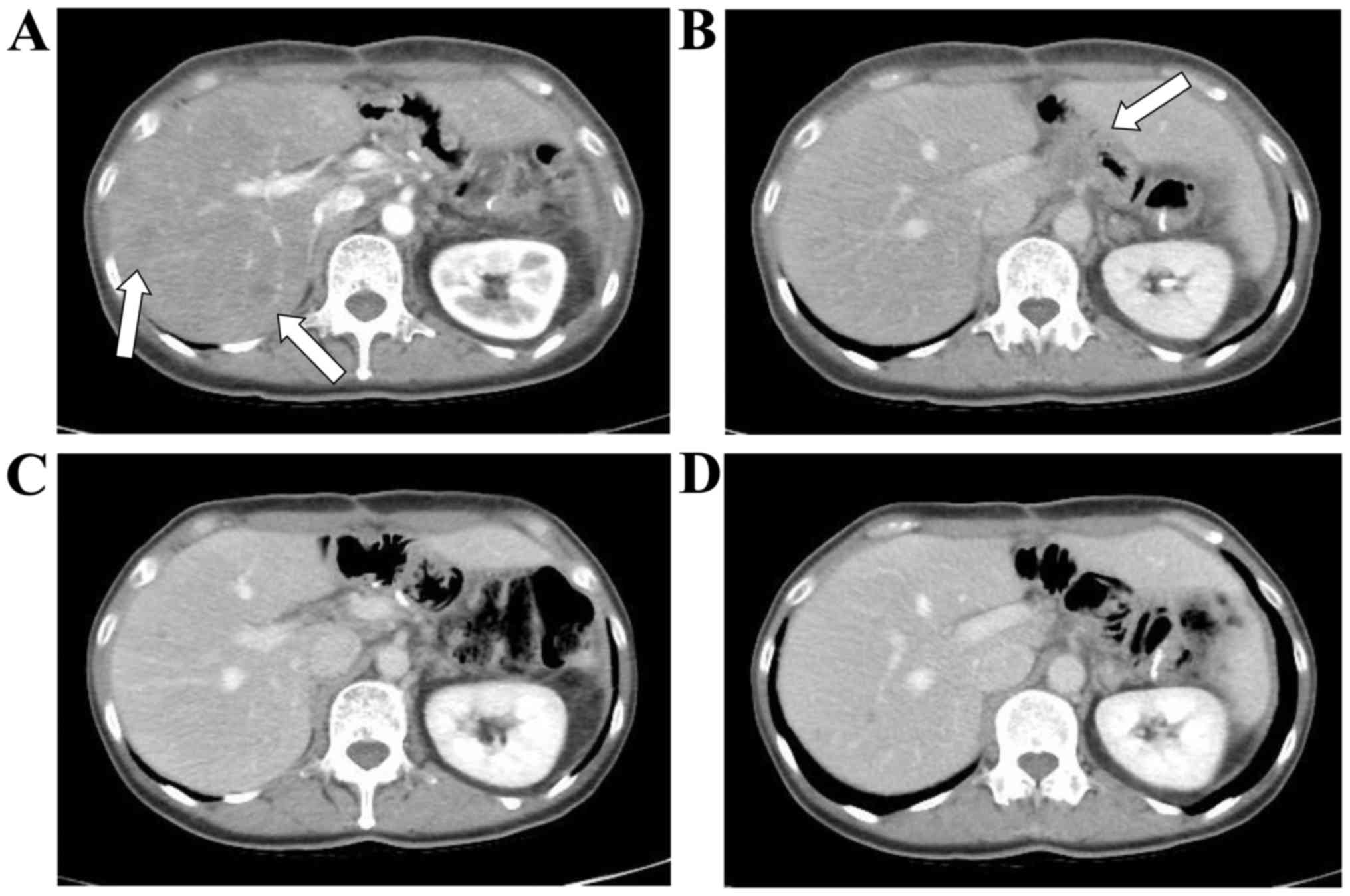
multiple metastases in the liver and a metastatic lymph node near
the celiac artery were detected on performing CT in November 2015
(Fig. 3A and B).
The patient's Eastern Cooperative Oncology Group
performance status was 0, and vital signs were within normal
ranges. Blood testing revealed a decreased red blood cell count of
329×104/µl and a decreased hemoglobin level of 10.8
g/dl, although the patient's general condition was good, and organ
functions were preserved. Second-line chemotherapy with sunitinib
(50 mg/body/day, days 1–28, every 6 weeks) was started. From the
second cycle of chemotherapy, the dose of sunitinib was reduced to
37.5 mg/body/day due to CTCAEv4.0 grade 1 fatigue. CTCAEv4.0 grade
1 diarrhea and grade 1 proteinuria were also observed, but no other
severe adverse events occurred. After four cycles of chemotherapy,
the liver and lymph node metastases disappeared, and a CR, as
defined by Response Evaluation Criteria In Solid Tumors version
1.1, was achieved in February 2016 (Fig.
3C and D). The patient then strongly demanded that chemotherapy
be stopped, although our recommendation was that it be continued.
CT at the end of August 2016 again revealed a CR. However, tumor
recurrence was detected in November 2016, indicating that CR had
been maintained for 6 months. The patient received sunitinib again,
and partial response was achieved after 3 months.
Discussion
This patient could be diagnosed with GIST
histologically because of positivity for CD34 and KIT (3). Risk classification is used for GIST
cases with no metastases. Historically, the Fletcher
classification, which consisted of tumor size and mitoses, was used
first (14). It was also reported
that the MIB-1 labeling index and the existence of tumor necrosis
were useful as indicators of tumor proliferation (15–17). The
prognosis of GIST has appeared to be different in different tumor
sites, so the Miettinen classification has been used as a standard
for predicting tumor recurrence (18). Furthermore, the modified Fletcher
classification, consisting of tumor size, mitoses, tumor sites, and
tumor rupture, was reported to be more useful for the selection of
high-risk recurrent cases (19).
This case was diagnosed as high-risk GIST with the modified
Fletcher classification. Though the modified Fletcher
classification is a discontinuous indicator for risk assessment,
the contour maps are considered to be continuous and useful for the
diagnosis of tumor recurrence (19).
In the present case study, the probability of tumor recurrence was
within 80–90% by the contour maps. Therefore, adjuvant chemotherapy
with imatinib was indicated for the present study.
For GIST patients whose tumors are totally resected,
imatinib is effective as adjuvant chemotherapy for primary
high-risk cases in order to improve recurrence-free survival
(8,9). For progressive disease cases treated
with 400 mg/body of imatinib therapy, dose escalation of imatinib
to 800 mg/body was reported to be effective in the EORTC62005 and
the S0033 trials (20,21). Increasing the dosage of imatinib
could have been effective for this patient, but Japanese patients
cannot receive 800 mg/body of imatinib as, due to the medical
insurance system of Japan, the maximal dose of imatinib for GIST is
400 mg/body.
Sunitinib is recommended for imatinib-resistant
GISTs in unresectable or metastatic cases; it is associated with a
significantly longer mPFS and higher RR (12). Clinical evaluation of continuous
daily dosing of sunitinib in patients with advanced GISTs following
imatinib failure has been analyzed (13); the disease control rate of sunitinib
was 53%, RR was 13%, mPFS was 34 weeks, and median overall survival
(OS) was 107 weeks. Sahu et al (22) reported no CR cases among 15 Indian
patients prospectively administered sunitinib as second-line
chemotherapy. Thus, in the prospective clinical trials examining
the effects of sunitinib, no cases demonstrated CR.
Retrospective reports of CR cases with second-line
chemotherapy involving sunitinib are also few in number. Dudeck
et al (23) retrospectively
identifed no CR cases among 51 German patients. To the best of our
knowledge, there have been three cases showing CR with sunitinib as
second-line chemotherapy for GISTs reported retrospectively: 2/199
(1.0%) patients showed CR in a Taiwanese study (24), and 1/48 patients (2.1%) showed CR in
a Chinese study (25). No individual
case reports have been published worldwide, so that the present
study is a very rare case report showing CR. Furthermore, CR could
have been maintained longer if the patient had not demanded that
chemotherapy be stopped.
This case report has several limitations. For
example, fluorodeoxyglucose-positron emission tomography (FDG-PET)
was not performed. This technique is able to detect metastatic
lesions that are negative on other modalities, and the patient in
this case study could have had other metastases prior to starting
sunitinib treatment. However, there are also FDG-PET-negative GIST
cases, so that FDG-PET is not considered a substitute for CT
(26). Furthermore, detection of a
KIT mutation was not performed. Imatinib has a less marked effect
for GIST cases with PDGFRA exon 18 D842V mutation (20), and decreases from the baseline in
plasma levels of soluble KIT following 20 and 24 weeks of dosing
were correlated with a longer OS (13). Finally, the four CR cases were all
Asian patients, suggesting that Asian patients may have certain
factors that are associated with the effects of sunitinib.
In conclusion, a very rare case of GIST that
achieved CR with second-line sunitinib was described. This
information should be of importance not only for the treatment of
GISTs, but also clinical research.
Acknowledgements
The authors would like to thank the medical staff
for their contribution to patient diagnosis and treatment.
References
|
1
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors-definition, clinical, histological,
immunohistochemical, and molecular genetic features and
differential diagnosis. Virchows Arch. 438:1–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Japanese Clinical Practice Guidelines for
Gastrointestinal Stromal Tumors (GIST). 3rd. Japan Society of
Clinical Oncology; pp. 1–16. 2004
|
|
3
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
et al: Gain-of-function mutations of c-kit in human
gastrointestinal stromal tumors. Science. 279:577–580. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miettinen M, Sobin LH and Sarlomo-Rikala
M: Immunohistochemical spectrum of GISTs at different sites and
their differential diagnosis with a reference to CD117 (KIT). Mod
Pathol. 13:1134–1142. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pierie JP, Choudry U, Muzikansky A, Yeap
BY, Souba WW and Ott MJ: The effect of surgery and grade on outcome
of gastrointestinal stromal tumors. Arch Surg. 136:383–389. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DeMatteo RP, Lewis JJ, Leung D, Mudan SS,
Woodruff JM and Brennan MF: Two hundred gastrointestinal stromal
tumors: Recurrence patterns and prognostic factors for survival.
Ann Surg. 231:51–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plaat BE, Hollema H, Molenaar WM, Broers
GH Torn, Pijpe J, Mastik MF, Hoekstra HJ, van den Berg E, Scheper
RJ and van der Graaf WT: Soft tissue leiomyosarcomas and malignant
gastrointestinal stromal tumors: Differences in clinical outcome
and expression of multidrug resistance proteins. J Clin Oncol.
18:3211–3220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joensuu H, Eriksson M, Hall K Sundby,
Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster
J, Al-Batran SE, et al: One vs three years of adjuvant imatinib for
operable gastrointestinal stromal tumor: A randomized trial. JAMA.
307:1265–1272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanda T, Nishida T, Wada N, Kobayashi O,
Yamamoto M, Sawaki A, Boku N, Koseki M, Doi T, Toh Y, et al:
Adjuvant therapy with imatinib mesylate after resection of primary
high-risk gastrointestinal stromal tumors in Japanese patients. Int
J Clin Oncol. 18:38–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demetri GD, von Mehren M, Blanke CD, Van
den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA,
Singer S, Janicek M, et al: Efficacy and safety of imatinib
mesylate in advanced gastrointestinal stromal tumors. N Engl J Med.
347:472–480. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verweij J, Casali PG, Zalcberg J, LeCesne
A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC,
van Glabbeke M, et al: Progression-free survival in
gastrointestinal stromal tumours with high-dose imatinib:
Randomised trial. Lancet. 364:1127–1134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Demetri GD, van Oosterom AT, Garrett CR,
Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich
MC, Morgan JA, et al: Efficacy and safety of sunitinib in patients
with advanced gastrointestinal stromal tumour after failure of
imatinib: A randomised controlled trial. Lancet. 368:1329–1338.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
George S, Blay JY, Casali PG, Le Cesne A,
Stephenson P, Deprimo SE, Harmon CS, Law CN, Morgan JA, Ray-Coquard
I, et al: Clinical evaluation of continuous daily dosing of
sunitinib malate in patients with advanced gastrointestinal stromal
tumour after imatinib failure. Eur J Cancer. 45:1959–1968. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fletcher CD, Berman JJ, Corless C,
Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti
H, Rubin BP, et al: Diagnosis of gastrointestinal stromal tumors: A
consensus approach. Hum Pathol. 33:459–465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong NA, Young R, Malcomson RD, Nayar AG,
Jamieson LA, Save VE, Carey FA, Brewster DH, Han C and Al-Nafussi
A: Prognostic indicators for gastrointestinal stromal tumours: A
clinicopathological and immunohistochemical study of 108 resected
cases of the stomach. Histopathology. 43:118–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujimoto Y, Nakanishi Y, Yoshimura K and
Shimoda T: Clinicopathologic study of primary malignant
gastrointestinal stromal tumor of the stomach, with special
reference to prognostic factors: Analysis of results in 140
surgically resected patients. Gastric Cancer. 6:39–48. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hasegawa T, Matsuno Y, Shimoda T and
Hirohashi S: Gastrointestinal stromal tumor: Consistent CD117
immunostaining for diagnosis, and prognostic classification based
on tumor size and MIB-1 grade. Hum Pathol. 33:669–676. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: Pathology and prognosis at different sites. Semin
Diagn Pathol. 23:70–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joensuu H, Vehtari A, Riihimäki J, Nishida
T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C,
et al: Risk of recurrence of gastrointestinal stromal tumour after
surgery: An analysis of pooled population-based cohorts. Lancet
Oncol. 13:265–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zalcberg JR, Verweij J, Casali PG, Le
Cesne A, Reichardt P, Blay JY, Schlemmer M, van Glabbeke M, Brown M
and Judson IR; EORTC Soft Tissue and Bone Sarcoma Group, the
Italian Sarcoma Group, : Australasian Gastrointestinal Trials
Group: Outcome of patients with advanced gastro-intestinal stromal
tumours crossing over to a daily imatinib dose of 800 mg after
progression on 400 mg. Eur J Cancer. 41:1751–1757. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blanke CD, Rankin C, Demetri GD, Ryan CW,
von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki
RG, et al: Phase III randomized, intergroup trial assessing
imatinib mesylate at two dose levels in patients with unresectable
or metastatic gastrointestinal stromal tumors expressing the kit
receptor tyrosine kinase: S0033. J Clin Oncol. 26:626–632. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sahu A, Godbole S, Jain P, Ghosh J,
Shrikhande S, Ramadwar M, Goyal M, Gulia S, Bajpai J, Kembhavi Y,
et al: Sunitinib in patients with imatinib-resistant
gastrointestinal stromal tumor: A single center experience study.
Indian J Cancer. 52:320–323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dudeck O, Zeile M, Reichardt P and Pink D:
Comparison of RECIST and Choi criteria for computed tomographic
response evaluation in patients with advanced gastrointestinal
stromal tumor treated with sunitinib. Ann Oncol. 22:1828–1833.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen YY, Yeh CN, Cheng CT, Chen TW, Rau
KM, Jan YY and Chen MF: Sunitinib for Taiwanese patients with
gastrointestinal stromal tumor after imatinib treatment failure or
intolerance. World J Gastroenterol. 17:2113–2119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Jiang WZ, Guan GX, Chen ZF, Chi P
and Lu HS: [Efficacy and safety of sunitinib on patients with
imatinib-resistant gastrointestinal stromal tumor]. Zhonghua Wei
Chang Wai Ke Za Zhi. 16:221–225. 2013.PubMed/NCBI
|
|
26
|
Demetri GD, von Mehren M, Antonescu CR,
DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF,
Schuetze S, et al: NCCN Task Force report: Update on the management
of patients with gastrointestinal stromal tumors. J Natl Compr Canc
Netw. 8 Suppl 2:S1–S41; quiz S42-S44. 2010. View Article : Google Scholar : PubMed/NCBI
|