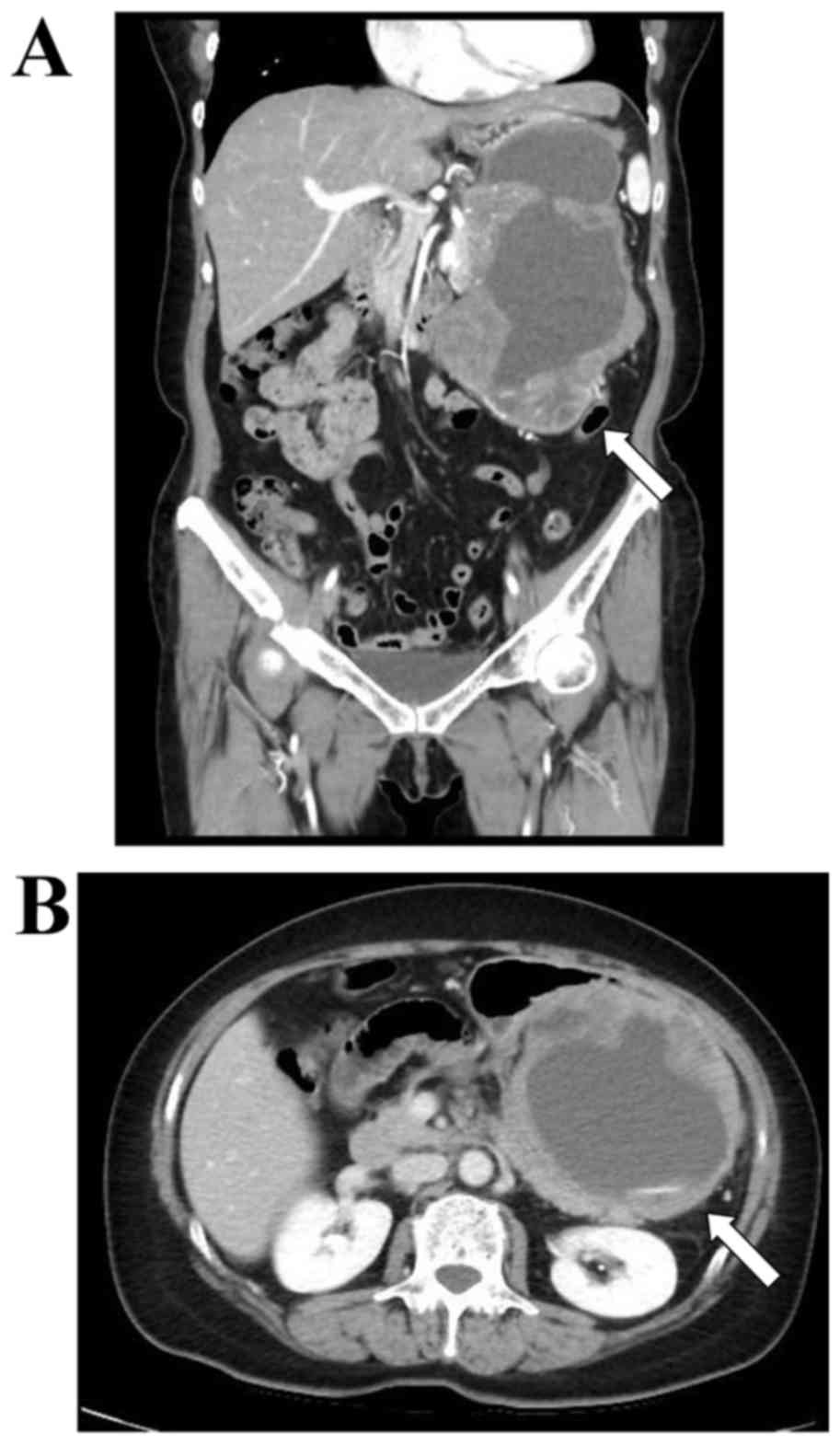
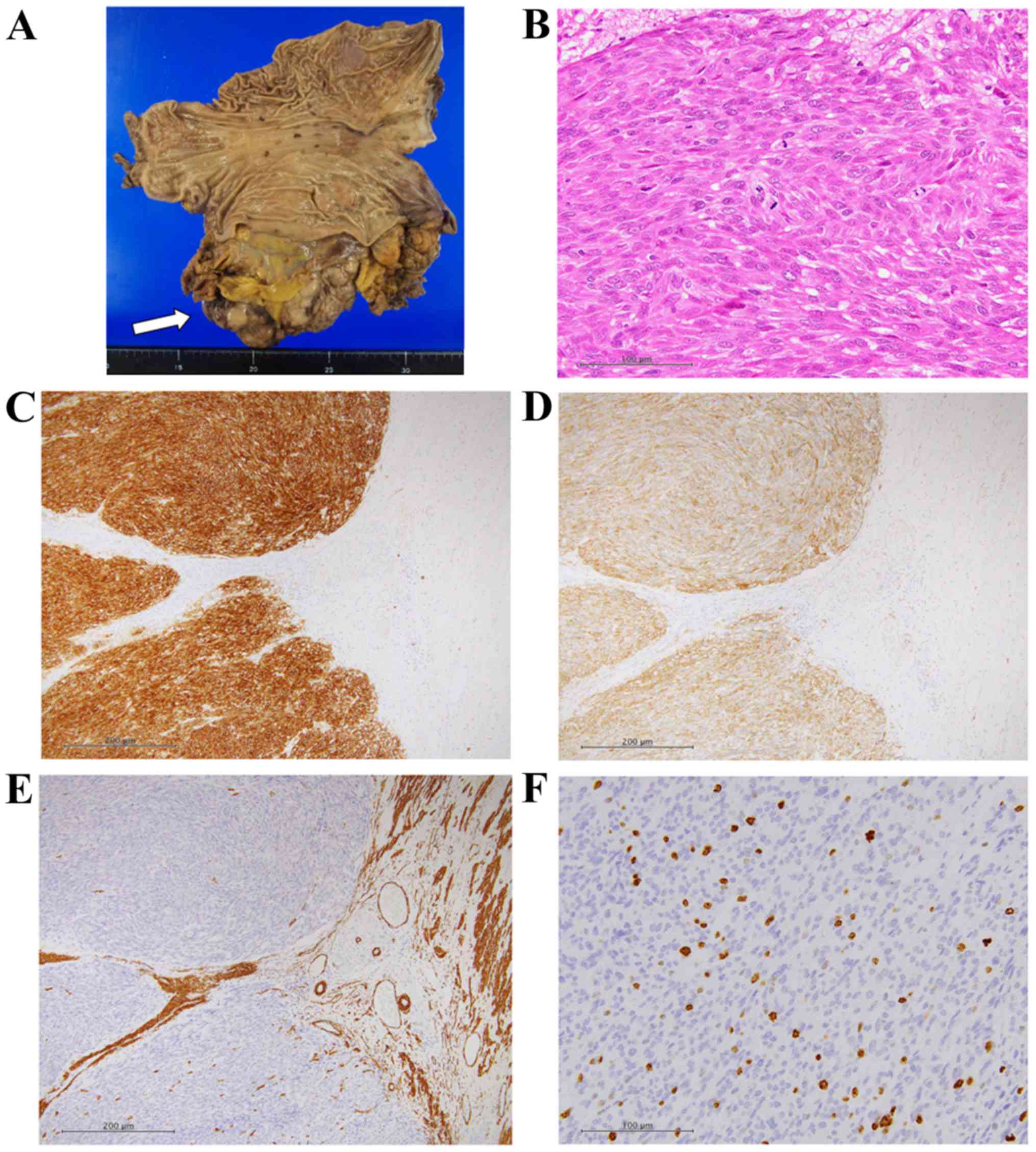
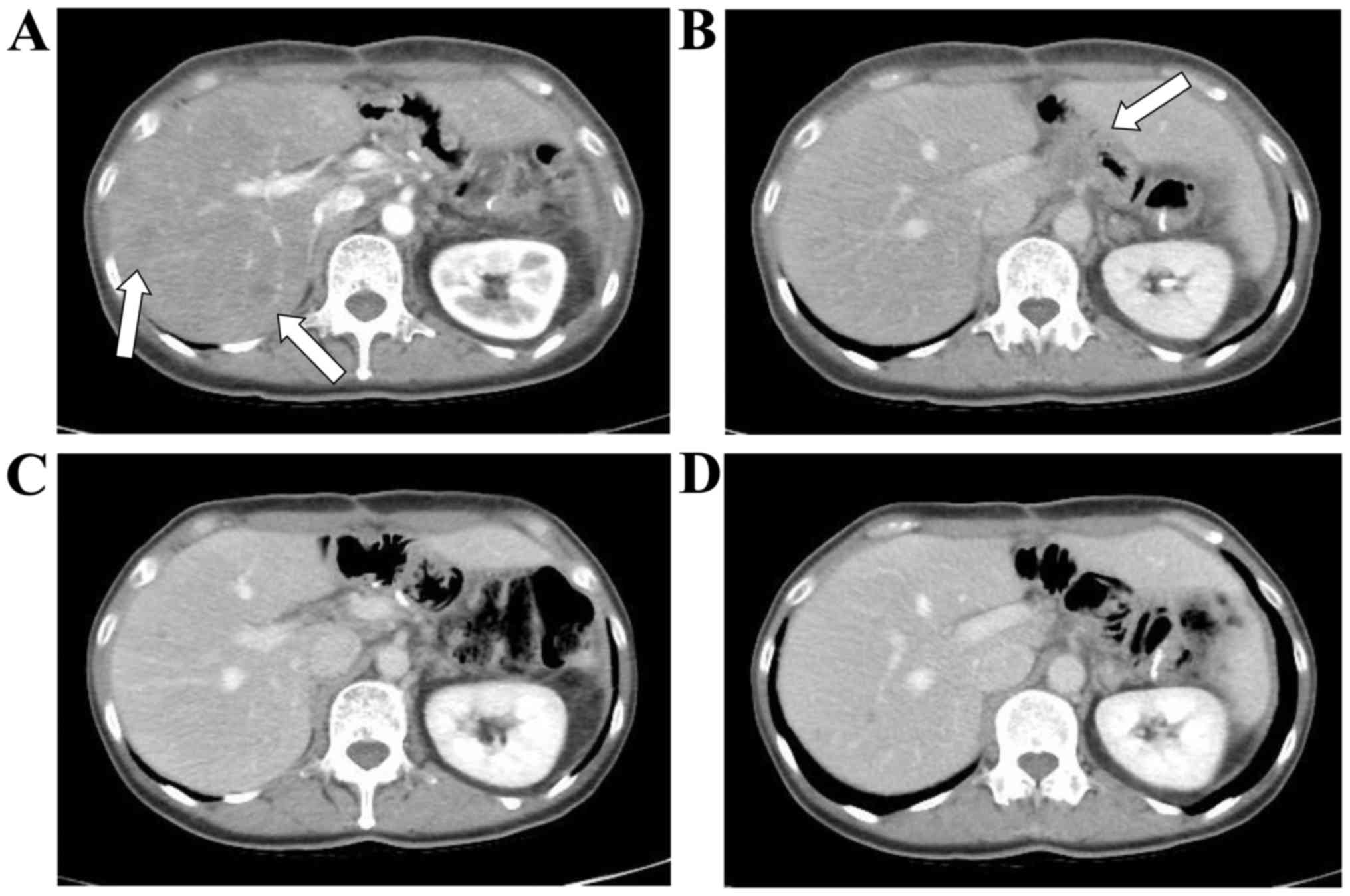
|
1
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors-definition, clinical, histological,
immunohistochemical, and molecular genetic features and
differential diagnosis. Virchows Arch. 438:1–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Japanese Clinical Practice Guidelines for
Gastrointestinal Stromal Tumors (GIST). 3rd. Japan Society of
Clinical Oncology; pp. 1–16. 2004
|
|
3
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
et al: Gain-of-function mutations of c-kit in human
gastrointestinal stromal tumors. Science. 279:577–580. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miettinen M, Sobin LH and Sarlomo-Rikala
M: Immunohistochemical spectrum of GISTs at different sites and
their differential diagnosis with a reference to CD117 (KIT). Mod
Pathol. 13:1134–1142. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pierie JP, Choudry U, Muzikansky A, Yeap
BY, Souba WW and Ott MJ: The effect of surgery and grade on outcome
of gastrointestinal stromal tumors. Arch Surg. 136:383–389. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DeMatteo RP, Lewis JJ, Leung D, Mudan SS,
Woodruff JM and Brennan MF: Two hundred gastrointestinal stromal
tumors: Recurrence patterns and prognostic factors for survival.
Ann Surg. 231:51–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plaat BE, Hollema H, Molenaar WM, Broers
GH Torn, Pijpe J, Mastik MF, Hoekstra HJ, van den Berg E, Scheper
RJ and van der Graaf WT: Soft tissue leiomyosarcomas and malignant
gastrointestinal stromal tumors: Differences in clinical outcome
and expression of multidrug resistance proteins. J Clin Oncol.
18:3211–3220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joensuu H, Eriksson M, Hall K Sundby,
Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster
J, Al-Batran SE, et al: One vs three years of adjuvant imatinib for
operable gastrointestinal stromal tumor: A randomized trial. JAMA.
307:1265–1272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanda T, Nishida T, Wada N, Kobayashi O,
Yamamoto M, Sawaki A, Boku N, Koseki M, Doi T, Toh Y, et al:
Adjuvant therapy with imatinib mesylate after resection of primary
high-risk gastrointestinal stromal tumors in Japanese patients. Int
J Clin Oncol. 18:38–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demetri GD, von Mehren M, Blanke CD, Van
den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA,
Singer S, Janicek M, et al: Efficacy and safety of imatinib
mesylate in advanced gastrointestinal stromal tumors. N Engl J Med.
347:472–480. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verweij J, Casali PG, Zalcberg J, LeCesne
A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC,
van Glabbeke M, et al: Progression-free survival in
gastrointestinal stromal tumours with high-dose imatinib:
Randomised trial. Lancet. 364:1127–1134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Demetri GD, van Oosterom AT, Garrett CR,
Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich
MC, Morgan JA, et al: Efficacy and safety of sunitinib in patients
with advanced gastrointestinal stromal tumour after failure of
imatinib: A randomised controlled trial. Lancet. 368:1329–1338.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
George S, Blay JY, Casali PG, Le Cesne A,
Stephenson P, Deprimo SE, Harmon CS, Law CN, Morgan JA, Ray-Coquard
I, et al: Clinical evaluation of continuous daily dosing of
sunitinib malate in patients with advanced gastrointestinal stromal
tumour after imatinib failure. Eur J Cancer. 45:1959–1968. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fletcher CD, Berman JJ, Corless C,
Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti
H, Rubin BP, et al: Diagnosis of gastrointestinal stromal tumors: A
consensus approach. Hum Pathol. 33:459–465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong NA, Young R, Malcomson RD, Nayar AG,
Jamieson LA, Save VE, Carey FA, Brewster DH, Han C and Al-Nafussi
A: Prognostic indicators for gastrointestinal stromal tumours: A
clinicopathological and immunohistochemical study of 108 resected
cases of the stomach. Histopathology. 43:118–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujimoto Y, Nakanishi Y, Yoshimura K and
Shimoda T: Clinicopathologic study of primary malignant
gastrointestinal stromal tumor of the stomach, with special
reference to prognostic factors: Analysis of results in 140
surgically resected patients. Gastric Cancer. 6:39–48. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hasegawa T, Matsuno Y, Shimoda T and
Hirohashi S: Gastrointestinal stromal tumor: Consistent CD117
immunostaining for diagnosis, and prognostic classification based
on tumor size and MIB-1 grade. Hum Pathol. 33:669–676. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: Pathology and prognosis at different sites. Semin
Diagn Pathol. 23:70–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joensuu H, Vehtari A, Riihimäki J, Nishida
T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C,
et al: Risk of recurrence of gastrointestinal stromal tumour after
surgery: An analysis of pooled population-based cohorts. Lancet
Oncol. 13:265–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zalcberg JR, Verweij J, Casali PG, Le
Cesne A, Reichardt P, Blay JY, Schlemmer M, van Glabbeke M, Brown M
and Judson IR; EORTC Soft Tissue and Bone Sarcoma Group, the
Italian Sarcoma Group, : Australasian Gastrointestinal Trials
Group: Outcome of patients with advanced gastro-intestinal stromal
tumours crossing over to a daily imatinib dose of 800 mg after
progression on 400 mg. Eur J Cancer. 41:1751–1757. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blanke CD, Rankin C, Demetri GD, Ryan CW,
von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki
RG, et al: Phase III randomized, intergroup trial assessing
imatinib mesylate at two dose levels in patients with unresectable
or metastatic gastrointestinal stromal tumors expressing the kit
receptor tyrosine kinase: S0033. J Clin Oncol. 26:626–632. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sahu A, Godbole S, Jain P, Ghosh J,
Shrikhande S, Ramadwar M, Goyal M, Gulia S, Bajpai J, Kembhavi Y,
et al: Sunitinib in patients with imatinib-resistant
gastrointestinal stromal tumor: A single center experience study.
Indian J Cancer. 52:320–323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dudeck O, Zeile M, Reichardt P and Pink D:
Comparison of RECIST and Choi criteria for computed tomographic
response evaluation in patients with advanced gastrointestinal
stromal tumor treated with sunitinib. Ann Oncol. 22:1828–1833.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen YY, Yeh CN, Cheng CT, Chen TW, Rau
KM, Jan YY and Chen MF: Sunitinib for Taiwanese patients with
gastrointestinal stromal tumor after imatinib treatment failure or
intolerance. World J Gastroenterol. 17:2113–2119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Jiang WZ, Guan GX, Chen ZF, Chi P
and Lu HS: [Efficacy and safety of sunitinib on patients with
imatinib-resistant gastrointestinal stromal tumor]. Zhonghua Wei
Chang Wai Ke Za Zhi. 16:221–225. 2013.PubMed/NCBI
|
|
26
|
Demetri GD, von Mehren M, Antonescu CR,
DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF,
Schuetze S, et al: NCCN Task Force report: Update on the management
of patients with gastrointestinal stromal tumors. J Natl Compr Canc
Netw. 8 Suppl 2:S1–S41; quiz S42-S44. 2010. View Article : Google Scholar : PubMed/NCBI
|