Introduction
α-fetoprotein (AFP) is a glycoprotein synthesized
from the fetal yolk sac and liver during the gestational period
(1). In adults, a high level of AFP
is considered abnormal, and a good tumor marker for monitoring or
screening yolk sac tumors and hepatocellular carcinoma.
Additionally, there is evidence that its level may be increased in
solid tumors, including gastric carcinoma (2,3).
In 1970, Bourreille et al (4) reported a case of gastric carcinoma with
a high AFP level and synchronic liver metastasis for the first
time. Thereafter, various studies have emphasized that patients
with AFP-secreting gastric carcinoma (AFP-SGC) have a high
incidence of liver metastasis, increased lymphovascular invasion
(LVI), advanced stage cancer and poor prognosis (5–7).
Furthermore, a previous study demonstrated that AFP-SGC has a
higher proliferation index, lower apoptosis and richer
neovascularization compared with non-AFP-SGC (8). These data gave rise to the
consdieration that AFP-SGC is a special subtype of gastric
carcinoma with a high malignant potential. However, few studies
have been reported that assess the clinicopathological features and
prognostic importance of this special subtype. The majority of them
included non-metastatic patients who were given curative surgery
(5). Therefore, the aim of the
present study was to evaluate and compare the clinicopathological
characteristics, treatment and prognostic features of patients with
AFP-SGC and non-AFP-SGC independently of the disease stage
(non-metastatic or metastatic).
Patients and methods
Between 2009 and 2015, the health records of 1,328
patients who were given a histopathological diagnosis of gastric
cancer in our hospital (the Ankara Numune Education and Reearch
Hospital, Ankara, Turkey) were investigated retrospectively. Of
those, the medical data of 404 patients whose AFP levels were
measured at the time of diagnosis were evaluated. The AFP cut-off
value was defined as 9 ng/ml (normal range, 0–9 ng/ml), as
determined from a UniCel Dxl 600–800 assay system (Beckman Coulter,
Inc., Brea, CA, USA) in our laboratory. The patients that had an
AFP level >9 ng/ml at the time of diagnosis were defined as
‘AFP-SGC’ patients, and those with an AFP level <9 ng/ml were
categorized as ‘non-AFP-SGC’ patients, accordingly. A total of 13
patients who had diseases that may have caused high levels of AFP,
including chronic liver diseases, cirrhosis, yolk sac tumor,
teratoma and hepatocellular carcinoma, were excluded from the
study. The analysis also excluded 29 patients who did not
follow-up.
The patients with AFP-SGC and non-AFP-SGC were
evaluated comparatively in terms of pathological parameters, such
as LVI, perineural invasion (PNI), the disease stage, and tumor
size; clinical parameters, such as age, weight loss at the time of
diagnosis, smoking history, and performance status; and treatment
modalities, such as curative surgery, adjuvant chemotherapy and
radiotherapy, no surgery, and palliative chemotherapy.
To evaluate the performance status, the Eastern
Cooperative Oncology Group (ECOG) performance scale was used; for
the staging and node status, the American Joint Committee on Cancer
staging system was used (9). The
staging in patients who underwent surgery was performed via a
histopathological examination, whereas, for metastatic patients who
did not undergo surgery, clinical staging was used. Additionally,
endoscopic and histopathological findings were recorded according
to the Borrmann and Lauren histological classification systems,
respectively (10).
The health status of the patients was determined by
accessing the health records of the patients at the hospital and
the registrations in the Central Population Administration System
of the Turkish Republic.
Statistical analysis
The computer programme ‘Statistical Package for the
Social Sciences’, version 18.0 for Windows (SPSS, Inc., Chicago,
IL, USA) was used for the statistical analysis. The variables were
investigated according to visual (histograms, probability plots)
and analytical (Kolmogorov Simirnov/Shapiro-Wilk's test) methods to
determine whether they were normally distributed or not.
Non-parametric variables are presented as the median and range, and
categorical variables are presented as the frequency with
percentages. Continuous variables were analyzed using the
Mann-Whitney U test. Categorical variables were analyzed using the
Chi-square or Fisher exact test. Survival analysis was performed
according to the Kaplan-Meier method, whereas log-rank statistics
was used to compare the subgroups. The possible factors identified
with univariate analyses were further entered into the Cox
regression analysis, with backward selection, to determine
independent predictors of survival. Overall survival (OS) was
defined as the interval between diagnosis and death or date last
known alive, whereas the duration between the end of primary
treatment and recurrence was defined as the disease-free survival
(DFS) rate. P<0.05 was considered to indicate a statistically
significant value.
Results
Clinicopathological parameters
A total of 362 patients were included in the present
study. Of those 362 patients, 53 had AFP-SGC and 309 had
non-AFP-SGC. Clinicopathological features of the patients are shown
in Table I. The median age of all
patients was 58 (range, 22–88), and 73.8% of them were male; 26.2%
were female.
 | Table I.Demographic characteristics of the
patients. |
Table I.
Demographic characteristics of the
patients.
| Characteristics | AFP-SGC (%)
(n=53) | Non-AFP-SGC
(%)(n=309) | P-value |
|---|
| Age (median,
range) | 58 (22–87) | 59 (23–88) | 0.72 |
| Sex |
|
|
|
|
Female | 13 (24.5) | 82 (26.5) | 0.75 |
| Male | 40 (75.5) | 227 (73.5) |
|
| Smoking | 35 (66.0) | 193 (62.5) | 0.61 |
| ECOG |
|
|
|
| 0–1 | 33 (62.3) | 243 (78.6) | 0.01 |
| 2–4 | 20 (37.7) | 66 (21.4) |
|
| Weight loss | 20 (37.7) | 139 (45.0) | 0.32 |
| Comorbidity | 23 (43.4) | 139 (45) | 0.83 |
| Lauren
classification |
|
|
|
|
Intestinal | 15 (34.9) | 107 (37) | 0.59 |
|
Diffuse | 16 (37.2) | 121 (41.9) |
|
|
Mixed | 12 (27.9) | 61 (21.1) |
|
| Depth of
invasion |
|
| 0.88 |
| T1 +
T2 | 4 (21.1) | 42 (19.7) |
|
| T3 +
T4 | 15 (78.9) | 171 (80.3) |
|
| Tumor
size, cm (median, range) | 4 (1–13) | 4 (4–17) | 0.78 |
| TNM stage |
|
| <0.001 |
| 1–2 | 5 (9.4) | 83 (26.9) |
|
| 3 | 10 (18.9) | 93 (30.1) |
|
| 4 | 38 (71.7) | 133 (43.0) |
|
| Borrmann type |
|
| 0.37 |
| I +
II | 8 (15.1) | 63 (20.4) |
|
| III +
IV | 45 (84.9) | 246 (79.6) |
|
| Tumor location |
|
| 0.89 |
| Fundus
cardia diffuse | 21 (39.6) | 114 (36.9) |
|
|
Corpus | 17 (32.1) | 98 (31.7) |
|
|
Antrum | 15 (28.3) | 97 (31.4) |
|
| LVI | 27 (77.1) | 156 (60.2) | 0.05 |
| PNI | 26 (74.3) | 145 (56.2) | 0.04 |
| Location of
metastases at diagnosis | 38 | 133 |
|
|
Liver | 31 (81.6) | 61 (45.9) |
<0.001 |
|
Peritoneum | 6 (15.8) | 46 (34.6) | 0.02 |
|
Intra-abdominal distant
LAP | 11 (28.9) | 47 (35.3) | 0.46 |
|
Lung | 6 (15.8) | 14 (10.5) | 0.39 |
|
Bone | 5 (13.2) | 12 (9) | 0.53 |
|
Others | 6 (15.8) | 38 (28.6) | 0.11 |
Although no significant differences were identified
in terms of the clinical and pathological parameters of age, sex,
smoking history, comorbidities, Lauren classification, tumor size,
Borrmann type or tumor localization between the patients with
AFP-SGC and non-AFP-SGC, the presence of LVI or PNI, an ECOG
performance score of ≥2, stage IV disease [according to the
tumor-lymph node-metastasis (TNM) staging system], and liver
metastasis were significantly more frequent in the AFP-SGC group
(P<0.05). The non-AFP-SGC group had more frequent peritoneal
metastasis (P<0.05) (Table
I).
Comparing the laboratory analyses between the two
groups, no statistically significant differences were identified in
the levels of lactate dehydrogenase, hemoglobin and albumin;
however, high levels of carcinoembryonic antigen (CEA) were present
more frequently in the AFP-SGC group (64.2 vs. 28.5%;
P<0.001).
OS analysis in the whole patient
group
Median follow-up durations for the patients with
AFP-SGC or non-AFP-SGC were 11.7 (range, 1.0–54.2) and 15.6 (range,
0.4–83.0) months, respectively. The median OS was 12.6 months
(range, 6.1–19.1) in the AFP-SGC group, and 22.1 months (range,
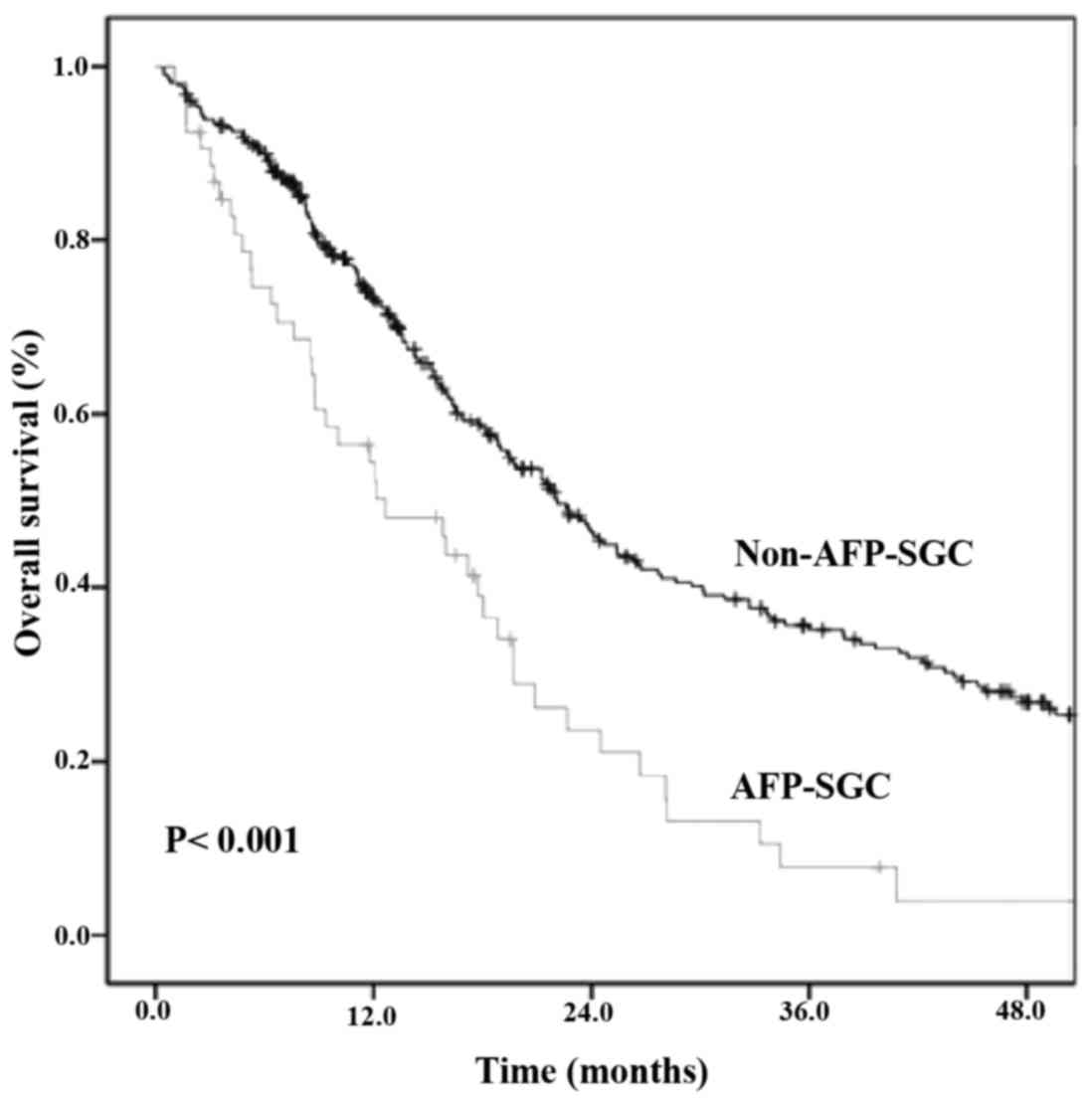
19.0–25.2) in the non-AFP-SGC group (P<0.001) (Fig. 1), whereas the 1- and 2-year survival
rates were 55 and 25%, respectively, in the AFP-SGC group; they
were 73 and 47%, respectively, in the non-AFP-SGC group
(P<0.001).
The median survival in the whole group was 20.9
months [95% confidence interval (CI), 18.3–23.4]. Prognostic
factors affecting the OS were evaluated using the long-rank test.
An ECOG performance score of ≥2 (P<0.001), weight loss at time
of diagnosis (P<0.001), AFP-SGC type (P<0.001), presence of
PNI (P<0.001), presence of LVI (P=0.02), high levels of CEA at
the time of diagnosis (P<0.001), metastatic disease at the time
of diagnosis (P<0.001), and lymph node positivity (P=0.06) were
the factors that influenced the OS. In the multivariate analyis, an
ECOG performance score of ≥2, lymph node positivity, the presence
of PNI, high levels of CEA, and metastatic disease at the time of
diagnosis were identified to be independent prognostic factors
(Table II).
 | Table II.Univariate and multivariate analysis
of the clinicopathological characteristics of all the patients. |
Table II.
Univariate and multivariate analysis
of the clinicopathological characteristics of all the patients.
| Characteristic | No. of patients
(%) | Univariate analysis
for OS | P-value
(univariate) | Multivariate
analysis |
|---|
| Age (mean,
years) |
|
| 0.34 |
|
|
<60 | 186 (51.4) | 21.9 |
|
|
|
≥60 | 176 (48.6) | 19.3 |
|
|
| Sex |
|
| 0.59 |
|
|
Female | 95 (26.2) | 18.7 |
|
|
|
Male | 267 (73.8) | 21.2 |
|
|
| ECOG |
|
|
<0.001 | P=0.003,
HR=1.957, 95% CI=1.263–3.032 |
|
0–1 | 276 (76.2) | 23.9 |
|
|
|
2–4 | 86 (23.8) | 10.0 |
|
|
| Weight loss |
|
|
<0.001 | P=0.309, HR=1.230,
95% CI=0.826–1.832 |
|
Yes | 159 (43.9) | 15.9 |
|
|
| No | 203 (56.1) | 25.3 |
|
|
| Tumor type |
|
|
<0.001 | P=0.640, HR=1.165,
95% CI=0.614–2.211 |
|
AFP-SGC | 53 (14.6) | 12.6 |
|
|
|
Non-AFP-SGC | 309 (85.4) | 22.1 |
|
|
| Comorbidity |
|
| 0.36 |
|
Yes | 162 (44.8) | 18.8 |
|
|
| No | 200 (55.2) | 22.7 |
|
|
| Smoking |
|
| 0.76 |
|
|
Yes | 228 (63.0) | 22.0 |
|
|
| No | 134 (37.0) | 18.8 |
|
|
| Tumor location |
|
| 0.08 | P=0.767, HR=1.072,
95% CI=0.678–1.694 |
| Fundus
cardia diffuse | 136 (37.6) | 19.7 |
|
|
|
Corpus | 114 (31.5) | 16.3 |
|
|
|
Antrum | 112 (30.9) | 23.7 |
|
|
| Tumor size
(cm) |
|
| 0.75 |
|
|
<5 | 226 (62.4) | 18.7 |
|
|
| ≥5 | 136 (37.6) | 23.8 |
|
|
| Node |
|
| 0.06 | P=0.031,
HR=1.746, 95% CI=1.053–2.895 |
|
Positive | 169 (70.1) | 26.5 |
|
|
|
Negative | 72 (29.9) | 45.7 |
|
|
| PNI |
|
|
<0.001 | P=0.049,
HR=1.579, 95% CI=1.003–2.486 |
|
Yes | 171 (58.3) | 22.6 |
|
|
| No | 122 (41.7) | 39.6 |
|
|
| LVI |
|
| 0.02 | P=0.331, HR=1.285,
95% CI=0.775–2.132 |
|
Yes | 183 (62.2) | 21.9 |
|
|
| No | 111 (37.8) | 28.6 |
|
|
| Bormann type |
|
| 0.86 |
| I +
II | 71 (19.6) | 17.9 |
|
|
| III +
IV | 291(80.4) | 21.2 |
|
|
| CEA (ng/ml) |
|
|
<0.001 | P=0.018,
HR=1.687, 95% CI=1.093–2.604 |
|
>5 | 122 (33.7) | 13.4 |
|
|
| ≤5 | 240 (66.3) | 25.4 |
|
|
| Metastases at
diagnosis |
|
|
<0.001 | P<0.001,
HR=2.323, 95% CI=1.496–3.608 |
|
Yes | 171 (47.2) | 11.4 |
|
|
| No | 191 (52.8) | 40.0 |
|
|
Survival data in the stage I–III
patient group
The OS and DFS data of 191 patients were at stages
I–III at the time of diagnosis, and who had curative surgery, were
evaluated. Of those patients, 15 of them (7.8%) were in the AFP-SGC
group, and 176 patients (92.2%) were in the non-SFP-SGC group. A
total of 28.3% of the AFP-SGC patients (n=15) and 57% of the
non-AFP-SGC patients (n=176) underwent curative surgery
(P<0.001). Metastatic or recurrent disease occurred in 60% (n=9)
of the 15 AFP-SGC patients, and the liver was the most common site
of metastasis (66.7%). In the non-AFP-SGC group, metastatic or
recurrent disease occurred in 42% (n=74) of the 176 patients, and
the most common site of metastasis was the peritoneum (22.9%,
n=17). No differences were identified in the proportions of
patients taking adjuvant treatment, comparing between the AFP-SGC
and non-AFP-SGC groups (66.7% vs. 73.3%; P=0.55). The DFS of
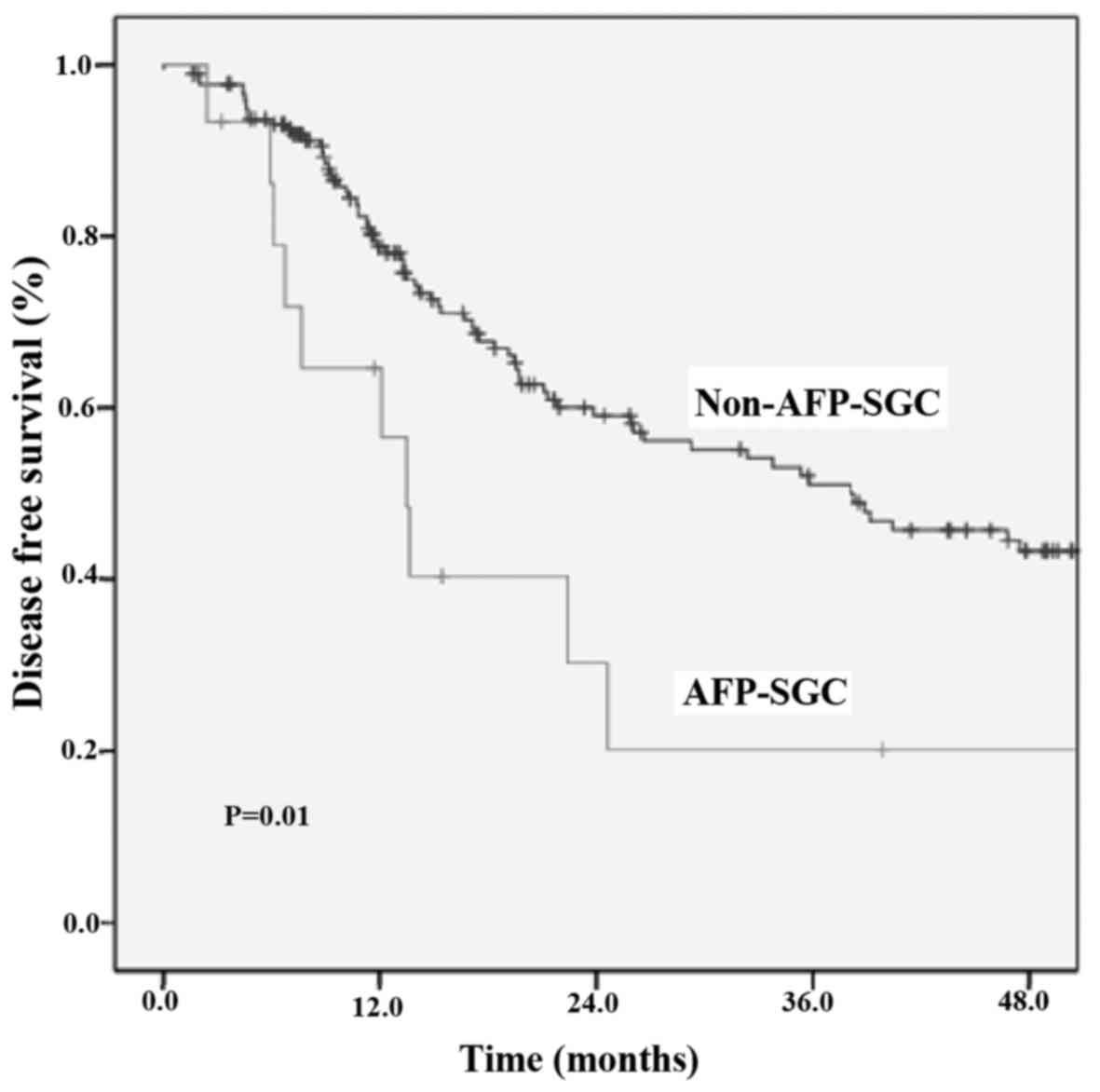
patients with stage I–III AFP-SGC or non-AFP-SGC was 13.4 (95% CI,
10.9–15.9) and 38.0 (95% CI, 23.2–52.9) months, respectively
(P=0.01) (Fig. 2). Additionally, the
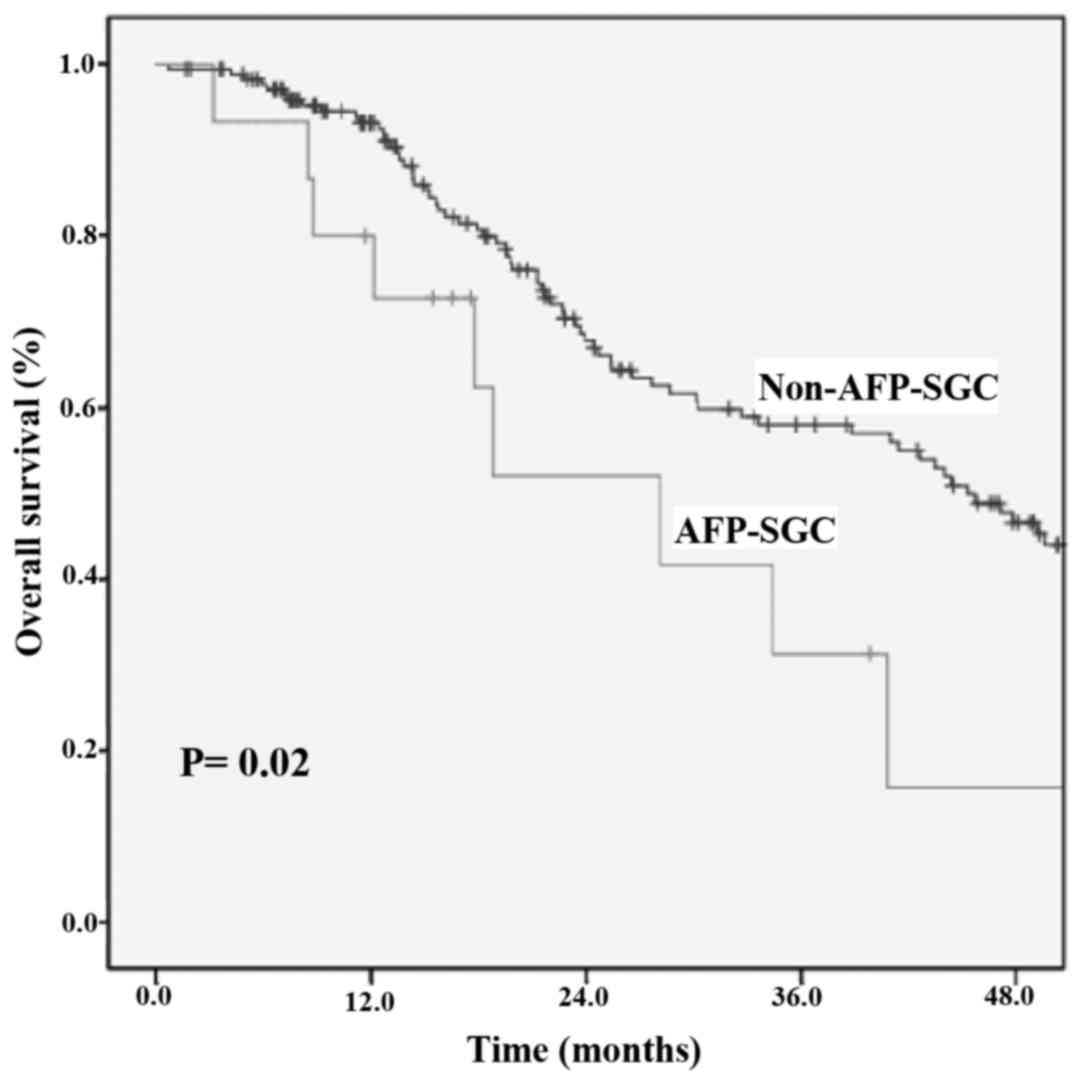
median OS of patients with stage I–III AFP-SGC was 28.1 months (95%
CI, 13.3–42.9), whereas, for non-AFP-SGC patients, it was 45.3
months (95% CI, 38.7–51.8; P=0.02) (Fig.
3).
Univariate and multivariate analyses were performed
with respect to the factors affecting the DFS. The AFP-SGC type
(P=0.01), an ECOG performance score of ≥2 (P=0.02), the presence of
PNI (P=0.03), lymph node positivity (P<0.001) and weight loss at
the time of diagnosis (P=0.03) were significant parameters for DFS
in the univariate analysis. For the multivariate analysis, an ECOG
performance score of ≥2, lymph node positivity, AFP-SGC and weight
loss at the time of diagnosis were independent prognostic factors
(Table III).
 | Table III.Univarite and multivariate analysis
of the clinicopathological characteristics of the patients with
stage I–III cancer. |
Table III.
Univarite and multivariate analysis
of the clinicopathological characteristics of the patients with
stage I–III cancer.
| Characteristic | Univariate analysis
for OS | P-value
(univariate) | Multivariate
analysis for OS | Univariate analysis
for DFS | P-value
(univariate) | Multivariate
analysis for DFS |
|---|
| Age (mean,
years) |
| 0.63 |
|
| 0.75 |
|
|
<60 | 47.1 |
|
| 38.0 |
|
|
|
≥60 | 40.8 |
|
| 26.0 |
|
|
| Sex |
| 0.67 |
|
| 0.24 |
|
|
Female | 38.8 |
|
| 24.6 |
|
|
|
Male | 44.0 |
|
| 35.3 |
|
|
| ECOG |
| 0.01 | P=0.019,
HR=1.918, 95% CI=1.112–3.306 |
| 0.02 | P=0.033,
HR=1.838, 95% CI=1.051–3.213 |
|
0–1 | 45.7 |
|
| 38.0 |
|
|
|
2–4 | 23.3 |
|
| 17.4 |
|
|
| Weight loss |
| 0.08 | P=0.120, HR=1.439,
95% CI=0.910–2.276 |
|
| P=0.028, HR=1.656,
95% CI=1.056–2.596 |
|
Yes | 26.5 |
|
| 17.4 | 0.03 |
|
| No | 47.1 |
|
| 38.8 |
|
|
| Tumor type |
| 0.02 | P=0.307, HR=1.492,
95% CI=0.693–3.216 |
| 0.01 | P=0.021,
HR=2.205, 95% CI=1.087–4.473 |
|
AFP-SGC | 28.1 |
|
| 13.4 |
|
|
|
Non-AFP-SGC | 45.3 |
|
| 38.0 |
|
|
| Tumor size
(cm) |
| 0.008 | P=0.066, HR=1.856,
95% CI=0.971–2.494 |
| 0.13 |
|
|
<5 | 67.6 |
|
| 39.1 |
|
|
| ≥5 | 32.6 |
|
| 24.6 |
|
|
| Lymph node |
| 0.004 | P=0.039,
HR=1.886, 95% CI=1.031–3.449 |
<0.001 |
| P<0.001,
HR=4.156, 95% CI=2.074–8.330 |
|
Positive | 32.6 |
|
| 24.4 |
|
|
|
Negative | N.R.a |
|
| N.R.a |
|
|
| PNI |
| 0.003 | P=0.056, HR=1.588,
95% CI=0.988–2.552 |
| 0.003 | P=0.158,
HR=1.401, 95% CI=0.877–2.239 |
|
Yes | 30.2 |
|
| 21.0 |
|
|
| No | 67.6 |
|
| 67.2 |
|
|
| LVI |
| 0.26 |
|
| 0.10 |
|
|
Yes | 34.3 |
|
| 24.6 |
|
|
| No | 47.8 |
|
| 47.4 |
|
|
| Bormann type |
| 0.92 |
|
|
|
|
| I +
II | 43.4 |
|
| N.R.a | 0.14 |
|
| III +
IV | 44.0 |
|
| 26.6 |
|
|
| CEA (ng/ml) |
| 0.16 |
|
| 0.14 |
|
|
>5 | 24.4 |
|
| 38.8 |
|
|
| ≤5 | 45.3 |
|
| 35.3 |
|
|
In patients with stage I–III cancer, univariate
analyse for OS demonstrated that an ECOG performance score of ≥2
(P=0.01), the AFP-SGC type (P=0.02), a tumor size of ≥5 cm
(P=0.008), lymph node positivity (P=0.004) and the presence of PNI
(P=0.003) were statistically significant. As for the multivariate
analysis, an ECOG performance score of ≥2 (P=0.019) and lymph node
positivity (P=0.039) were significant for the OS (Table III).
Survival data in the stage IV patient
group
At the time of diagnosis, 171 patients (47.2%) from
the two groups presented with stage IV disease; of these, 38
(22.2%) had AFP-SGC and 133 (77.8%) had non-AFP-SGC. The most
frequently implemented first-line chemotherapy regimen was modified
docetaxel/cisplatin/5-flourouracil (DCF) for patients with either
AFP-SGC or non-AFP-SGC (85 and 79.4%, respectively). No
statistically significant differences were identified for the
first- or second-line chemotherapy choice, comparing between the
two groups (P=0.82 and 0.72, respectively). The treatment features
of the patients who developed metastasis during diagnosis, or at a
later stage, are shown in Table
IV.
 | Table IV.Treatment characteristics of the
patients with AFP-SGC or non-AFP-SGC. |
Table IV.
Treatment characteristics of the
patients with AFP-SGC or non-AFP-SGC.
| Characteristic | AFP-SGC (%)
(n=53) | Non-AFP-SGC (%)
(n=309) | P-value |
|---|
| Curative
surgery |
|
|
<0.001 |
|
Yes | 15 (28.3) | 176 (57.0) |
|
| No | 38 (71.7) | 133 (43.0) |
|
|
Lymphadenectomy |
|
| 0.57 |
| D1 | 3 (15.8) | 48 (23.6) |
|
| D2 | 16 (84.2) | 155 (76.4) |
|
| Adjuvant
CRT/RT | 10 (66.7) | 129 (73.3) | 0.55 |
|
| First-line |
|
| 0.82 |
| chemotherapy |
|
DCF | 34 (85.0) | 135 (79.4) |
|
|
EOX | 3 (7.5) | 20 (11.8) |
|
|
FOLFOX | 1 (2.5) | 7 (4.1) |
|
|
CFF | 2 (5.0) | 8 (4.7) |
|
| Second-line
chemotherapy |
|
| 0.72 |
|
EOX | 12 (70.6) | 47 (66.2) |
|
|
FOLFIRI | 3 (17.6) | 10 (14.1) |
|
|
Capesitabine | 2 (11.8) | 14 (19.7) |
|
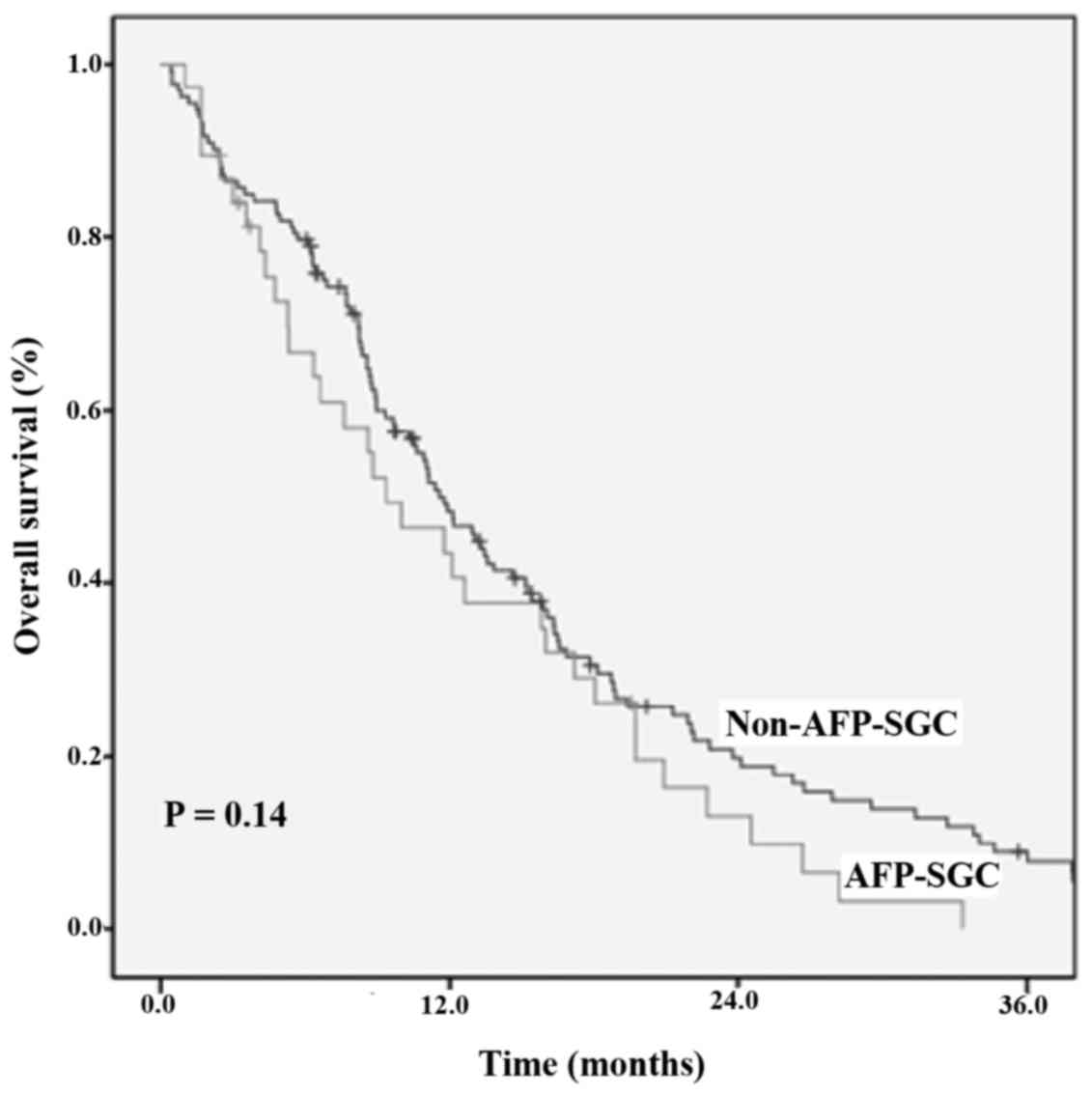
The OS analysis between the stage IV patients
revealed that the median OS for the AFP-SGC group was 9.3 months
(95% CI, 4.8–13.8), and that of non-AFP-SGC group was 11.5 months
(95% CI, 9.4–13.6) (P=0.14) (Fig.
4).
Discussion
Serum AFP levels may not often be raised in various
types of solid tumors, other than the well-known hepatocellular
carcinoma and yolk sac tumors (2,11–16). The
most common of these is stomach adenocarcinoma (13). According to the literature, there are
geographical differences associated with the incidence of AFP-SGC:
It been reported most frequently in Western countries (up to 15%),
whereas in Middle Eastern countries, the incidence is 1.3–6.3%
(7,17). In the current study, similar results
were identified as for the Western countries, with a rate of 14.6%.
The wide range of incidence rates of AFP-SGC reported in the
literuature, as for numerous other types of tumor, may be explained
by differences in ethnicity, geographic localization and
measurement techniques.
There are clinical and prognostic indicators that
have a well-known prognostic value, including LVI, PNI, and the
disease stage in gastric cancer (18–20). In
the current study, these indicators were evaluated in the two
groups, and patients with AFP-SGC were revealed to be diagnosed at
more advanced stages, and to have more LVI and PNI compared with
the non-AFP-SGC group. Similar findings were demonstrated in a
study by Chang et al (7), who
analyzed the data of 27 patients with AFP-SGC, and 478 with
non-AFP-SGC. They identified that the AFP-secreting group had
higher rates of LVI, PNI and advanced stage disease compared with
the non-AFP-secreting group (7).
Furthermore, Liu et al (21)
reported that 104 patients with AFP-SGC had higher rates of
vascular invasion and lymph node metastasis. Similar findings have
supported that the AFP-SGC type exhibits a more aggressive
behaviour, although the molecular mechanism of this aggressive
behaviour has yet to be fully elucidated. Several studies have
suggested that hepatocyte growth factor and c-Met receptor are
important for cell proliferation and migration (22–25).
Amemiya et al (26)
demonstrated more frequent c-Met overexpression for the AFP-SGC
group compared with the non-AFP-SGC group in a series of cases
(26). Based on their results, they
proposed that the aggressive behaviour of the AFP-SGC tumors may be
associated with c-Met overexpression. However, further studies are
necessary to elucidate the exact mechanism in this tumor group.
Liver metastasis is one of the main features of
AFP-SGC tumors, and the liver is the most common location for
metastasis. In various studies, the rates of liver metastasis of
the AFP-SGC patients have been reported to be between 14.3–75.6%
(27–30). In the current study, patients with
AFP-SGC had a higher rate of liver metastasis (81.6 vs. 45.9%;
P<0.001), whereas patients with non-AFP-SGC had peritoneal
metastasis more frequently (15.8 vs. 34.6%; P=0.02). The liver was
also the most common site of metastases in patients with AFP-SGC
who underwent curative surgery, whereas patients with non-AFP-SGC
had the peritoneum as the most common site of metastasis.
Similarly, Hirajima et al (31) also demonstrated that the AFP-SGC
group had a higher liver metastasis rate, and the non-AFP-SGC group
had a higher peritoneal metastasis rate (31). The data in the literature are
generally consistent in reporting that AFP secretion is accompanied
by a tendency towards liver metastasis. However, the underlying
cause of this tendency of liver metastasis has yet to be fully
elucidated.
Treatments based on 5-fluorouracil provide a
standard therapy for gastric tumors, with respect to adjuvant or
paliative strategies. However, in the available literature, data
are lacking on the features and effectiveness of treatments for
AFP-SGC. In the current study, the treatment choices were also
evaluated, and in the two patient groups who received chemotherapy
and chemoradiotherapy, the rates were similar. The first-line
treatment choice was most commonly modified DCF chemotherapy for
the two groups. No statistically significant differences were
identified between the two groups in terms of first- or second-line
chemotherapy choices. This was one of the important points that
differentiated the current study from previous studies.
Long-term survival rates are low in patients with
AFP-SGC, as they have the worse prognostic factors. Adachi et
al (32) reported that 270
patients with stage I–IV AFP-SGC had a 5-year OS rate of 22%,
whereas Chang et al (33)
reported a case series of AFP-SGC patients (stage I–IV) who had 1-
and 3-year survival rates of 37.5 and 8.3%, respectively. Wang
et al (34) determined that
45 patients with AFP-SGC had 1, 2, and 3-year survival rates that
were lower than those of the AFP-negative group. The present study
has also supported these data: The patients from all stages had 1-
and 2-year survival rates of 55 and 25% in the AFP-SGC group,
respectively; whereas the non-AFP-SGC group had rates of 73 and 47%
(P<0.001). However, the prognostic value of AFP secretion was
not significant in the multivariate analyses for all patient
groups. When the patients were categorized in terms of their stages
at diagnosis and analyzed seperately, it was revealed that AFP
secretion was a prognostic factor for both DFS and OS in the
non-metastatic patient group in the univariate analysis. In
addition, AFP-SGC was shown to be an independent prognostic factor
for DFS in the multivariate analysis. Findings reported previously
in the literatüre, and those in the current study, indicated that
AFP secretion is accompanied by a short OS. Apart from well-known
prognostic parameters for gastric tumors, molecular classification
studies are currently in progress that may lead to changes being
effected in the course of the treatment (35). Under these circumstances, AFP might
become a prognostic marker that could easily be applicable, with
low costs.
There were several limitations of the current study.
First, for patients with high levels of basal AFP, the AFP levels
were not measured following the chemotherapy cycles to evaluate the
response to chemotherapy. Secondly, the data were retrospectively
evaluated from patients’ files, and therefore the possibility of
benign etiologies could not be eliminated, such as detailed
mediation histories that may have led to the measurement of high
levels of AFP.
In conclusion, AFP-SGC has the more aggressive
clinicopathological features and biological behavior, with an
increased tendency of liver metastasis. This tendency was revealed,
with early recurrences, particularly in patients who underwent
curative surgery. In the near future, by the increasing number of
studies regarding this topic, AFP may become an important surrogate
marker to manage therapies of patients with gastric cancer.
References
|
1
|
Bergstrand CG and Czar B: Demonstration of
a new protein fraction in serum from the human fetus. Scand J Clin
Lab Invest. 8:174–179. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamanaka W, Yoneda S, Shirakusa T,
Shirahama H, Tashiro Y, Iwasaki A, Shiraishi T and Tsuru H:
Alpha-fetoprotein (AFP)-producing adrenocortical carcinoma-long
survival with various therapeutic strategies including a lung
resection: Report of a case. Surg Today. 38:275–278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saito S, Hatano T, Hayakawa M, Koyama Y,
Ohsawa A and Iwamasa T: Studies on alpha-fetoprotein produced by
renal cell carcinoma. Cancer. 63:544–549. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bourreille J, Metayer P, Sauger F, Matray
F and Fondimare A: [Existence of alpha feto protein during
gastric-origin secondary cancer of the liver]. Presse Med.
78:1277–1278. 1970.PubMed/NCBI
|
|
5
|
Chang YC, Nagasue N, Abe S, Kohno H,
Yamanoi A, Uchida M and Nakamura T: The characters of AFP-producing
early gastric cancer. Nihon Geka Gakkai Zasshi. 91:1574–1580.
1990.(In Japanese). PubMed/NCBI
|
|
6
|
Motoyama T, Aizawa K, Watanabe H, Fukase M
and Saito K: alpha-Fetoprotein producing gastric carcinomas: A
comparative study of three different subtypes. Acta Pathol Jpn.
43:654–661. 1993.PubMed/NCBI
|
|
7
|
Chang YC, Nagasue N, Abe S, Taniura H,
Kumar DD and Nakamura T: Comparison between the clinicopathologic
features of AFP-positive and AFP-negative gastric cancers. Am J
Gastroenterol. 87:321–325. 1992.PubMed/NCBI
|
|
8
|
Koide N, Nishio A, Igarashi J, Kajikawa S,
Adachi W and Amano J: Alpha-fetoprotein-producing gastric cancer:
Histochemical analysis of cell proliferation, apoptosis, and
angiogenesis. Am J Gastroenterol. 94:1658–1663. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Greene FL, Page DL and Fleming ID: AJCC
cancer staging manual. 6th. Springer; New York: 2002, View Article : Google Scholar
|
|
10
|
Laurén PA and Nevalainen TJ: Epidemiology
of intestinal and diffuse types of gastric carcinoma. A time-trend
study in Finland with comparison between studies from high- and
low-risk areas. Cancer. 71:2926–2933. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamagata T, Yamagata Y, Nakanishi M,
Matsunaga K, Minakata Y and Ichinose M: A case of primary lung
cancer producing alpha-fetoprotein. Can Respir J. 11:504–506. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsueda K, Yamamoto H, Yoshida Y and
Notohara K: Hepatoid carcinoma of the pancreas producing protein
induced by vitamin K absence or antagonist II (PIVKA-II) and
alpha-fetoprotein (AFP). J Gastroenterol. 41:1011–1019. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kinjo T, Taniguchi H, Kushima R, Sekine S,
Oda I, Saka M, Gotoda T, Kinjo F, Fujita J and Shimoda T:
Histologic and immunohistochemical analyses of
α-fetoprotein-producing cancer of the stomach. Am J Surg Pathol.
36:56–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cappetta A, Bergamo F, Mescoli C, Lonardi
S, Rugge M and Zagonel V: Hepatoid adenocarcinoma of the colon:
What should we target? Pathol Oncol Res. 18:93–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawamura N, Hatano K, Kakuta Y, Takada T,
Hara T and Yamaguchi S: [A case of hepatoid adenocarcinoma of the
urinary bladder]. Hinyokika Kiyo. 55:619–622. 2009.PubMed/NCBI
|
|
16
|
Isonishi S, Ogura A, Kiyokawa T, Suzuki M,
Kunito S, Hirama M, Tachibana T, Ochiai K and Tanaka T:
Alpha-fetoprotein (AFP)-producing ovarian tumor in an elderly
woman. Int J Clin Oncol. 14:70–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McIntire KR, Waldmann TA, Moertel CG and
Go VL: Serum alpha-fetoprotein in patients with neoplasms of the
gastrointestinal tract. Cancer Res. 35:991–996. 1975.PubMed/NCBI
|
|
18
|
Deng J, You Q, Gao Y, Yu Q, Zhao P, Zheng
Y, Fang W, Xu N and Teng L: Prognostic value of perineural invasion
in gastric cancer: A systematic review and meta-analysis. PLoS One.
9:e889072014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dicken BJ, Graham K, Hamilton SM, Andrews
S, Lai R, Listgarten J, Jhangri GS, Saunders LD, Damaraju S and
Cass C: Lymphovascular invasion is associated with poor survival in
gastric cancer: An application of gene-expression and tissue array
techniques. Ann Surg. 243:64–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Talamonti MS, Kim SP, Yao KA, Wayne JD,
Feinglass J, Bennett CL and Rao S: Surgical outcomes of patients
with gastric carcinoma: The importance of primary tumor location
and microvessel invasion. Surgery. 134:720–727; discussion 727–729.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long
Z, Zhu H and Wang Y: Clinicopathologic features and prognostic
factors in alpha-fetoprotein-producing gastric cancers: Analysis of
104 cases. J Surg Oncol. 102:249–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsumoto K and Nakamura T: Hepatocyte
growth factor (HGF) as a tissue organizer for organogenesis and
regeneration. Biochem Biophys Res Commun. 239:639–644. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Halaban R, Rubin JS, Funasaka Y, Cobb M,
Boulton T, Faletto D, Rosen E, Chan A, Yoko K, White W, et al: Met
and hepatocyte growth factor/scatter factor signal transduction in
normal melanocytes and melanoma cells. Oncogene. 7:2195–2206.
1992.PubMed/NCBI
|
|
24
|
Tajima H, Matsumoto K and Nakamura T:
Regulation of cell growth and motility by hepatocyte growth factor
and receptor expression in various cell species. Exp Cell Res.
202:423–431. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsumoto K and Nakamura T: Hepatocyte
growth factor: Molecular structure, roles in liver regeneration,
and other biological functions. Crit Rev Oncog. 3:27–54.
1992.PubMed/NCBI
|
|
26
|
Amemiya H, Kono K, Mori Y, Takahashi A,
Ichihara F, Iizuka H, Sekikawa T and Matsumoto Y: High frequency of
c-Met expression in gastric cancers producing alpha- fetoprotein.
Oncology. 59:145–151. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang JF, Shi SS, Shao YF and Zhang HZ:
Clinicopathological and prognostic features of hepatoid
adenocarcinoma of the stomach. Chin Med J (Engl). 124:1470–1476.
2011.PubMed/NCBI
|
|
28
|
Chun H and Kwon SJ: Clinicopathological
characteristics of alpha-fetoprotein-producing gastric cancer. J
Gastric Cancer. 11:23–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ucar E, Semerci E, Ustun H, Yetim T,
Huzmeli C and Gullu M: Prognostic value of preoperative CEA CA
19-9, CA 72-4, and AFP levels in gastric cancer. Adv Ther.
25:1075–1084. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Cheng Y, Sheng W, Lu H, Xu X, Xu Y,
Long Z, Zhu H and Wang Y: Analysis of clinicopathologic features
and prognostic factors in hepatoid adenocarcinoma of the stomach.
Am J Surg Pathol. 34:1465–1471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hirajima S, Komatsu S, Ichikawa D, Kubota
T, Okamoto K, Shiozaki A, Fujiwara H, Konishi H, Ikoma H and Otsuji
E: Liver metastasis is the only independent prognostic factor in
AFP-producing gastric cancer. World J Gastroenterol. 19:6055–6061.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adachi Y, Tsuchihashi J, Shiraishi N,
Yasuda K, Etoh T and Kitano S: AFP-producing gastric carcinoma:
Multivariate analysis of prognostic factors in 270 patients.
Oncology. 65:95–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang YC, Nagasue N, Kohno H, Taniura H,
Uchida M, Yamanoi A, Kimoto T and Nakamura T: Clinicopathologic
features and long-term results of alpha-fetoprotein-producing
gastric cancer. Am J Gastroenterol. 85:1480–1485. 1990.PubMed/NCBI
|
|
34
|
Wang D, Li C, Xu Y, Xing Y, Qu L, Guo Y,
Zhang Y, Sun X and Suo J: Clinicopathological characteristics and
prognosis of alpha-fetoprotein positive gastric cancer in Chinese
patients. Int J Clin Exp Pathol. 8:6345–6355. 2015.PubMed/NCBI
|
|
35
|
Chen T, Xu XY and Zhou PH: Emerging
molecular classifications and therapeutic implications for gastric
cancer. Chin J Cancer. 35:492016. View Article : Google Scholar : PubMed/NCBI
|