Introduction
The recent introduction of the anticancer drug
cetuximab [an epidermal growth factor receptor (EGFr) inhibitor:
C-mab] as a molecular-targeted therapy for treating head and neck
cancers has expanded the scope of anticancer drugs for treating
these cancers. However, no biomarkers are currently available that
can predict C-mab efficacy against oral squamous cell carcinoma
(OSCC); therefore, it is typically administered irrespective of
patient sensitivity (1). Hence, we
investigated C-mab use with the collagen gel droplet-embedded
culture drug sensitivity test (CD-DST). Kobayashi et al
(2,3)
developed CD-DST which combines the collagen gel droplet culture
method, a simple method of three-dimensional (3D) culture that
allows a very small number of clinical samples to be tested with a
serum-free medium step and quantitative evaluation by image
analysis. CD-DST has little effect on non-cancerous cells, allowing
accurate measurements of cancerous cells only. This method has been
primarily used on tumors of the digestive system (4–6).
Compared to such cancers of the primary organs, OSCC and other oral
cancers generally have a smaller tumor volume. CD-DST is,
therefore, likely to be a suitable method for testing the
sensitivity of anticancer drugs on OSCC. However, there is little
application of the CD-DST method to OSCC, with no evaluation of
molecularly targeted drugs. In the present study, the CD-DST method
was performed using a patient biopsy specimen of hard palate cancer
to discern chemotherapy combined with a molecularly targeted drug
at retrospective.
Case report
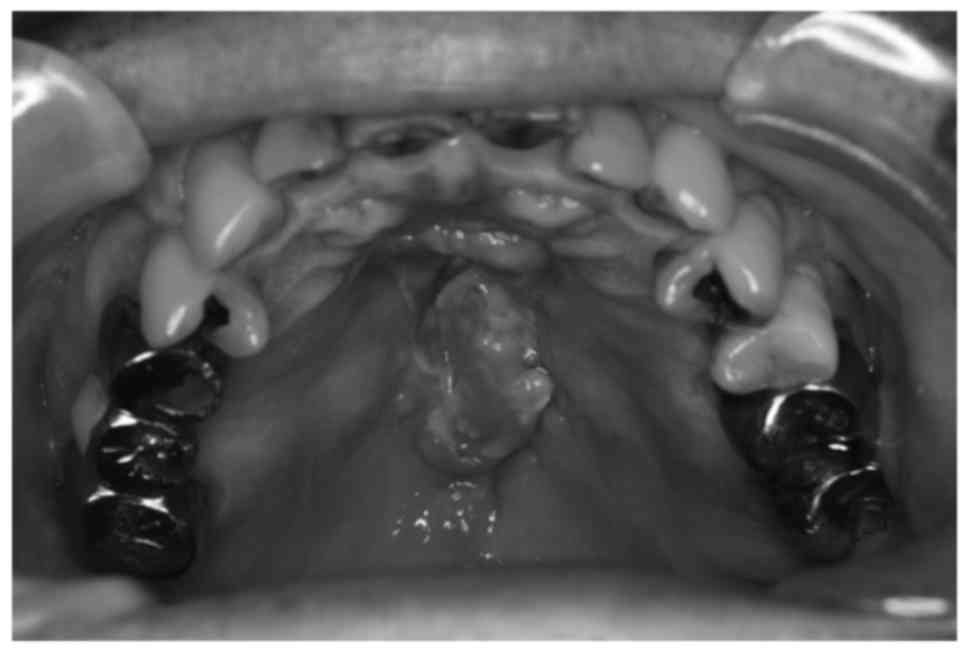
A 55-year-old man with hard palatal pain was
referred to our institution in July 2012. An ulcerative mass
measuring 35×17×8 mm3 with induration at the border of
the hard palate was observed (Fig.
1). The patient also had bilateral neck metastasis at level
IIa. The hard palatal mass was diagnosed as well-differentiated
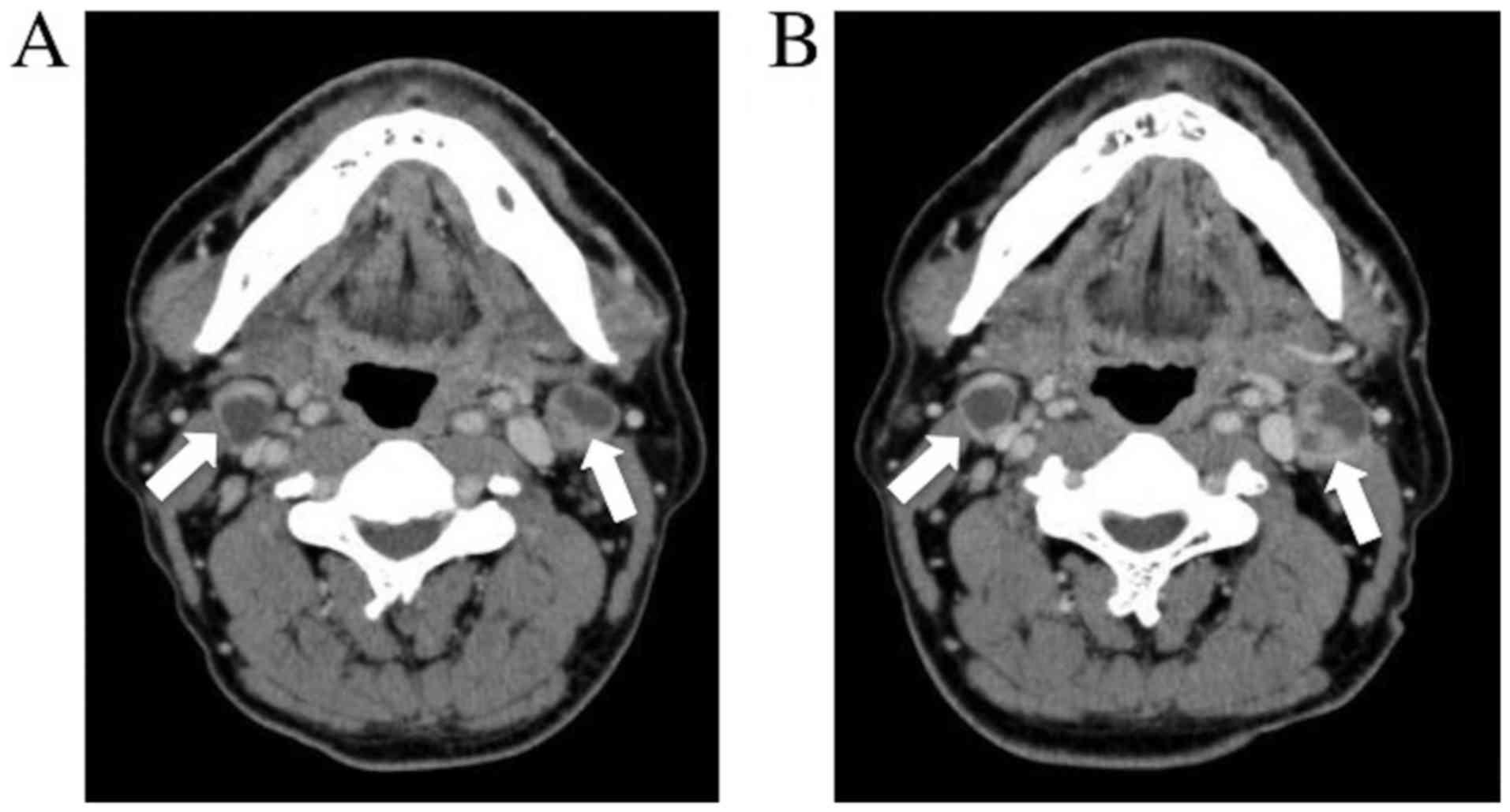
squamous cell carcinoma. Contrast-enhanced computed tomography
(e-CT) showed a rim-enhanced mass at level IIa of the bilateral
cervical area. In addition, it was close to the internal carotid
artery (Fig. 2A). Positron emission
tomography-computed tomography demonstrated high
18-fluorodeoxyglucose uptake at the hard palatal and cervical lymph
nodes. The distant metastasis workup was negative. Diagnosis was
hard palatal cancer, cervical lymph node metastasis (T2N2cM0: Stage
IVA). CD-DST results revealed that the tumor was sensitive to
various chemotherapeutic agents. The patients underwent one course
of induction chemotherapy [docetaxel (DOC): 80 mg/body; cisplatin
(CDDP): 80 mg/body; 5-fluorouracil (5-FU): 4,000 mg/body] following
the diagnosis of hard palate cancer. The treatment effect of
chemotherapy was confirmed with the Response Evaluation Criteria in
Solid Tumors (RECIST) guideline version 1.1 (7). The size of the lymph node metastasis
did not change as per e-CT after neoadjuvant chemotherapy. The
therapy effect determination was stable disease (SD) (Fig. 2B). We performed maxillary malignant
tumor resection and bilateral radical neck dissection under general
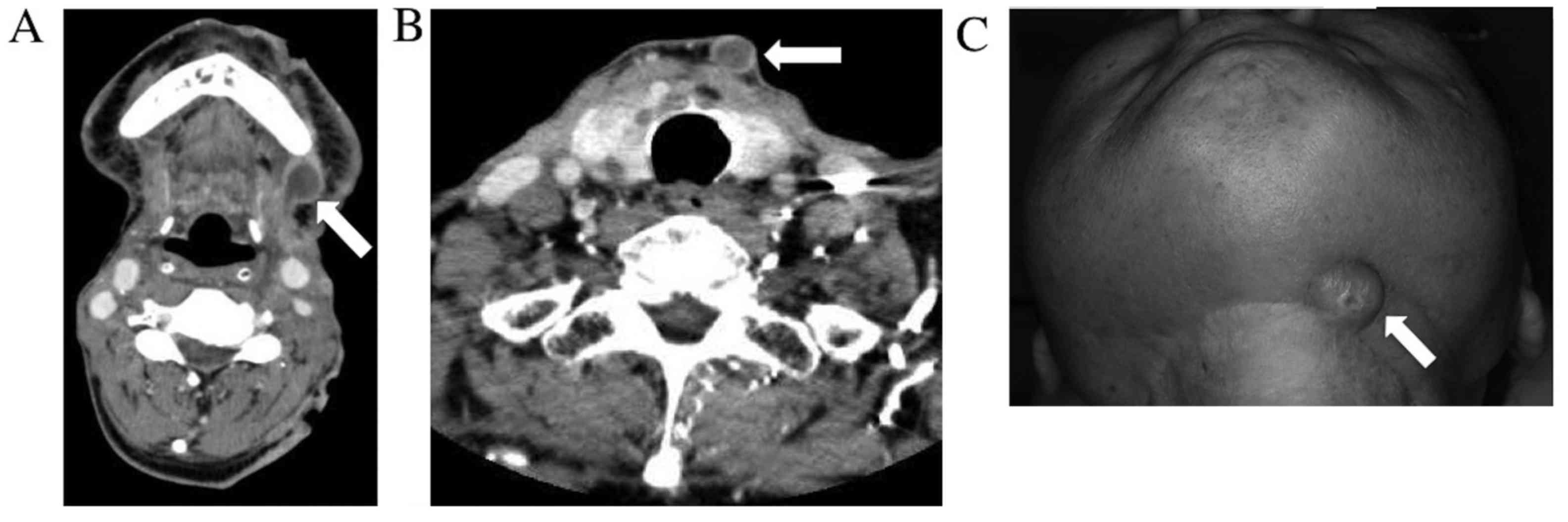
anesthesia. However, left cervical lymph node metastasis recurrence
and neck skin metastasis were observed eight months after surgery
(Fig. 3). In accordance with the
report of Vermorken et al (1), C-mab + CDDP + 5-FU (C-mab: 1450
mg/body; CDDP: 150 mg/body; 5-FU: 5,000 mg/body) was started and a
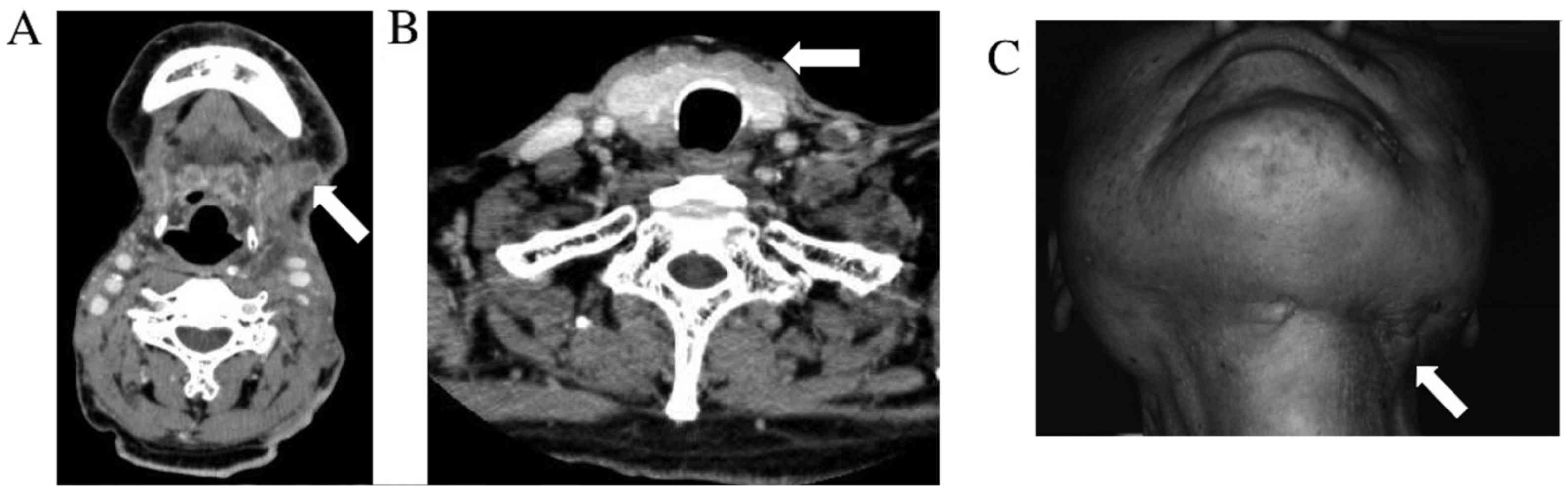
total of six courses were performed. At the end of the six courses,
we confirmed by CT that cervical lymph node recurrence and skin
metastasis showed marked reduction of tumors, and therapy effect
determination was partial response (PR) (Fig. 4). Thereafter, once weekly
administration of C-mab alone was continued three times; however,
there was a rapid increase in cervical skin metastatic tumors. The
therapy effect determination became progressive disease (PD), and
the treatment was canceled. As the symptoms worsened, it shifted to
best support care focused on pain management. He died 330 days
after cervical skin metastasis was confirmed.
The ethics committee of Nippon Dental University,
School of Life Dentistry at Niigata (approval no. ECNG-H-119)
approved study. CDDP, 5-FU, DOC, and C-mab, which are
chemotherapeutic drugs frequently used for oral cancer, were
assessed for in vitro chemosensitivity via the CD-DST
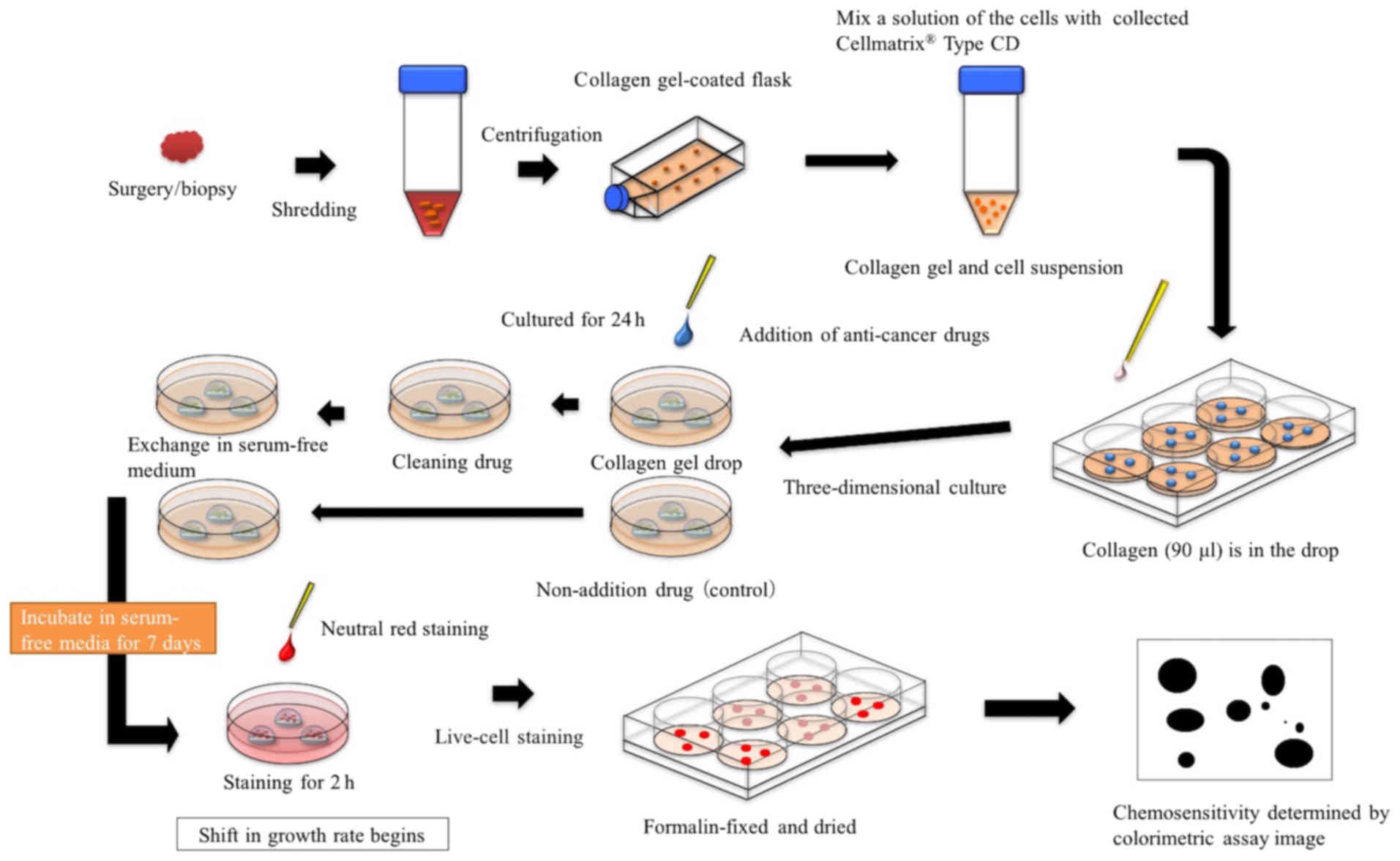
method. CD-DST was performed according to the method described by
Kobayashi et al (2,3), using the Primaster® human cancer cell
primary culture kit (Kurabo Industries Ltd., Osaka, Japan)
(Fig. 5). Contact concentration and
time of anticancer agents were as follows: 0.5 µg/ml CDDP (8), 0.7 µg/ml 5-FU (8), 0.1 µg/ml DOC (9) contacted for 24 h. The contact
concentration of C-mab was set at 250 µg/ml for 144 h, which is the
maximum blood concentration during clinical administration. In
addition, the test was also performed with a dose of 500 µg/ml.
Drug efficacy was determined via image analysis (Solution Systems
Inc., Chiba, Japan). The growth rates of control incubations were
calculated as the total volume on day 7/total volume on day 0. The
in vitro sensitivity was expressed as the percentage T/C,
where T was the total volume of the treated group and C was the
total volume of the control group. When T/C was less than or equal
to 50%, the in vitro drug sensitivity was regarded as
effective. In contrast, when T/C was greater than 50%, sensitivity
was considered as not effective (2,3).
Clinical responses were assessed according to RESIST, where tumors
demonstrating a complete response (CR) or a PR were considered
clinically responsive.
Result
Results of CD-DST and clinical response are shown in
Table I. In CDDP single agent, T/C
was 97.6%, which was a low sensitivity. In DOC single agent, T/C
was 76.9%, which was a low sensitivity. In 5-FU single agent, T/C
was 83.2%, which was a low sensitivity. In C-mab 250 µg/ml single
agent and C-mab 500 µg/ml single agent, T/C was 65.5 and 60.1%,
which was a low sensitivity. In multiple drug combination PF, T/C
was 93.2%, which was a low sensitivity. In C-mab + PF, T/C was
32.4%, which was a high sensitivity. In multiple drug combination
TPF, T/C was 71.6%, which was a low sensitivity. The clinical
response of neoadjuvant chemotherapy (TPF) was SD. The clinical
response of C-mab + PF was PR. In addition, the clinical response
of C-mab single agent was PD.
 | Table I.Results of drug sensitivity assessed
by CD-DST and clinical response were compared. |
Table I.
Results of drug sensitivity assessed
by CD-DST and clinical response were compared.
| Drug | T/C | Clinical
response |
|---|
| CDDP | 97.6 | – |
| DOC | 76.9 | – |
| 5-FU | 83.2 | – |
| C-mab 250 µg/ml | 65.5 | PD |
| C-mab 500 µg/ml | 60.1 |
|
| CDDP+5-FU (PF) | 93.2 | – |
|
C-mab+CDDP+5-FU(C-mab+PF) | 32.4 | PR |
| CDDP+5-FU+DOC
(TPF) | 71.6 | SD |
Discussion
C-mab, which specifically binds to EGFr, is a
promising novel chemotherapy drug for treating OSCC. However, there
are no established predictors of OSCC therapeutic responses, and
this inability to predict tumor response has resulted in drugs
being administered to patients irrespective of tumor sensitivity.
C-mab reportedly causes infusion reactions and other serious
adverse events (1,10). Therefore, identifying a predictor of
OSCC patient therapeutic response is essential to eliminating
ineffective drug administration and associated patient risks. To
further increase the effective rate of chemotherapy, an anticancer
drug sensitivity test that accurately reflects the clinical
prognosis is necessary.
Presently, various chemotherapy sensitivity tests
have been developed and performed, such as HTCA, SDI, and HDRA
(11–13). HDRA is an anticancer drug sensitivity
test using 3D cell culture, but it entails problems such as the
requirement for a large number of cells and the influence of
contaminating fibroblasts. In contrast, CD-DST is capable of
analyzing a small number of cells (1×105) in 3D cell
cultures that create an environment close to that of the body and
is unaffected by contaminating fibroblasts, thus achieving high
positive and negative predictive values (2,3).
Measurement success rates of ≥80% have been obtained
for cancers, including colorectal cancer (14), lung cancer (15), and breast cancer (9), and a high clinical efficacy prediction
rate of 91% has been obtained via CD-DST (3). This study evaluated its application to
chemosensitivity testing for OSCC. In this study, it was possible
to judge susceptibility to anticancer drugs without concerns such
as the lack of cell numbers and bacterial contamination. OSCC and
other oral cancers generally have a smaller tumor volume. Thus,
CD-DST is likely to be a suitable method for testing the
sensitivity of anticancer drugs on OSCC.
We performed CD-DST method on metastatic lymph node
resected after neoadjuvant chemotherapy. In this study, the
combination of TPF showed low sensitivity (T/C %: 71.6). The effect
of preoperative chemotherapy (TPF) was determined as SD as lymph
node metastasis did not change. In a retrospective examination, the
CD-DST method and clinical response were consistent, suggesting
that evaluation of multiple drug combination chemotherapy is
possible in OSCC.
Furthermore, PF and C-mab each had low sensitivity
with a single agent; however, when tested in combination, T/C was
32.4%, which was a high sensitivity value. The therapy effect
determination was PR at the end of six cycles, consistent with the
CD-DST method result. The CD-DST method could reproduce the
synergistic effect of C-mab on PF therapy for OSCC. In this
respect, synergistic antitumor activity has been reported in
combination experiments with chemotherapeutic drugs or
molecular-targeted therapeutic drugs. Combination treatment with
cisplatin and gefitinib has confirmed in vitro sufficient
additive inhibitory action on the survival of cancer cells (head
and neck cancer) (16,17). However, the putative mechanism
through which C-mab combination therapy enhances antitumor response
remains to be demonstrated. Therefore, if the effect of PF + C-mab
can be evaluated by the CD-DST method, antitumor effect can be
expected to be comprehensively judged without considering factors
such as EGFr expression level and resistance gene. In addition, in
this study CD-DST method, C-mab alone has a low sensitivity, but
based on the EXTREME trial (1), we
continued administering C-mab alone once a week after six cycles of
C-mab + PF. Cervical skin metastasis increased rapidly in about one
month after changing to C-mab single administration. The clinical
response was PD, and the susceptibility test of C-mab single agent
was consistent with low sensitivity (C-mab 250 µg/ml: 65.5%, 500
µg/ml: 60.1%). In this respect, our case report demonstrated that
it was possible to evaluate even multi-drug-combined chemotherapy
regimens, including C-mab. However, Anti-tumor effects of C-mab
include antibody-dependent cell-mediated cytotoxicity (ADCC)
activity and the like in addition to the signal inhibitory effect
on EGFr (18), further study is
necessary. The accumulation and analyses of additional cases are
required to conduct a precise evaluation of CD-DST for patients
with OSCC.
References
|
1
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kobayashi H, Tanisaka K, Kondo N, Mito Y,
Koezuka M, Yokouchi H, Higashiyama M, Kodama K, Doi O, Yamada M, et
al: Development of new in vitro chemosensitivity test using
collagen gel droplet embedded culture and its clinical usefulness.
Gan To Kagaku Ryoho. 22:1933–1939. 1995.(In Japanese). PubMed/NCBI
|
|
3
|
Kobayashi H, Tanisaka K, Doi O, Kodama K,
Higashiyama M, Nakagawa H, Miyake M, Taki T, Hara S, et al: An in
vitro chemosensitivity test for solid human tumors using collagen
gel droplet embedded cultures. Int J Oncol. 11:449–455.
1997.PubMed/NCBI
|
|
4
|
Naitoh H, Yamamoto H, Murata S, Kobayashi
H, Inoue K and Tani T: Stratified phase II trial to establish the
usefulness of the collagen gel droplet embedded culture-drug
sensitivity test (CD-DST) for advanced gastric cancer. Gastric
Cancer. 17:630–637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ochiai T, Nishimura K, Watanabe T,
Kitajima M, Nakatani A, Inou T, Washio M, Sakuyama N, Sato T,
Kishine K, et al: Individualized chemotherapy for colorectal cancer
based on the collagen gel droplet-embedded drug sensitivity test.
Oncol Lett. 4:621–624. 2012.PubMed/NCBI
|
|
6
|
Mekata E, Sonoda H, Shimizu T, Tatsuta T,
Yamaguchi T, Endo Y and Tani T: Clinical predictive value of in
vitro anticancer drug sensitivity test for the therapeutic effect
of adjuvant chemotherapy in patients with stage II–III colorectal
cancer. Mol Clin Oncol. 1:763–767. 2013.PubMed/NCBI
|
|
7
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakuma K, Tanaka A and Mataga I: Collagen
gel droplet-embedded culture drug sensitivity testing in squamous
cell carcinoma cell lines derived from human oral cancers: Optimal
contact concentrations of cisplatin and fluorouracil. Oncol Lett.
12:4643–4650. 2016.PubMed/NCBI
|
|
9
|
Takamura Y, Kobayashi H, Taguchi T,
Motomura K, Inaji H and Noguchi S: Prediction of chemotherapeutic
response by collagen gel droplet embedded culture-drug sensitivity
test in human breast cancers. Int J Cancer. 98:450–455. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshino T, Hasegawa Y, Takahashi S, Monden
N, Homma A, Okami K, Onozawa Y, Fujii M, Taguchi T, de Blas B, et
al: Platinum-based chemotherapy plus cetuximab for the first-line
treatment of Japanese patients with recurrent and/or metastatic
squamous cell carcinoma of the head and neck: Results of a phase ii
trial. Jpn J Clin. 43:524–531. 2013. View Article : Google Scholar
|
|
11
|
Salmon SE, Mamburger AW, Soehnlen B, Durie
BG, Alberts DS and Moon TE: Quantitation of differential
sensitivity of human tumor stem cells to anticancer drugs. N Engl J
Med. 298:1321–1327. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanigawa N, Kern DH, Hisaka Y and Morton
DL: Rapid assay for evaluating the chemosensitivity of human tumors
in soft agar culture. Cancer Res. 42:2159–2164. 1982.PubMed/NCBI
|
|
13
|
Kern DH, Dogemullar CR, Kennedy MC,
Hildebrand-Zanki SU, Tanigawa N and Sondak VK: Development of
miniaturized, improved nucleic acid precursor incorporation assay
for chemosensitivity testing of human solid tumors. Cancer Res.
45:5436–5441. 1985.PubMed/NCBI
|
|
14
|
Araki Y, lsomoto H, Matsumoto A, Kaibara
A, Yasunaga M, Hayashi K, Yatsugi H and Yamauchi K: An in vitro
chemosensitivity test for colorectal cancer using collagen-gel
droplet embedded cultures. Kurume Med J. 46:163–166. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawamura M, Inoue Y, Oyama T and Kobayashi
K: Chemosensitivity test for unresectable non-small cell lung
cancer. Nihon Geka Gakkai Zasshi. 103:229–232. 2002.(In Japanese).
PubMed/NCBI
|
|
16
|
Prewett M, Rockwell P, Rose C and
Goldstein N: Anti-tumor and cell cycle responses in KB cells
treated with a chimeric anti-EGFR monoclonal antibody in
combination with cisplatin. Int J Oncol. 9:217–224. 1996.PubMed/NCBI
|
|
17
|
Huang S, Armstrong EA, Benavente S,
Chinnaiyan P and Harari PM: Dual-agent molecular targeting of the
epidermal growth factor receptor (EGFR): Combining anti-EGFR
antibody with tyrosine kinase inhibitor. Cancer Res. 64:5355–5362.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kimura H, Sakai K, Arao T, Shimoyama T,
Tamura T and Nishio K: Antibody- dependent cellular cytotoxicity of
cetuximab against tumor cells with wild-type or mutant epidermal
growth factor receptor. Cancer Sci. 98:1275–1280. 2007. View Article : Google Scholar : PubMed/NCBI
|