Introduction
Laryngeal squamous cell carcinoma (LSCC) is the
second most common malignant neoplasm of head and neck squamous
cell carcinoma. It is an aggressive malignancy associated with high
rates of metastasis, recurrence and a low 5-year survival rate. It
has a high incidence and primarily involves therapeutic failure
especially for the advanced cases (1,2).
Therefore, identifying novel potential prognostic markers may lead
to an improved clinical management of patients with laryngeal
cancer.
Krüppel-like factors (KLFs) are a family of DNA
binding transcriptional regulators expressed in a wide variety of
human tissues. These factors have diverse and essential functions
in multiple cell processes, including proliferation, inflammation,
differentiation, migration, pluripotency, maintenance of
homeostasis, and apoptosis (3,4). KLF4 is
a bifunctional transcription factor able to either activate or
repress transcription using different mechanisms, depending on the
target gene. Thus, depending on the cell type or cell context or
cancer stage, KLF4 may act either as a tumor suppressor gene or as
an oncogene. KLF4 is involved in cell cycle, where it can induce
cycle arrest in some molecular contexts, while favoring
proliferation in others (5). In
colorectal and gastric cancers, KLF4 expression decreases at early
stages due to different mechanisms including loss of
heterozygosity, hypermethylation of the promoter and point
mutations in the KLF4 gene, and may be lost with tumor
growth and progression (6–8). Moreover, it was found that KLF4 is
downregulated at the mRNA and protein levels in several non-small
cell lung carcinoma cell lines, partially due to promoter
hypermethylation. The restoration of KLF4 expression inhibits the
clone formation and induces a delay in in vivo tumor growth
(9).
KLF4 is overexpressed in 70% of primary mammary
cancers at the stage of ductal carcinoma, where it plays an
oncogenic role. In addition, nuclear localization of KLF4 in ductal
carcinoma predicts an unfavorable outcome (10,11).
Previous findings showed the beneficial side of knocking down KLF4,
as p53-dependent cell death is restored (12). In head and neck squamous cell
carcinoma (HNSCC) tissues, persistent KLF4 expression predicts poor
prognosis and confers aggressiveness (13). Recent studies showed that KLF4 is
upregulated in small cell lung carcinoma tissues and has a
potential tumor-promoting role in this type of lung malignancy
(14).
Heat shock proteins (HSPs) are highly conserved
molecular chaperones with principal roles in protein homeostasis,
transport processes and signal transduction. Recently, heat shock
proteins, found to be overexpressed in a wide range of
malignancies, have been considered as promising candidate
biomarkers for some cancers (15–17).
Heat shock protein 27 (HSP27) is a molecular chaperone highly
expressed in aggressive cancers, where it is involved in numerous
pro-tumorigenic signaling pathways (18,19).
Overexpression of HSP27 was observed across different types of
cancer including breast, ovarian, prostate, bladder, gastric, and
oral squamous cell carcinoma and many others (18,20). Its
overexpression contributes to cancer progression via different
mechanisms, and its anti-apoptotic and pro-survival activities play
crucial roles in tumorigenesis. HSP27 increases proliferation by
facilitating cell cycle progression and enhances migration and
invasion via several mechanisms (21,22).
Additionally, high levels of HSP27 have been associated with poor
prognosis and chemo- and radio- resistance in various cancers
including prostate, breast, head and neck and lung cancer (21,23–27).
HSP27 is now considered an attractive therapeutic target for cancer
treatment. In vitro and in vivo studies have shown
that the downregulation of HSP27 protein expression using antisense
oligonucleotides or siRNA contributes in the reduction of tumor
progression, induction of apoptosis and tumor sensitization to
treatment (27–29). The strategy of HSP27 gene inhibition,
using Apatorsen (OGX-427) a 2′-methoxyethyl-modified antisense
oligonucleotide, has shown a promising therapeutic effect in
clinical application. A phase I dose-escalation study showed a good
tolerance of Apatorsen, associated with a decrease in tumor markers
and in circulating tumor cells and a stable measurable disease in
patients with castration-resistant prostate, breast, ovary, lung,
and bladder cancer (30).
Only a few studies have investigated the potential
role and the profile of mRNA and protein expression of HSP27 in
laryngeal squamous cell carcinoma tissues (31,32).
Nevertheless, no studies have assessed the role and the expression
of KLF4 in laryngeal cancer tissues. In this study, we examined the
KLF4 and HSP27 mRNA and protein levels, by RT-PCR and
immunohistochemical analyses, respectively, in laryngeal tumors
(n=44) and normal tissues (n=21). We also evaluated the
combinational clinical significance of KLF4 and HSP27 expression
for the diagnosis or prognosis and treatment decision-making in
laryngeal cancers.
Materials and methods
Sample collection
Forty-four formalin-fixed paraffin-embedded larynx
carcinoma samples and 21 normal tissue samples were collected from
the Department of Pathological Anatomy of the Notre Dame de Secours
University Hospital (Byblos, Lebanon) and the National Institute of
Pathology (Baabda, Lebanon). Following surgical removal, all the
tissue samples were fixed in formalin and embedded in paraffin
prior to sectioning for histological, immunohistochemical and gene
expression analyses. The cancer tissue samples were graded
independently by a pathologist and histologically classified.
Epidemiological and clinical data were collected from patient
records and registries (Table I).
This study was approved by the Institutional Review Board of the
Notre Dame de Secours University Hospital.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
Characteristics | No. (%) |
|---|
| Total Subjects | 65 |
| Normal
tissues | 21 (32.3) |
| Tumor
tissues | 44 (67.7) |
| Sex |
|
Male | 83.1 |
|
Female | 16.9 |
| Age median (range),
years | 65 (47–88) |
| Stage |
| I | 9 (20.5) |
| II | 11 (25) |
|
III | 6 (13.6) |
| IV | 18 (40.9) |
Immunohistochemistry and
immunoscoring
Sections of paraffin-embedded tissue specimens (4
µm) were subjected to immunostaining using the Ventana automated
stainer (BenchMark XT; Roche Diagnostics GmbH, Mannheim, Germany)
at the National Institute of Pathology (Baabda, Lebanon). The
tissue sections were deparaffinized using xylene, rehydrated
through graded ethanols and equilibrated in phosphate-buffered
saline before undergoing antigen retrieval. The endogenous
peroxidase activity was blocked with 0.3% hydrogen peroxide for 5
min. The tissue sections were subsequently incubated with the
primary mouse monoclonal antibodies at a dilution of 1:200 for 1 h
at room temperature: anti-KLF4 antibody (SAB5300069; clone 1E6),
and anti-HSP27 antibody (SAB3701437; clone G3.1) (both from
Sigma-Aldrich, St. Louis, MO, USA). The appropriate secondary
antibody was horseradish peroxidase (HRP)-conjugated rabbit
anti-mouse IgG (A9044; Sigma-Aldrich) at a dilution of 1:200 for 1
h at room temperature. The HRP detection was achieved with
3,3′-diaminobenzidine substrate (Sigma-Aldrich) and counterstained
with hematoxylin.
Two investigators (G.A. and E.H.) independently
scored the slides in a blinded manner. A quantitative score was
performed by adding the score of the staining area and the score of
staining intensity for each case to assess the expression levels of
KLF4 and HSP27. The quantitative score was estimated by calculating
the percentage of immunopositive cells as follows: 0, no staining
of cells in any microscopic fields; 1+, <30% of tissue stained
positive; 2+, between 30 and 60% stained positive; and 3+, >60%
stained positive. The intensity of staining was scored by
evaluating the average staining intensity of the positive cells: 0,
no staining; 1+, mild staining; 2+, moderate staining; and 3+,
intense staining.
RNA extraction and reverse
transcriptase-quantitative PCR analysis
Total RNA extraction from formalin-fixed
paraffin-embedded tissue sections (20 µm) was performed using
GenElute™ FFPE RNA Purification kit (RNB400; Sigma-Aldrich)
according to the manufacturer's instructions. RNA (2 µg) was
reverse transcribed using iScript™ cDNA Synthesis kit (Bio-Rad,
Hercules, CA, USA). Reverse transcriptase-quantitative PCR
(RT-qPCR) was performed in a CFX96™ Real-Time System using iQ™
SYBR® Green Supermix (Bio-Rad). The primer sequences
used in RT-PCR were: HSP27 reverse, 5′-TCGAAGGTGACTGGGATGGT-3′ and
forward, 5′-CCCCCATGCCCAAGCTA-3′; KLF4 reverse,
5′-ATGTGTAAGGCGAGGTGGTC-3′ and forward, 5′-ACCCACACAGGTGAGAAACC-3′;
GAPDH reverse, 5′-TGGTGGTCCAGGGGTCTTAC-3′ and forward,
5′-TTGCCCTCAACGACCAGTTT-3′ (Sigma-Aldrich). The GAPDH gene
was used as an internal control for the relative mRNA amount. All
the experiments were performed in triplicate and normalized to
GAPDH mRNA expression. The relative RNA level was automatically
calculated with the ∆∆Cq method.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5. The χ2 test, paired t-test, and Fisher test
were used to compare the protein and mRNA expression level of KLF4
and HSP27 between tumors and normal tissues and between the
different tumor stages. P<0.05 was considered to indicate
statistically significant differences (P<0.05, P<0.01,
P<0.001).
Results
Patient characteristics
Table I shows the
characteristics of the patients. The median age of patients was 65
years and 83.1% of the patients were male. According to the TNM
staging system, 20.5% were stage I (n=9), 25% were stage II (n=11),
13.6% were stage III (n=6), and 40.9% were stage IV (n=18).
Expression levels of KLF4
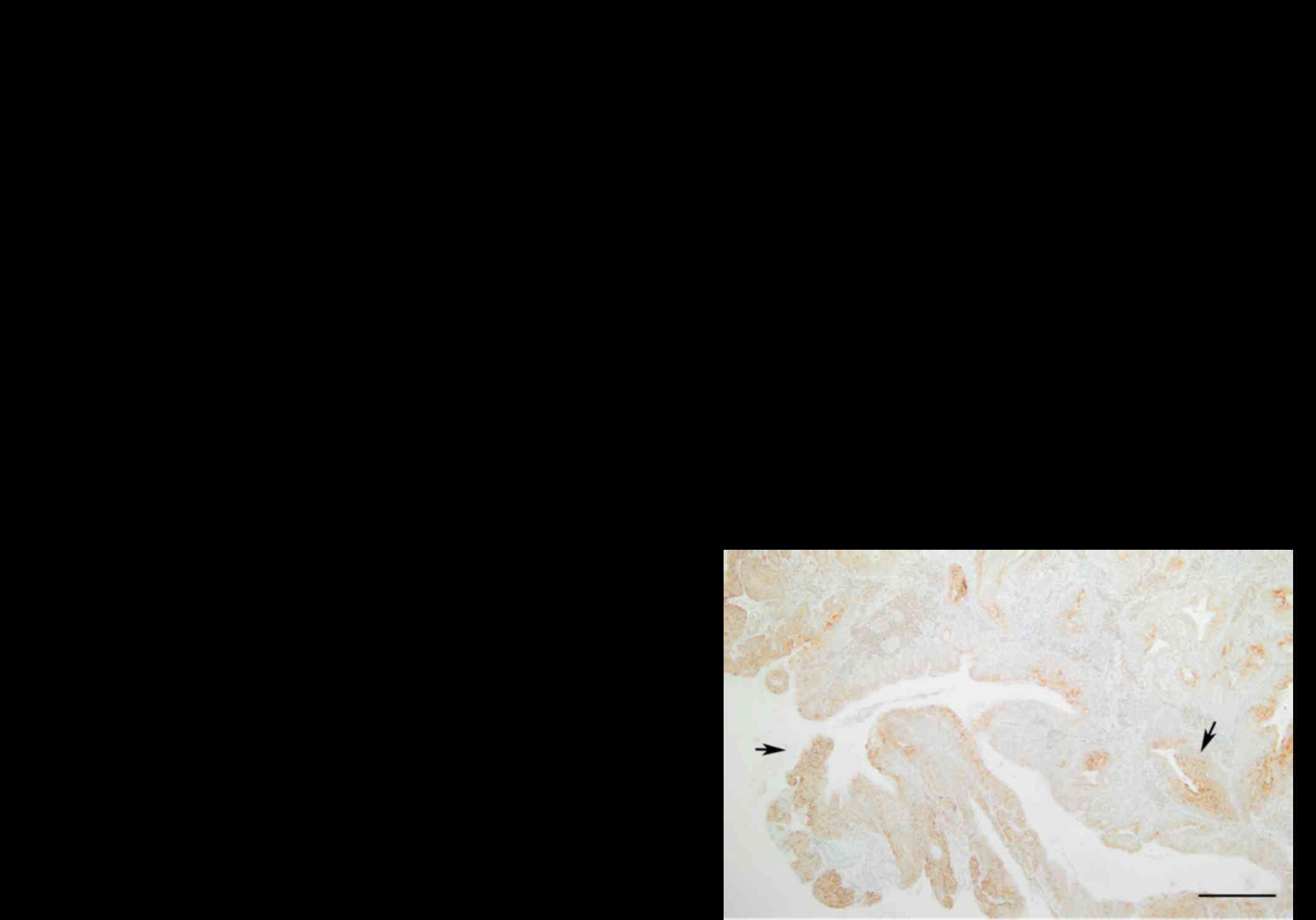
The immunohistochemical analysis showed that KLF4
was expressed in the nucleus of tumor and normal cells.
Representative findings of the immunohistochemical staining are
shown in Fig. 1A and B. A
significant difference in KLF4 protein expression was observed
between normal and cancer tissues (P<0.001) (Table II). KLF4 expression was
significantly lower in tumor tissues compared to normal tissues.
The profile of KLF4 protein expression in each tumor stage is shown
in Table III. No significant
difference of expression was observed between stages. The protein
expression of KLF4 in stage I is similar to that in normal tissues.
It decreases in stages II and III, and was mostly downregulated in
stage IV tumors (Table III). To
determine whether this decrease of the protein expression occurred
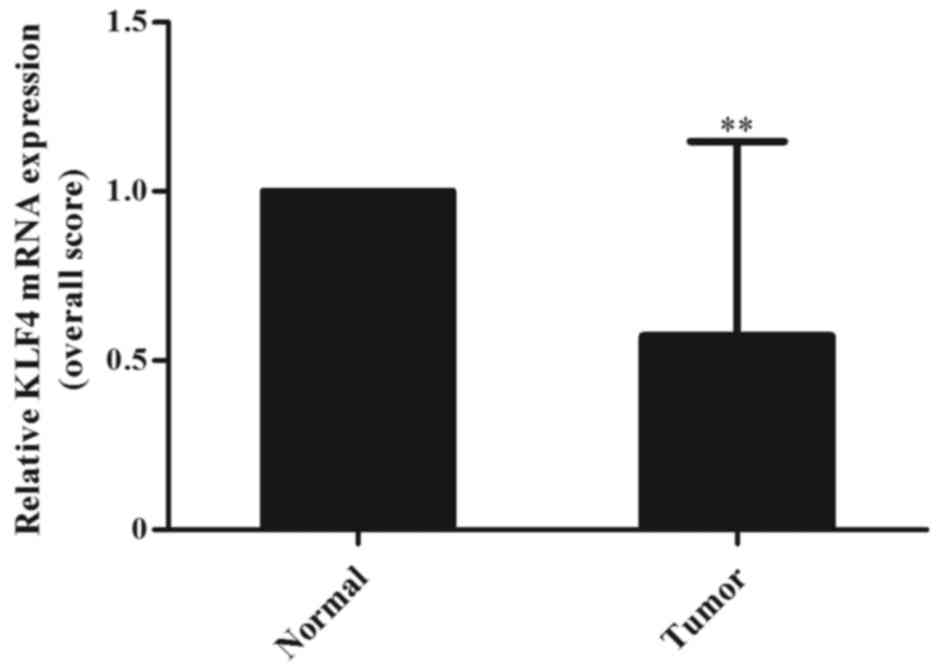
at the transcriptional level, the KLF4 mRNA levels were evaluated
in normal and cancer tissues by quantitative real-time PCR
analysis. A significant lower KLF4 mRNA copy numbers were found in
tumor tissues compared to normal tissues (P=0.0058), in the same
manner as for the protein expression (Fig. 2). These results showed that KLF4
expression was downregulated in laryngeal tumors not only at the
protein level but also at the RNA level.
 | Table II.Results of KLF4 immunostaining in
normal and tumor tissues (cases per intensity of expression). |
Table II.
Results of KLF4 immunostaining in
normal and tumor tissues (cases per intensity of expression).
| Protein
expression | Normal tissue
(%) | Tumor tissue
(%) | P-value (normal vs.
tumor) |
|---|
| 0 | 3 (14.3) | 27 (61.4) | <0.001 |
| 1+ | 14 (66.7) | 7 (15.9) | <0.001 |
| 2+ | 4 (19) | 7 (15.9) | >0.05 |
| 3+ | 0 (0) | 3 (6.8) | >0.05 |
 | Table III.Profile of KLF4 protein expression in
the tumor stages (cases per intensity of expression). |
Table III.
Profile of KLF4 protein expression in
the tumor stages (cases per intensity of expression).
|
| KLF4 protein
expression, n (%) |
|
|---|
|
|
|
|
|---|
| Stage (n) | 0 (no
staining) | 1+ (mild
staining) | 2+ (moderate
staining) | 3+ (intense
staining) |
|---|
| I (9) | 4 (44.5) | 2 (22.2) | 1 (11.1) | 2 (22.2) |
| II (11) | 5 (45.4) | 3 (27.3) | 3 (27.3) | 0 |
| III (6) | 3 (50.0) | 1 (16.7) | 2 (33.3) | 0 |
| IV (18) | 15 (83.2) | 1 (5.6) | 1 (5.6) | 1 (5.6) |
Expression levels of HSP27
The immunohistochemical staining showed that HSP27
is mainly expressed in the cytoplasm at a significant difference of
intensities between normal and tumor sections. Examples of the
immunohistochemical staining for HSP27 are shown in the Fig. 1C and D. As shown in Table IV, HSP27 was significantly
overexpressed in tumor tissues compared to normal tissues
(P<0.001). A significant gradual increase in the HSP27 protein
expression was observed from stage I to stage IV (Table V) (P=0.0039). These results were
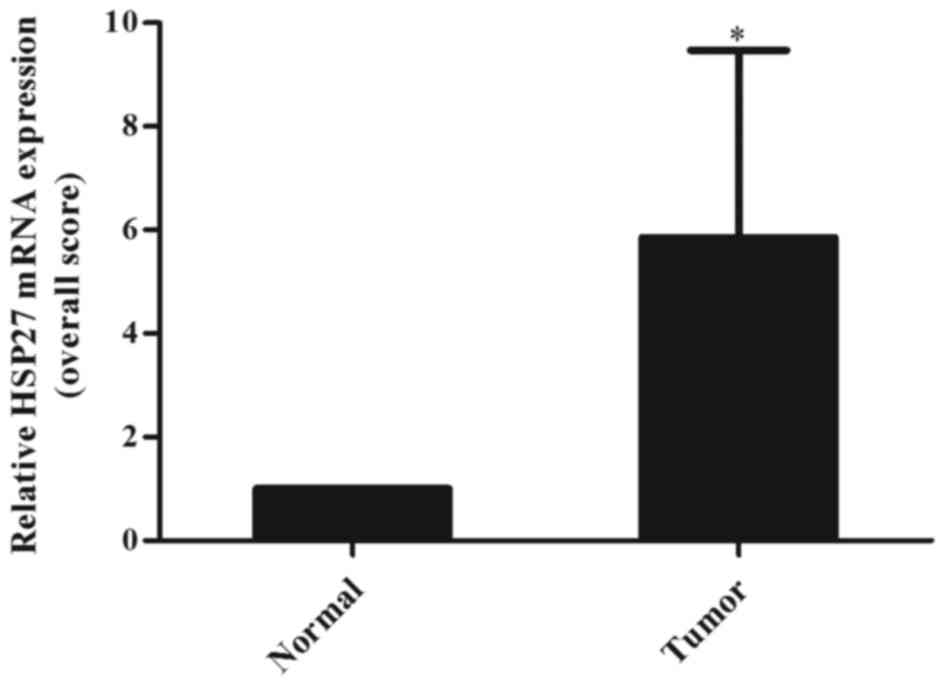
confirmed by quantitative real-time PCR analysis showing that HSP27
was also up-regulated at RNA level in larynx cancers (Fig. 3). The HSP27 copy numbers were
significantly higher in cancer tissue sections compared to normal
tissue sections (P=0.0115).
 | Table IV.Results of HSP27 immunostaining in
normal and tumor tissues (cases per intensity of expression). |
Table IV.
Results of HSP27 immunostaining in
normal and tumor tissues (cases per intensity of expression).
| Protein
expression | Normal tissue
(%) | Tumor tissue
(%) | P-value (normal vs.
tumor) |
|---|
| 0 | 5 (23.8) | 0 (0) | <0.001 |
| 1+ | 15 (71.4) | 1 (2.3) | >0.05 |
| 2+ | 1 (4.8) | 19 (43.2) | <0.001 |
| 3+ | 0 (0) | 24 (54.5) | <0.001 |
 | Table V.Profile of HSP27 protein expression
in the tumor stages (cases per intensity of expression). |
Table V.
Profile of HSP27 protein expression
in the tumor stages (cases per intensity of expression).
|
| Hsp27 protein
expression, n (%) |
|---|
|
|
|
|---|
| Stage (n) | 0 (no
staining) | 1+ (mild
staining) | 2+ (moderate
staining) | 3+ (intense
staining) |
|---|
| I (9) | 0 | 1 (11.1) | 6 (66.7) | 2 (22.2) |
| II (11) | 0 | 0 | 5 (45.5) | 6 (54.5) |
| III (6) | 0 | 0 | 4 (66.7) | 2 (33.3) |
| IV (18) | 0 | 0 | 4 (22.2) | 14 (77.8) |
Factors associated with KLF4 and HSP27
expression levels
In order to determine any correlation of KLF4 and
HSP27 expression levels with age and gender, a statistical analysis
was performed. KLF4 and HSP27 expression levels were not found to
be significantly associated with age or gender.
Discussion
Laryngeal carcinoma is the second most common
malignancy among head and neck tumors. Although the clinical
outcome of laryngeal carcinoma has gradually improved, the
prognosis of this tumor remains poor. A better understanding of the
molecular mechanisms and key molecules driving laryngeal
carcinogenesis may aid in the identification of novel predictive
and prognostic biomarkers, and in the development of novel
treatment strategies for this cancer. The role of KLF4 and HSP27 as
possible biomarkers and therapy targets has been extensively
investigated in various types of cancer (33,34).
However, the aberrant expression of these proteins in laryngeal
squamous cell carcinoma (LSCC) is poorly understood. In the present
study, the expression profile and the potential role of these
proteins as possible biomarkers of LSCC were investigated.
In this study, we examined the expression of KLF4
and HSP27 in a series of human laryngeal tumors and normal tissues.
The expression profile of these proteins indicated that they are
significantly regulated in LSCC. The protein and mRNA expression
levels of KLF4 were significantly decreased in LSCC compared to
those in normal tissue, while HSP27 was significantly overexpressed
in tumor compared to normal tissues, at the protein and mRNA
levels. Regarding tumor stages, the expression of the two proteins
varies in an opposite manner. The KLF4 expression decreases
gradually with tumor progression suggesting that KLF4 expression is
lost as the tumor progresses, while HSP27 expression increases with
stages, showing a significant difference between stages I and IV.
These findings suggest that KLF4 and HSP27 may be opposite
functions and roles in the carcinogenic process of LSCC. KLF4 seems
to play a tumor suppressing role in LSCC, while HSP27 seems to play
an oncogenic role and its overexpression may contribute in the
initiation of the disease and its progression and aggressiveness.
The downregulation of KLF4 in LSCC may be associated with promoter
hypermethylation, a loss of heterozygosity of the KLF4 locus, or
with point mutations in the coding region. Epigenetic control and
gene network may play a role in the decrease of KLF4 levels in
laryngeal cancers. The decrease in its expression in stage IV was
associated with increased tumor differentiation and aggressiveness.
The mechanisms underlying this downregulation require elucidation
by future studies.
The aberrant expression of KLF4 has been reported in
various forms of cancer. However, no previous studies have examined
the expression level of KLF4 in laryngeal carcinoma. To the best of
our knowledge, the present study is the first to provide a
preliminary description of the profile of expression of KLF4 and
its potential role in laryngeal carcinogenesis. This is the first
study showing a significant difference in KLF4 protein and mRNA
expression levels between normal and cancerous laryngeal tissues.
Our findings were consistent with those reported by different
studies showing a tumor suppressor role of KLF4 in several types of
cancer, including gastric and colon cancers (6–8), bladder
cancer (35), esophageal cancer
(36), and non small cell lung
carcinoma (14,37). Of note, in head and neck squamous
cell carcinoma malignancies, KLF4 can exert different roles in
different types. In oral squamous cell carcinoma, the expression of
KLF4 increases at the early stages of the disease where it plays an
oncogenic role (38). However, in
our study on LSCC, opposite results were obtained. The expression
of KLF4 appears to exert a dual effect depending on the cell
context and gene network. Our actual in vitro study aims to
determine the underlying mechanisms and the potential factors that
regulate the gene or the protein expression of KLF4.
The expression level and role of HSP27 have been
considerably investigated by several studies that reported a higher
expression level of this protein in tumors from various origins,
associated with tumor aggressiveness and poor survival of patients.
HSP27 is clearly involved in the tumorigeneis process, tumor
resistance and progression, and metastasis. It is considered as a
promising therapeutic target (39).
Its gene inhibition using antisense oligonucleotide showed a
promising therapeutic effect in clinical application (30). HSP27 has been identified as a
candidate biomarker. Some studies have explored its potential
prognostic value and its role in predicting the adequate therapy
(16). However, studies regarding
the role of HSP27 in larynx cancer are limited (31,32). In
the present study, the expression of HSP27 increases significantly
in larynx tumor tissues compared with normal tissues. This
expression is correlated to tumor stages, confirming the role of
this protein in oncogenic transformation and tumor progression. The
level of HSP27 seems to be associated with the level of tumor
differentiation. These findings are consistent with recent studies
investigating HSP27 expression in cancer and elucidating its
prognostic role.
Our results were not consistent with those of Xu
et al, that showed an absence of significant difference in
HSP27 protein expression between laryngeal carcinoma and normal
controls (32). This discrepancy may
be due to the marginally larger tumor sample collection in our
study, potentially contributing to more relevant results, and to
the different method used to evaluate HSP27 expression. Our
observations were also not consistent with those of Kaigorodova
et al who demonstrated a high nuclear expression of the
phosphorylated and unphosphorylated forms of HSP27 in the biopsies
of patients with lymph node metastases (31). However, the cytoplasmic expression of
HSP27 in these patients did not differ statistically, and in their
study, they did not evaluate the mRNA expression level of HSP27 in
their larynx tissues.
Since HSP27 is upregulated from early stages of the
disease and KLF4 is downregulated progressively and specially in
advanced stages, this implies that HSP27 might suppress the
expression of KLF4 probably through indirect mechanisms. In fact, a
study showed that HSP27 interacts with SP1 in the brain (40) which is a transcription factor of the
KLF/SP family that can activate the transcription of KLF4 (41,42).
This interaction was shown to potentiate the transcription activity
of SP1, and had a cytoprotective role for the neurons (40). In laryngeal carcinoma, HSP27 could
interact with SP1 and thus preventing SP1 from activating the
transcription of KLF4. Our actual in vitro study aims to
elucidate the presence of this link between these two proteins and
the underlying mechanisms and factors or protein partners implied
in this regulation. To date, neither KLF4 nor HSP27 have been
extensively studied in laryngeal cancer. This is the first study
that investigated the expression and role of KLF4 in larynx cancer,
and that showed a potential association between KLF4 and HSP27 in
this type of cancer. However, due to the lack of patient survival
data, we were unable to investigate any correlation between
immunohistochemical and RTPCR findings and patient survival.
Understanding the molecular mechanisms underlying
the pathogenesis of larynx cancer is required to achieve better
patient outcomes. The role of HSP27 and KLF4 in larynx cancer
initiation and progression highlights their use as potential future
targets for prognosis and treatment.
Acknowledgements
The present study has been funded with support from
the National Council for Scientific Research in Lebanon and from
the Lebanese University.
References
|
1
|
Santos TS, Estêvão R, Antunes L, Certal V,
Silva JC and Monteiro E: Clinical and histopathological prognostic
factors in locoregional advanced laryngeal cancer. J Laryngol Otol.
130:948–953. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marur S and Forastiere AA: Head and neck
squamous cell carcinoma: Update on epidemiology, diagnosis, and
treatment. Mayo Clin Proc. 91:386–396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ding B, Liu P, Liu W, Sun P and Wang CL:
Emerging roles of Krüppel-like factor 4 in cancer and cancer stem
cells. Asian Pac J Cancer Prev. 16:3629–3633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Evans PM and Liu C: Roles of Krüpel-like
factor 4 in normal homeostasis, cancer and stem cells. Acta Biochim
Biophys Sin (Shanghai). 40:554–564. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rowland BD and Peeper DS: KLF4, p21 and
context-dependent opposing forces in cancer. Nat Rev Cancer.
6:11–23. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao W, Hisamuddin IM, Nandan MO, Babbin
BA, Lamb NE and Yang VW: Identification of Krüppel-like factor 4 as
a potential tumor suppressor gene in colorectal cancer. Oncogene.
23:395–402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei D, Gong W, Kanai M, Schlunk C, Wang L,
Yao JC, Wu TT, Huang S and Xie K: Drastic down-regulation of
Krüppel-like factor 4 expression is critical in human gastric
cancer development and progression. Cancer Res. 65:2746–2754. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei D, Kanai M, Huang S and Xie K:
Emerging role of KLF4 in human gastrointestinal cancer.
Carcinogenesis. 27:23–31. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu W, Hofstetter WL, Li H, Zhou Y, He Y,
Pataer A, Wang L, Xie K, Swisher SG and Fang B: Putative
tumor-suppressive function of Kruppel-like factor 4 in primary lung
carcinoma. Clin Cancer Res. 15:5688–5695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pandya AY, Talley LI, Frost AR, Fitzgerald
TJ, Trivedi V, Chakravarthy M, Chhieng DC, Grizzle WE, Engler JA,
Krontiras H, et al: Nuclear localization of KLF4 is associated with
an aggressive phenotype in early-stage breast cancer. Clin Cancer
Res. 10:2709–2719. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen CJ, Lin SE, Lin YM, Lin SH, Chen DR
and Chen CL: Association of expression of kruppel-like factor 4 and
kruppel-like factor 5 with the clinical manifestations of breast
cancer. Pathol Oncol Res. 18:161–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rowland BD, Bernards R and Peeper DS: The
KLF4 tumour suppressor is a transcriptional repressor of p53 that
acts as a context-dependent oncogene. Nat Cell Biol. 7:1074–1082.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tai SK, Yang MH, Chang SY, Chang YC, Li
WY, Tsai TL, Wang YF, Chu PY and Hsieh SL: Persistent Krüppel-like
factor 4 expression predicts progression and poor prognosis of head
and neck squamous cell carcinoma. Cancer Sci. 102:895–902. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fadous-Khalifé MC, Aloulou N, Jalbout M,
Hadchity J, Aftimos G, Paris F and Hadchity E: Krüppel-like factor
4: A new potential biomarker of lung cancer. Mol Clin Oncol.
5:35–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zoubeidi A and Gleave M: Small heat shock
proteins in cancer therapy and prognosis. Int J Biochem Cell Biol.
44:1646–1656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lianos GD, Alexiou GA, Mangano A, Mangano
A, Rausei S, Boni L, Dionigi G and Roukos DH: The role of heat
shock proteins in cancer. Cancer Lett. 360:114–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kaigorodova EV and Bogatyuk MV: Heat shock
proteins as prognostic markers of cancer. Curr Cancer Drug Targets.
14:713–726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sherman M and Multhoff G: Heat shock
proteins in cancer. Ann N Y Acad Sci. 1113:192–201. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ciocca DR, Arrigo AP and Calderwood SK:
Heat shock proteins and heat shock factor 1 in carcinogenesis and
tumor development: An update. Arch Toxicol. 87:19–48. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lo WY, Tsai MH, Tsai Y, Hua CH, Tsai FJ,
Huang SY, Tsai CH and Lai CC: Identification of over-expressed
proteins in oral squamous cell carcinoma (OSCC) patients by
clinical proteomic analysis. Clin Chim Acta. 376:101–107. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cordonnier T, Bishop JL, Shiota M, Nip KM,
Thaper D, Vahid S, Heroux D, Gleave M and Zoubeidi A: Hsp27
regulates EGF/β-catenin mediated epithelial to mesenchymal
transition in prostate cancer. Int J Cancer. 136:E496–E507. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shiota M, Bishop JL, Nip KM, Zardan A,
Takeuchi A, Cordonnier T, Beraldi E, Bazov J, Fazli L, Chi K, et
al: Hsp27 regulates epithelial mesenchymal transition, metastasis
and circulating tumor cells in prostate cancer. Cancer Res.
73:3109–3119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stope MB, Weiss M, Preuss M, Streitbörger
A, Ritter CA, Zimmermann U, Walther R and Burchardt M: Immediate
and transient phosphorylation of the heat shock protein 27
initiates chemoresistance in prostate cancer cells. Oncol Rep.
32:2380–2386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hansen RK, Parra I, Lemieux P, Oesterreich
S, Hilsenbeck SG and Fuqua SA: Hsp27 overexpression inhibits
doxorubicin-induced apoptosis in human breast cancer cells. Breast
Cancer Res Treat. 56:187–196. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsu HS, Lin JH, Huang WC, Hsu TW, Su K,
Chiou SH, Tsai YT and Hung SC: Chemoresistance of lung cancer
stemlike cells depends on activation of Hsp27. Cancer.
117:1516–1528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ciocca DR and Calderwood SK: Heat shock
proteins in cancer: Diagnostic, prognostic, predictive, and
treatment implications. Cell Stress Chaperones. 10:86–103. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hadchity E, Aloy MT, Paulin C, Armandy E,
Watkin E, Rousson R, Gleave M, Chapet O and Rodriguez-Lafrasse C:
Heat shock protein 27 as a new therapeutic target for radiation
sensitization of head and neck squamous cell carcinoma. Mol Ther.
17:1387–1394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aloy MT, Hadchity E, Bionda C, Diaz-Latoud
C, Claude L, Rousson R, Arrigo AP and Rodriguez-Lafrasse C:
Protective role of Hsp27 protein against gamma radiation-induced
apoptosis and radiosensitization effects of Hsp27 gene silencing in
different human tumor cells. Int J Radiat Oncol Biol Phys.
70:543–553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lelj-Garolla B, Kumano M, Beraldi E, Nappi
L, Rocchi P, Ionescu DN, Fazli L, Zoubeidi A and Gleave ME: Hsp27
inhibition with OGX-427 sensitizes non-small cell lung cancer cells
to erlotinib and chemotherapy. Mol Cancer Ther. 14:1107–1116. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chi KN, Yu EY, Jacobs C, Bazov J,
Kollmannsberger C, Higano CS, Mukherjee SD, Gleave ME, Stewart PS
and Hotte SJ: A phase I dose-escalation study of apatorsen
(OGX-427), an antisense inhibitor targeting heat shock protein 27
(Hsp27), in patients with castration-resistant prostate cancer and
other advanced cancers. Ann Oncol. 27:1116–1122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaigorodova EV, Zavyalova MV, Bychkov VA,
Perelmuter VM and Choynzonov EL: Functional state of the Hsp27
chaperone as a molecular marker of an unfavorable course of larynx
cancer. Cancer Biomark. 17:145–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu X, Zhao M and Shi Y: Expression of heat
shock proteins in laryngeal carcinoma. Zhonghua Er Bi Yan Hou Ke Za
Zhi. 33:232–234. 1998.(In Chinese). PubMed/NCBI
|
|
33
|
Ghaleb AM and Yang VW: Krüppel-like factor
4 (KLF4): What we currently know. Gene. 611:27–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu J, Liu T, Rios Z, Mei Q, Lin X and Cao
S: Heat Shock Proteins and Cancer. Trends Pharmacol Sci.
38:226–256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohnishi S, Ohnami S, Laub F, Aoki K,
Suzuki K, Kanai Y, Haga K, Asaka M, Ramirez F and Yoshida T:
Downregulation and growth inhibitory effect of epithelial-type
Krüppel-like transcription factor KLF4, but not KLF5, in bladder
cancer. Biochem Biophys Res Commun. 308:251–256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang N, Liu ZH, Ding F, Wang XQ, Zhou CN
and Wu M: Down-regulation of gut-enriched Kruppel-like factor
expression in esophageal cancer. World J Gastroenterol. 8:966–970.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Z, Wang Z, Liu X, Shi M, Chen G,
Zhang B, Li Z and Song L: Correlation of KLF4 and SPARC expression
with the clinical characteristics of non-small cell lung cancer.
Zhongguo Fei Ai Za Zhi. 15:720–724. 2012.(In Chinese). PubMed/NCBI
|
|
38
|
Yoshihama R, Yamaguchi K, Imajyo I, Mine
M, Hiyake N, Akimoto N, Kobayashi Y, Chigita S, Kumamaru W,
Kiyoshima T, et al: Expression levels of SOX2, KLF4 and brachyury
transcription factors are associated with metastasis and poor
prognosis in oral squamous cell carcinoma. Oncol Lett.
11:1435–1446. 2016.PubMed/NCBI
|
|
39
|
Acunzo J, Andrieu C, Baylot V, So A and
Rocchi P: Hsp27 as a therapeutic target in cancers. Curr Drug
Targets. 15:423–431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Friedman MJ, Li S and Li XJ: Activation of
gene transcription by heat shock protein 27 may contribute to its
neuronal protection. J Biol Chem. 284:27944–27951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Deaton RA, Gan Q and Owens GK:
Sp1-dependent activation of KLF4 is required for PDGF-BB-induced
phenotypic modulation of smooth muscle. Am J Physiol Heart Circ
Physiol. 296:H1027–H1037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang JP, Zhang H, Wang HB, Li YX, Liu GH,
Xing S, Li MZ and Zeng MS: Down-regulation of Sp1 suppresses cell
proliferation, clonogenicity and the expressions of stem cell
markers in nasopharyngeal carcinoma. J Transl Med. 12:2222014.
View Article : Google Scholar : PubMed/NCBI
|