Introduction
Hemangiopericytoma (HPC) is a malignant vascular
tumor arising from mesenchymal cells that surround endothelial
tissue, which is known as Zimmerman's pericytes (1). HPC was first reported by Stout and
Murray in 1942 and represents only<1% of all vascular neoplasms
(1). Most of HPC are in soft tissues
and can be reclassified as a fibroblastic neoplasm similar to a
solitary fibrous tumors (2–4). The most common sites of HPC are the
lower extremities, followed by the pelvis and head and neck
(5,6). HPC has non-specific image features and
clinical manifestations. Pathological confirmation combined with
immunochemical analysis is mandatory for diagnosis of HPC (2). The histological appearance does not
reliably predict the biologic behavior of the tumor.
Primary bone HPC is extremely rare. In the radiology
literature, there are only case reports or small case series
reports of bone HPC (7–13). Although most of bone HPC demonstrate
as destructive or lytic lesions (7–13),
radiographic findings are non-specific as well (14).
Currently F18-fluorodeoxyglucose (FDG) positron
emission tomography/computed tomography (PET/CT) imaging is
standard care for staging, restaging and surveillance of primary
malignant bone neoplasms, but there is very little information
about its application in bone HPC. There were only limited case
reports about FDG PET/CT image findings of HPC, mostly non-osseous
lesions (15–20). This retrospective study presents FDG
PET/CT findings in 4 patients with primary bone HPC.
Patients and methods
Ethics
This retrospective study was approved by the
Institutional Review board. Relevant cases were identified through
a search of a computerized database of patients who underwent
PET/CT imaging at the Advanced Imaging Center, University Hospital
between January 2010 and June 2016.
Patients
The study group consisted of 4 subjects with
pathologically diagnosed bone HPC, who had FDG PET/CT for staging
and/or restaging or surveillance. Non-osseous HPCs were excluded
from the study.
Method
FDG PET/CT. Combined PET-CT was performed using a
PET-CT scanner (Discovery LS, GE Healthcare, Milwaukee, WI, USA)
and standard techniques. The patients had fasted for at least 6 h
prior to examination and their blood glucose level was <200
mg/dl. The patients received oral but not intravenous contrast
media. Spiral low-dose CT (80 mA, 140 kV and 4 mm section
thickness) was performed with the cranio-caudal direction covering
the areas from the vertex to the toes for the purpose of
attenuation correction and anatomic localization. Thereafter,
emission scan was conducted in a reverse direction.
Image analysis
An image software Mim (Mim Software Inc, Cleveland,
OH, USA) was used for image display and analysis. The whole-body
maximum-pixel-intensity projection was used for visual evaluation.
Maximum standardized uptake value (SUVmax) of lesions
was recorded.
PET/CT findings were correlated with the patients'
medical records including radiological, laboratory, pathologic and
follow-up information.
Results
Table I summarizes
patients' characteristics and clinical data including sites of the
bone lesions, purpose of FDG PET/CTs, SUVs of the lesions, adjacent
soft tissue involvement, and metastatic disease.
 | Table I.Patients' characteristics and FDG
PET/CT findings. |
Table I.
Patients' characteristics and FDG
PET/CT findings.
| Patient | Sex/age | Location | Bone change | PET indication |
SUVmax | Soft tissue
involvement | Metastasis |
|---|
| 1 | Male/54 | L. Ilium | Destructive | Staging | 11.8 | Yes | No |
|
|
|
|
| Restaging |
|
| No |
| 2 | Female/88 | R. Femur | Destructive | Staging | 9.0 | Yes | No |
| 3 | Female/30 | R. Tibia | Lytic | Staging | 14 | Yes | No, but 1 synchronous
lesion |
|
|
|
|
| Restaging |
|
| No |
| 4 | Female/78 | L. Femur | Lytic | Restaging | Varied, max. 8 | Yes (CT) | Yes on Restaging |
All 4 patients had surgical pathological diagnoses
of bone HPC, including positive CD34 immunochemical stain
analyses.
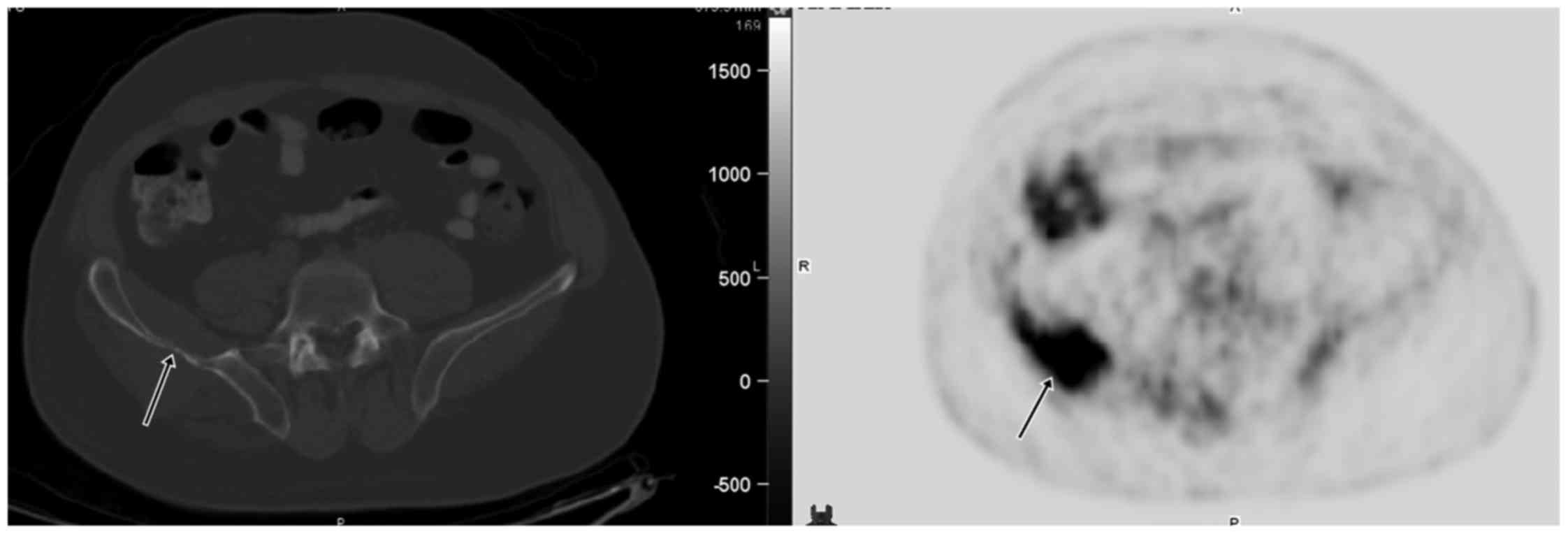
Patient 1 was a 54-year old man with the right hip
pain. CT and radiographs showed a large destructive lesion of the
right iliac wing with adjacent soft tissue involvement. Biopsy
suggested HPC. Staging FDG PET/CT showed a large, intensely FDG
avid (SUV 11.8) right iliac lesion with adjacent soft tissue
components (Fig. 1). The patient
underwent radical resection, plate/screw fixation and bone graft
reconstruction. Afterwards, 5 postoperative surveillance PET/CTs
were all negative for recurrent or metastatic disease. The patient
was disease-free for >3 years. On laboratory data, the patient
had persistent preoperative hypocalcemia (the lowest serum calcium
level 6.9 mg/dl), which was normalized 1 week postoperatively.
Patient 2 was an 88-year-old woman with pain of the
right lower extremity. Radiographic images showed a destructive
mass of the right femur. Staging FDG PET/CT demonstrated a large
lytic lesion with intense uptake (SUV 9.0) in the right distal
femur and adjacent posteromedial soft tissue. Surgical pathology
indicated HPC. The patient was lost to follow-up postoperatively.
No laboratory data were available on the medical record.
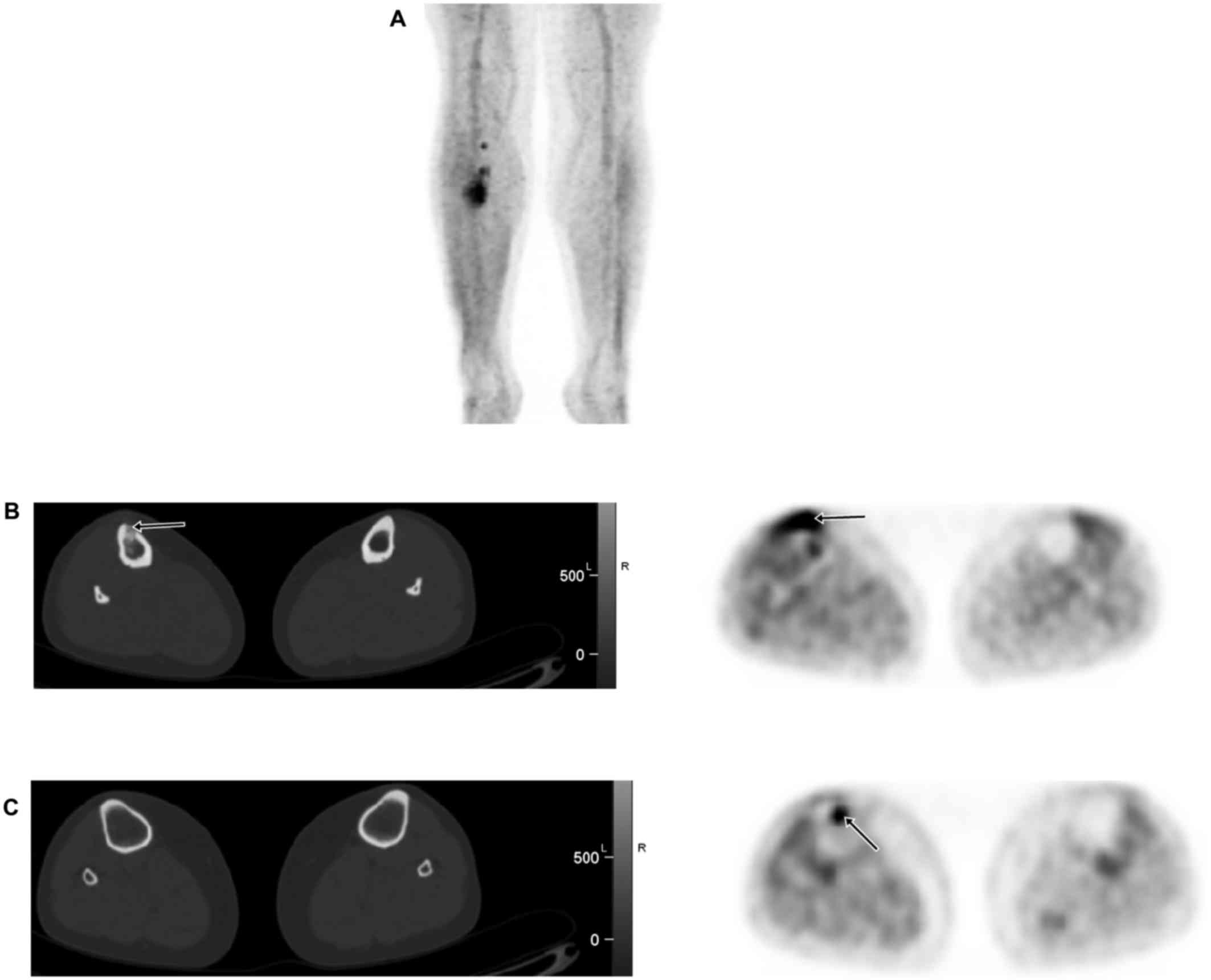
Patient 3 was a 30-year-old woman with newly
diagnosed HPC of the right tibia. Staging FDG PET/CT showed
abnormal uptake (SUV 14) in the known lesion of the right proximal
tibia. There was an additional 1.1 cm focus of uptake (SUV 9.0) in
the right proximal tibial metaphysis suspicious for synchronous
lesion, although no corresponding bone lesion was seen on the
integrated CT (Fig. 2). The patient
underwent radical resection and pathology confirmed multi-focal
lesions of HPC. Postoperative surveillance PET/CT were all negative
for recurrent or metastatic disease. The patient were disease-free
for 6.5 years. The patient had temporary mild hypocalcemia (serum
calcium level 8.3 mg/dl) preoperatively, but normal serum calcium
level 2 days postoperatively.
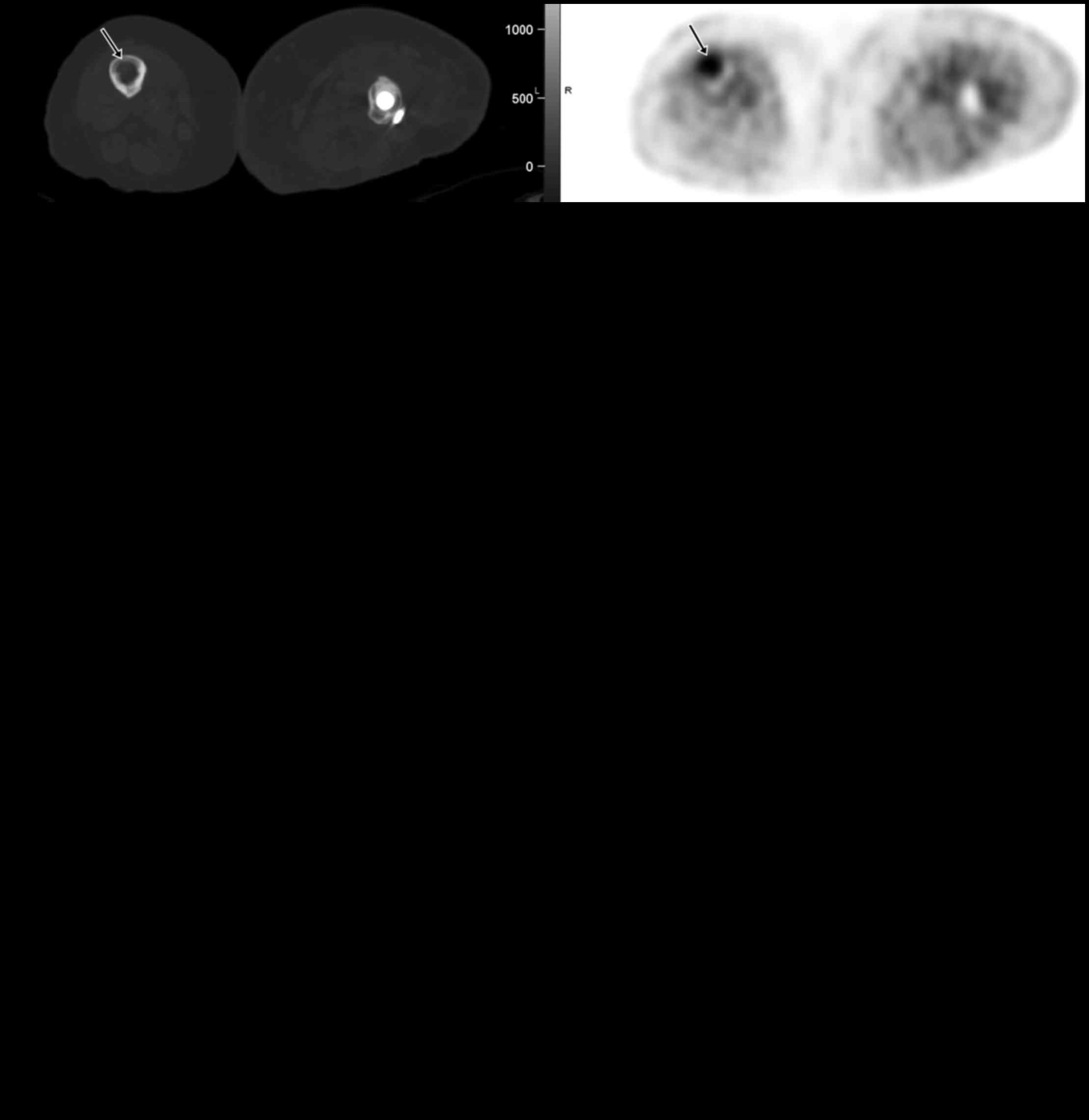
Patient 4 was a 78-year-old woman with a destructive
lesion and pathologic fracture of the left proximal femur. Surgical
pathology suggested bone HPC. There was no pre-surgical staging
PET/CT. Postsurgical surveillance PET/CTs were negative until 2
years later when FDG PET/CT showed positive left inguinal
lymphadenopathy and new pulmonary nodules. Excisional biopsy of the
left inguinal lymph node was positive for HPC metastasis.
Afterwards, the patient underwent chemotherapy with different
regimens (Doxil, Temodar, Avastin and irinotecan). Post-therapeutic
PET/CTs demonstrated continuous progression of metastatic disease
with new lesions in the lungs, liver, muscle and bone (Fig. 3). The patient declined further
treatment and was enrolled in hospice care. The patient had
persistent hypocalcemia (the lowest calcium level 7.0 mg/dl)
preoperatively, within 5 months postoperatively and 1 months after
metastases were diagnosed.
In the current case series, 3 had lesions in the
lower extremities (femora and tibia) and 1 had lesion in the pelvis
(ilium). All had destructive or lytic lesions on radiographic
images. All 3 primary bone HPC lesions (patients 1–3) and multiple
metastatic HPC lesions (patient 4) demonstrated high FDG avidity on
PET/CT imaging. The adjacent soft tissue involvement or invasion
was present in all 3 staging PET/CT images. One patient had a
synchronous lesion in the same bone on PET/CT. FDG PET/CT was
accurate in staging 3 patients (patient 1–3) and restaging 3
patients (patient 1, 3 and 4).
Three of four patients with available laboratory
data had hypocalcemia when HPC existed either as primary lesions
(patients 1, 3 and 4) or metastatic disease (patient 4), but normal
calcium levels when they were disease-free. All patients had normal
serum phosphorus levels when hypocalcemia existed. Unfortunately,
no patient had further evaluation of the other laboratory profile
such as parathyroid hormone or vitamin-D.
Discussion
Primary bone HPC is extremely rare, comprising only
0.1% of malignant primary bone tumors (14). Patient's symptoms and signs are
nonspecific in HPC. In current 4 cases, 3 patients had pain and 1
patient presented with pathological fracture. For the lesion sites,
2 were in the femur, 1 was in the tibia and 1 was in the ilium.
Findings are consistent with observation of previous case reports
that the lower extremities are the most common site of bone HPC
(14,21). On radiographic images including plain
X-ray, CT and/or MRI, all lesions were destructive or osteolytic
with cortical disruption and extension to the adjacent soft tissue.
However these features were nonspecific for HPC and
indistinguishable from either more benign-appearing tumors such as
giant cell tumor, chondromyxoid fibroma, or more
malignant-appearing tumors such as fibrosarcoma, angiosarcoma and
metastasis (14). HPC could not be
diagnosed until surgical pathology from biopsy or resection was
obtained. In all cases, final diagnoses were based on the
architectural pattern on pathology and immunochemical stain. In
addition to initial workup, CT and/or MRI are often used to define
the extent of bone lesion and soft tissue involvement.
The current data demonstrated high FDG avidity of
both primary bone and metastatic lesions of HPC. All lesions had
intense uptake on FDG PET/CT and adjacent soft tissue involvements.
In the patient 3, PET detected an additional small synchronous
lesion in the same bone as the original. In the patient 4, FDG/CT
accurately diagnosed multiple metastatic nodal, pulmonary, hepatic,
bone and muscle lesions which were all highly FDG avid. Therefore,
FDG PET/CT is a useful image modality for staging, surveillance and
detection of recurrent/metastatic disease in bone HPC.
Radical resection of the lesion is a mainstay of
treatment of HPC. In 3 of 4 patients with available follow-ups, the
patient 1 and 3 were disease-free 3 years and 6.5 years
postoperatively without any adjunct therapy, respectively. The
patient 4 developed metastases 2 years after surgical resection of
bone HPC and the metastatic lesions were irresponsive to
chemotherapy. Except for regional lymph nodes, the first and main
site of distant metastasis was the lung.
Tumor-induced osteomalacia (TIO) has been reported
to occur in HPC (14,15,20). TIO
is a rare paraneoplastic syndrome characterized by
hyperphosphaturia, hypophosphatemia, decreased serum Vitamin D3 and
osteomalacia. There were a few case reports that TIO was in
association with HPC and the serum and urine phosphate levels
returned to normal after excision of the tumors (14,15,20,22). It
was hypothesized that these tumors elaborate a substance that
decreases or interrupts the synthesis of 1,
25-dihydroxycholecalciferol, resulting in reduced tubular
reabsorption of phosphorus, which in turn induces osteomalacia
(14). There was also a case report
about association between HPC and hypoglycemia, which was caused by
increased circulating insulin-like activity from elevated free
insulin-like growth factor II (IGF-II) stimulating glucose uptake
primarily into muscle tissue (23).
However, the current case series, for the first time, demonstrates
different laboratory findings than that of TIO or paraneoplastic
syndrome. All 3 patients with available laboratory data had
hypocalcemia prior to treatment, and normalization of serum calcium
levels days or months after surgical resections of the HPC lesions
or when they were disease-free. Patient 4 had recurrent
hypocalcemia after development of metastatic disease. No patient
had hypophosphatemia. Unfortunately, no further laboratory workups
were obtained for these patients, such as parathyroid hormone,
urine calcium or phosphate clearance or serum/urine vitamin D
analyses. The mechanism of hypocalcemia in bone HPC is not clear.
It is reasonable to assume that both primary bone HPC lesion and
metastatic HPC lesions might produce a substance which could
interfere or damage normal metabolism of calcium. Further
biochemistry investigation is needed to clarify the association
between bone HPC and hypocalcemia.
In conclusion, bone HPC is extremely rare.
Radiographic features of the lesions are nonspecific and pathologic
diagnosis is warranted. Both primary bone and metastatic HPC
lesions demonstrated high FDG avidity on PET/CT, which could be
effectively used for staging, surveillance and detection of
recurrent/metastatic disease. On pre-therapeutic imaging, PET/CT
could reveal soft tissue involvement and synchronous lesion. All
three patients with available laboratory data had hypocalcemia
prior to treatment but normalization of serum calcium levels days
or months after surgical resections of the HPC lesions or when they
were disease-free. Recurrent hypocalcemia occurred after
development of metastatic disease in 1 patient. The findings are
different from that reported in the literature regarding
tumor-induced osteomalacia or paraneoplastic syndrome. Both primary
bone HPC lesion and metastatic HPC lesions may produce a substance
which could interfere or damage normal metabolism of calcium.
References
|
1
|
Stout AP and Murray MR:
Hemangiopericytoma: A vascular tumor featuring zimmermann's
pericytes. Ann Surg. 116:26–33. 1942. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verbeke SL and Bovée JV: Primary vascular
tumors of bone: A spectrum of entities? Int J Clin Exp Pathol.
4:541–551. 2011.PubMed/NCBI
|
|
3
|
Fletcher CD: The evolving classification
of soft tissue tumours: An update based on the new who
classification. Histopathology. 48:3–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gengler C and Guillou L: Solitary fibrous
tumour and haemangiopericytoma: Evolution of a concept.
Histopathology. 48:63–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Espat NJ, Lewis JJ, Leung D, Woodruff JM,
Antonescu CR, Shia J and Brennan MF: Conventional
hemangiopericytoma: Modern analysis of outcome. Cancer.
95:1746–1751. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Combs SE, Thilmann C, Debus J and
Schulz-Ertner D: Precision radiotherapy for hemangiopericytomas of
the central nervous system. Cancer. 104:2457–2465. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Unni KK, Ivins JC, Beabout JW and Dahlin
DC: Hemangioma, hemangiopericytoma and hemangioendothelioma
(angiosarcoma) of bone. Cancer. 27:1403–1414. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Cao L, Liu L, Guo S, Tai H and Chen
Z: Primary epidural hemangiopericytoma in the sacrum: A rare case
and literature review. Tumour Biol. 35:11655–11658. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang P, Hu J and Zhou D:
Hemangiopericytoma of the cervicothoracic spine: A case report and
literature review. Turk Neurosurg. 24:948–953. 2014.PubMed/NCBI
|
|
10
|
Lian YW, Yao MS, Hsieh SC, Lao WT, Fang CL
and Chan WP: MRI of hemangiopericytoma in the sacrum. Skeletal
Radiol. 33:485–487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dürr HR, Nerlich A, Lienemann A, Müller PE
and Refior HJ: Malignant hemangiopericytoma of the bone.
Langenbecks Arch Surg. 385:207–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sahin-Akyar G, Fitöz S, Akpolat I, Sağlik
Y and Erekul S: Primary hemangiopericytoma of bone located in the
tibia. Skeletal Radiol. 26:47–50. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin YJ, Tu YK, Lin SM and Shun CT: Primary
hemangiopericytoma in the axis bone: Case report and review of
literature. Neurosurgery. 39:397–399. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang JS, Gold RH, Mirra JM and Eckardt J:
Hemangiopericytoma of bone. Cancer. 62:848–859. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jain AS, Shelley S, Muthukrishnan I, Kalal
S, Amalachandran J and Chandran S: Diagnostic importance of
contrast enhanced (18)F-fluorodeoxyglucose positron emission
computed tomography in patients with tumor induced osteomalacia:
Our experience. Indian J Nucl Med. 31:14–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SJ, Kim ST, Park SH, Choi YL, Park JB,
Kim SJ and Lee J: Successful use of pazopanib for treatment of
refractory metastatic hemangiopericytoma. Clin Sarcoma Res.
4:132014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aras M, Dede F, Ones T, Atasoy BM, Inanir
S, Erdil TY and Turoglu HT: F-18 FDG PET/CT findings of primary
sinonasal hemangiopericytoma: Rare location in a young adult
patient. Clin Nucl Med. 36:473–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ito S, Yokoyama J, Yoshimoto H, Yazawa M,
Kazuo K, Hanaguri M, Ohba S, Fujimaki M and Ikeda K: Usefulness of
choline-PET for the detection of residual hemangiopericytoma in the
skull base: Comparison with FDG-PET. Head Face Med. 8:32012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hung TJ, Macdonald W, Muir T, Celliers L
and Al-Ogaili Z: 68Ga DOTATATE PET/CT of Non-FDG-Avid pulmonary
metastatic hemangiopericytoma. Clin Nucl Med. 41:779–780. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khadgawat R, Singh Y, Kansara S, Tandon N,
Bal C, Seith A and Kotwal P: PET/CT localisation of a scapular
haemangiopericytoma with tumour-induced osteomalacia. Singapore Med
J. 50:e55–e57. 2009.PubMed/NCBI
|
|
21
|
Verbeke SL, Fletcher CD, Alberghini M,
Daugaard S, Flanagan AM, Parratt T, Kroon HM, Hogendoorn PC and
Bovée JV: A reappraisal of hemangiopericytoma of bone; analysis of
cases reclassified as synovial sarcoma and solitary fibrous tumor
of bone. Am J Surg Pathol. 34:777–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fuentealba C, Pinto D, Ballesteros F,
Pacheco D, Boettiger O, Soto N, Fernandez W, Gabler F, Gonzales G
and Reginato AJ: Oncogenic hypophosphatemic osteomalacia associated
with a nasal hemangiopericytoma. J Clin Rheumatol. 9:373–379. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung J and Henry RR: Mechanisms of
tumor-induced hypoglycemia with intraabdominal hemangiopericytoma.
J Clin Endocrinol Metab. 81:919–925. 1996. View Article : Google Scholar : PubMed/NCBI
|