Introduction
Anthocyanins are the chemical components that result
in the intense color to many fruits and vegetables, such as
bilberries, blueberries, red cabbages and purple sweet potatoes.
Rapidly accumulating in vitro and in vivo evidence
indicates that anthocyanins have cancer preventive and anti-cancer
activity. Bilberry anthocyanins were shown to be effective cancer
preventive agents in 25 colorectal cancer patients (1). Anthocyanins treated cancer cells reveal
upregulation of tumor suppressor genes and intracellular-signaling
cascades as common molecular targets for anthocyanins (2). Defect in apoptosis has been implicated
as a major cause of resistance to chemotherapy. Anthocyanins
induced apoptosis in cancer cells via activation of redox-sensitive
caspase 3-pathways were observed in B-cell chronic lymphocytic
leukemia (B-CLL), but had no effect in normal peripheral blood
cells (3). Furthermore, Bilberry
extracts exhibited strong pro-apoptotic activity through
redox-sensitive caspase 3 activation-related mechanism in B-CLL
cells involving dysregulation of the Bad/Bcl-2 pathway (3). Interestingly, bilberry anthocyanins
also synergistically suppress growth and invasive potential of
human non-small-cell lung cancer cells (4). The development of a better delivery
system for Bilberry anthocyanins would significantly enhance their
cancer prevention and treatment effectiveness. Herein is reported
such a system utilizing the NutraNanoSphere™ (NNS) micellation of
Bilberry.
Materials and methods
Cell line and media production
Experiments were performed using a chronic
myelogenous leukemia K-652 cell line purchased from the American
Type Culture Collection.
The tissue culture media was made by adding 5 ml of
100X penicillin-streptomycin (10,000 units penicillin with 10 mg of
streptomycin/ml), 5 ml of 200 mM sterile-filtered L-Glutamine (both
from Sigma-Aldrich, St. Louis, MO, USA), 5 ml of Cellgro sodium
100X bicarbonate solution (Cellgro Mediatech, Herndon, VA, USA),
and 50 ml of fetal calf serum (Atlanta Biologicals, Flowery Branch,
GA, USA) to 500 ml of Minimum Essential Media, Alpha 1X, with
Earle's salts without ribonucleotides, deoxyribonucleotides, and no
L-glutamine (Cellgro Mediatech).
Viability stain
The viability stain used for analysis with the flow
cytometer was developed by Dr Jerry Thornthwaite. The evidence that
showed the viability stain, which uses a special medium and dye
exclusion with propidium iodide (PI) (Sigma-Aldrich), was effective
in measuring cell viability was shown in two ways. Firstly, 500 µl
portions of K562 cells (5×106/ml) were heated as
triplicates in a 56°C water bath for ≤20 min. At 5 min intervals,
500 µl of the viability reagent was added to the timed samples at
room temperature. All samples were run after a 2 min incubation at
room temperature on the Accuri Flow Cytometer (BD Biosciences, San
Jose, CA, USA). Also, K562 cell viability was measured directly
from cell cultures in which 100 µl samples from the cell culture
were added to 100 µl of the viability stain. After incubation for
five min at room temperature, the samples were suspended and
analyzed using flow cytometry. Forward light scatter was used to
gate on the K562 cells and analyze the number of viable cells
within the established control viability gate in the 585±20 nm
fluorescence red channel. Typical control cultures with 95–99%
viability were used to establish the boundary of the viable cells,
which only showed passive background staining on the cell
surface.
Encapsulated reagent
Bilberry NNS were obtained from Dr Lothar Haegeler
of X-Labs (Switzerland-Singapore) and had a concentration of had a
concentration of 2.5 mg/50 µl or 50 mg/ml. The anthocyanin
concentration in the micellized Bilberry was 25% (w/w).
Average diameter measurements of the
NNS
The samples were diluted by volume in a ratio of 1:6
with DI Water and filtered by a 0.45 µm Nylon membrane to remove
any dust contaminants. The Zetasizer ZSP (Malvern Instruments,
Malvern, UK) was used with a backscattering angle of 173 degrees to
measure the particle size by dynamic light scattering. A
non-negative least squares algorithm was used to generate the size
distribution by intensity, which indicated the diameter of the
major population for the NNS. The intensity data was then converted
to a mass or volume distribution to compare relative amounts of
each size population, which indicated the percentage of the sample
represented in the respective population.
Sample preparation and cell
counting
In all assays, viable cell counts were obtained by
mixing 100 µl of a cell culture with 100 µl of viability stain. All
samples were analyzed within an hour after room temperature
incubation for at least 5 min. The percentage viability was stable
for ≤2 h. A 10 µl portion of each sample was run through the Flow
Cytometer at a medium flow setting. The resulting number was
multiplied by 200 to determine the number of viable cells/ml.
Cell growth plate preparation
The cells that were counted were then diluted with
the media to a concentration of 1×105 cells/ml. A 500 µl
portion of the cells was added to each well of the 48-well plate.
The plates incubated in a Forma Scientific CO2
water-jacketed incubator at a temperature of 37.2°C for 48 h to
allow the cells to enter the exponential growth phase.
Addition of compounds
After incubation for 48 h, the stock sample
compounds were diluted accordingly, by a factor of two for up to
eight dilutions. A 50 µl sample was added to each well, and ≤6
replicates of each dilution to the wells were done. A total of 50
µl of cell culture media was added to each control well. Once
finished, every well contained 550 µl. The plates were incubated
for 48 h.
Cell processing, staining, and
analysis
Up to six replicates at each concentration, starting
with the controls that were used to set the gates for viability,
were suspended with a 500 µl pipet, and 100 µl portions were added
to 2 ml 96-well analysis tubes. After all of the samples were added
to the tubes, 100 µl of the viability stain were added using an
8-channel multipipettor, and the tray was shaken slightly and
incubated for ≥5 min. All samples were analyzed within an hour
after room temperature incubation for at least 5 min. The
viability-stained cells were stable for at least 2 h at room
temperature. A 10 µl portion of each sample was run through the
Flow Cytometer at a medium flow setting. The resulting number was
multiplied by 200 to determine the number of viable cells/ml.
Percentage cell viability
Control cells were used to set the forward angle
light scatter gate for the entire cell population gate for the
viable cells less debris to the left of the scatter peak. The
resulting fluorescent peak population of cells comprised the viable
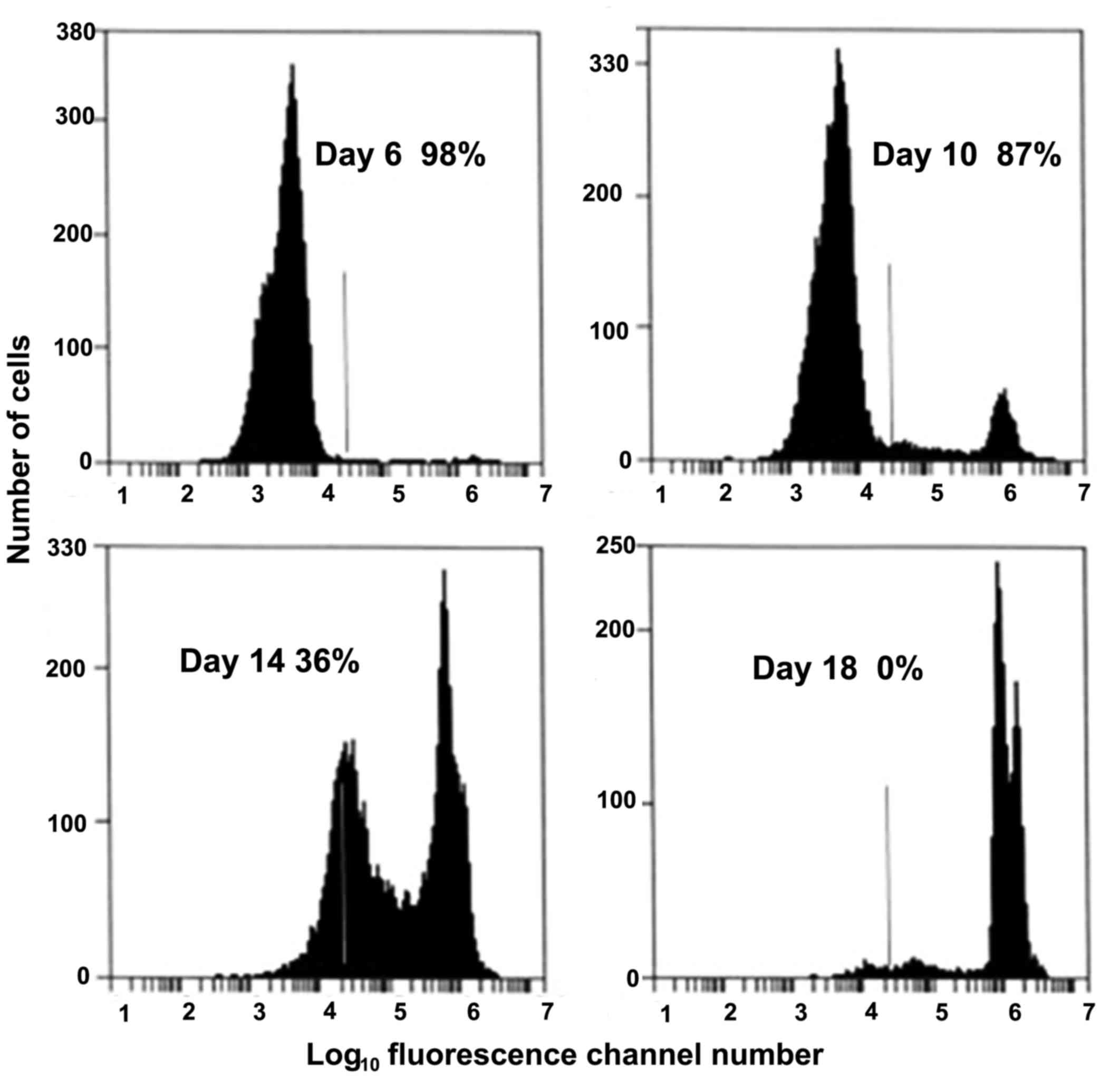
cells (95–97%) as seen as an example in the first frame of Fig. 1. Any fluorescent cells to the right
of the baseline comprised the PI stained, dead cell population. The
percentage viable cells were determined by dividing the number
cells in the viable fluorescent cell population as established by
the control cells divided number of viable + nonviable cells and
multiplying by 100. Fig. 2 showed
the decrease in the percentage of viable cells as the K562 cancer
cell cultures progressively die because of nutritional
depletion.
Data analysis
The data collected was then graphed using PSI-Plot
and whose standard procedure for graphing. The data was graphed in
the form of percentage inhibition vs. concentration of each
component. Proof of the quantitative ability of the viability stain
is shown in Figs. 1 and 2. To calculate percentage inhibition, first
the cell counts were multiplied by 200 to account for the 1:1
dilution of adding the PI stain, as well as the volume of the
analyzed sample being 10 µl. Multiplication by 200 resulted in
cells/ml. The values for the viable cells/ml were incorporated into
the equation, % inhibition=(1-X/Y) ×100%, where X was equal to the
cells in a particular well, and Y was the average number of cells
in the control wells. The mean of multiple replicates (4–6) ± the
standard deviation were then determined.
The LD50 values were reported in mg/ml
per well throughout this study. All well volumes were 550 µl, where
500 µl of the tumor cells had 50 µl of the supplements at different
concentrations added to their respective wells in 48-well plates
(Falcon Plastics, Brookings, SD, USA). A 48 h incubation started
with 5×104 cells and ended with about 6–7×104
cells. The initial 48 h incubation allowed the cells to enter
exponential growth. The test supplements at various concentrations
were added in 50 µl to the appropriate wells. Unless noted the
plates were incubated for an additional 48 h, where the control
population grew to 8–10×104 cells/ml.
Results
Cell viability assay
In the process of developing the viability assay
used in this paper, K562 cells were heated at 56°C in 500 µl
aliquots at 5 min intervals up to 20 min. Each sample had 500 µl of
the PI-viability stain added and analyzed by flow cytometry within
30 min at room temperature (22–24°C). In Fig. 1, gating on the K562 cancer cell
forward light scatter and analyzing by red fluorescence channel
(585±20 nm), showed the percentage of viable K562 cells decreased
in a near linear fashion as the cells were exposed to a 56°C water
bath for ≥20 min.
Furthermore as shown in Fig. 2, the viability of expended cells in
long term cultures showed two populations of dead cells: Those
where the cellular membranes were compromised, and a second more PI
intense population where the nuclear membranes were compromised and
intercalation of the PI into double stranded DNA occurred. The
percentage viability was determined from the ratio of the cells in
the viable cell peak (the first one) divided by the total cells
times 100%. The K562 cells starting culture was 1×105
cells/ml. The right of the vertical line was used as the cut-off of
viable (left) and non-viable (right) cells, which was established
for the control cultures (95–97% viable). The control, viable PI
population curve came to a baseline on the left of the curve, which
was the beginning of the viable cell population fluorescent
staining. The dead cells were to the right of the vertical line. At
Day 6, the cells were 98% viable, with up to 1.5–2.0×106
cells/ml. At Day 10, dead cells were detected in the positive PI
fluorescence portion of the histogram. By Days 14–18, two distinct
populations of dead cells were seen, which showed the cells where
the cell membrane was compromised (peak to the left of the vertical
line) and cells where the nuclear membrane was compromised (peak to
the right of the vertical line). This showed the PI had
intercalated into the double-stranded nucleic acids, causing a very
large increase in fluorescent yield. At Day 18, all of the cells
were dead. There is over a 1,000-fold increase in fluorescence,
which is indicative of the high fluorescence yield due to the
intercalation of the Pl into the double stained nucleic acids.
The enhanced anticancer effects of
encapsulating Bilberry in the NNS
The Bilberry NNS Measurements showed a greatly
enhanced potent activity when these compounds were encapsulated in
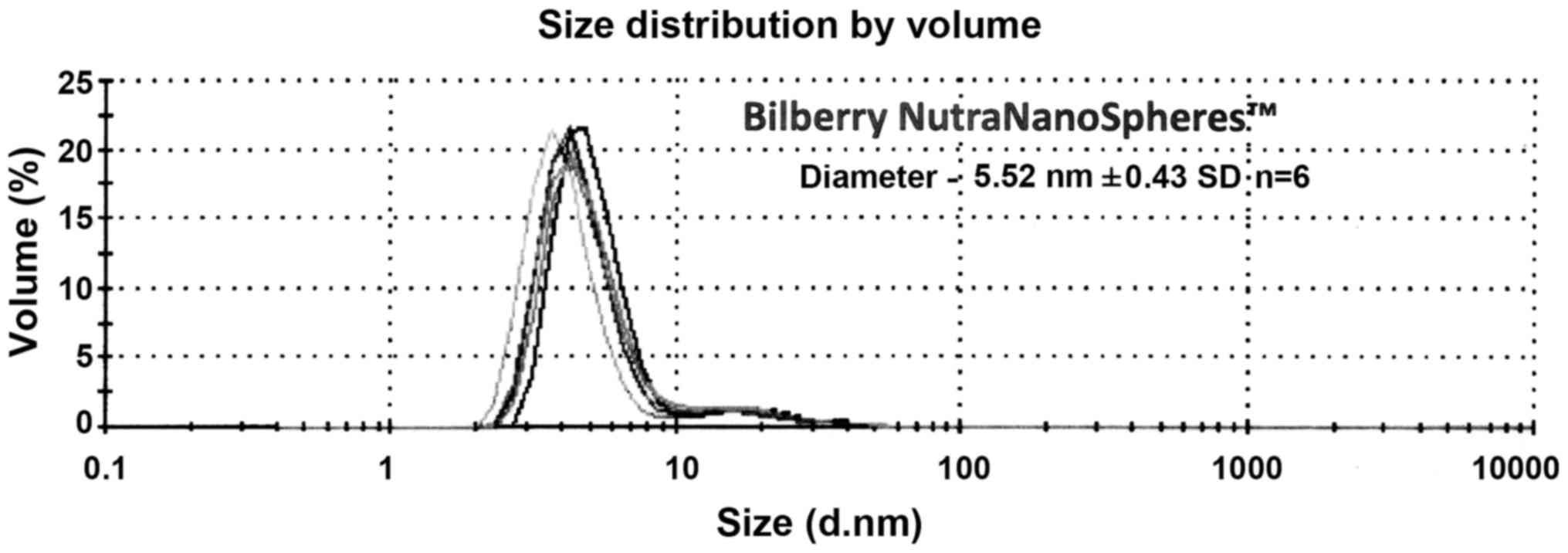
the liposomal structure. Fig. 3
shows the size distributions for the Bilberry NNS. The samples were
diluted by volume in a ratio of 1:6 with DI Water and filtered by a
0.45 µm Nylon membrane to remove any dust contaminants. These
samples were run on the Malvern Zetasizer ZSP with a backscattering
angle of 173 degrees to measure the particle size by dynamic light
scattering. A non-negative least squares algorithm was used to
generate the size distribution by intensity.
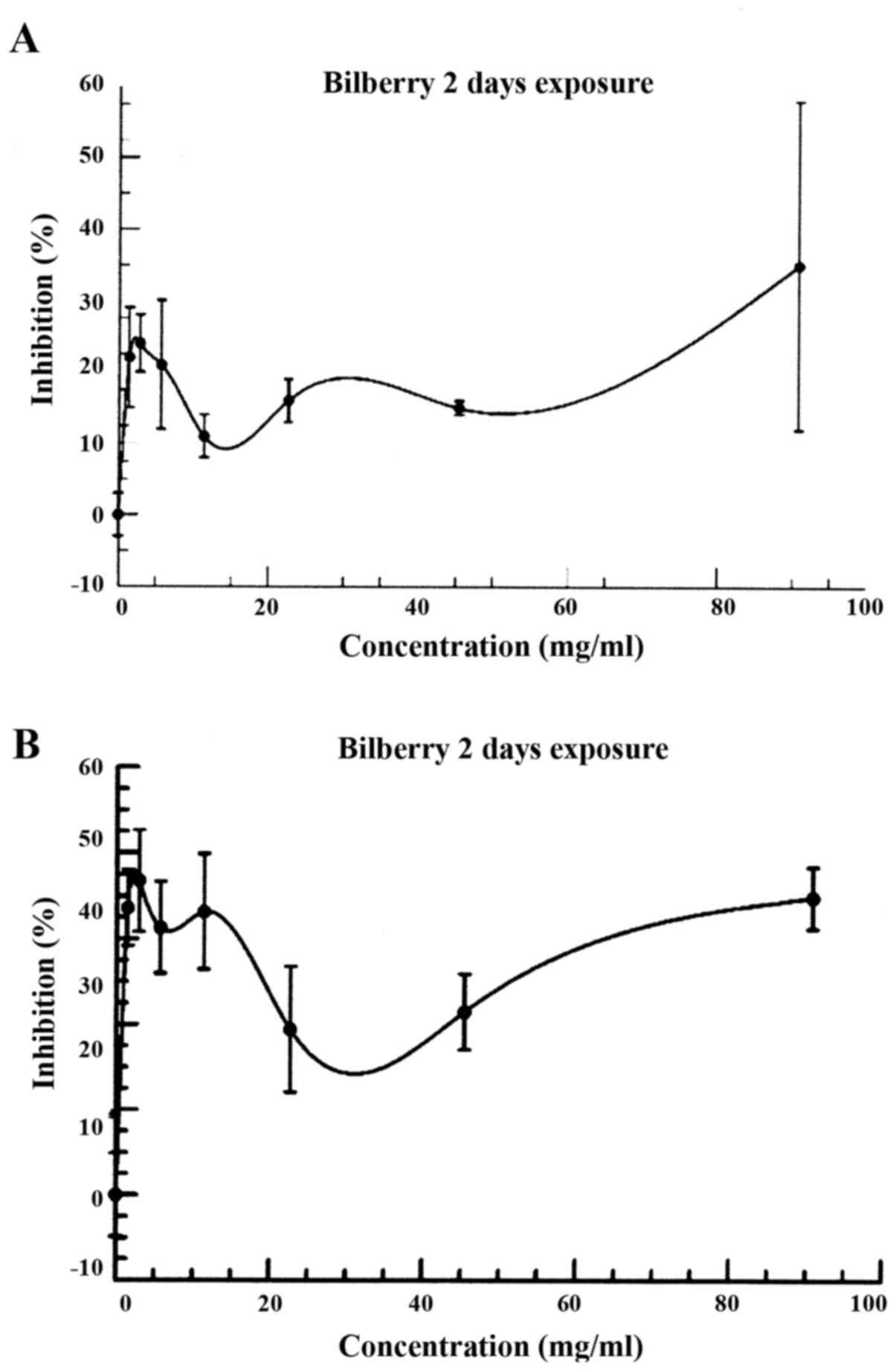
In Fig. 4, the
maximum inhibition results occurred after an exposure for 3 days
(72 h) as compared to 2 days (48 h), and a subsequent decrease by
day 4 (data not shown).
By Day 3, the percentage inhibition curve between 0
and 4.54 mg/ml showed two, possibly three regions of inhibition
about .25, .75, and 4.54 mg/ml. A dramatic increase in the
inhibition of the cancer cells was seen between Day 2 and 3. In
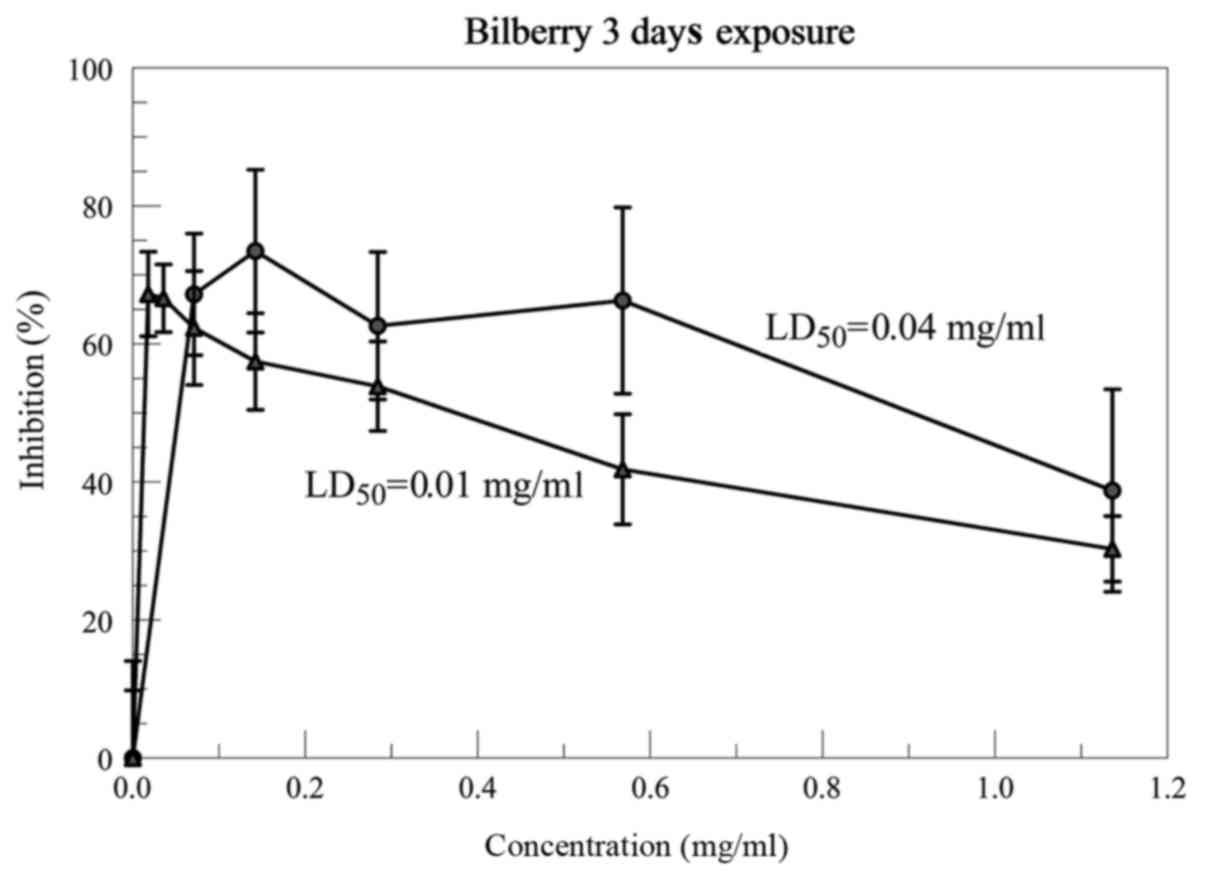
Fig. 5, the 0.25 mg/ml inhibition
region was expanded from 0.01–1.14 mg/ml for the Bilberry NNS. The
LD50 in two experiments were 0.01 and 0.04 mg/ml. The
published data shows the LD50 is ~0.3–0.4 mg/ml at
Bilberry concentrations of 50 mg/ml for the stock solution
(5). This shows an increase in the
cytotoxicity of the Bilberry NNS of 8–40 times the published free
bilberry.
Discussion
The most promising anticarcinogenic agents in plants
are phenolic compounds, which are abundantly present in Bilberries
(Vaccinium myrtillus), and a variety of others including
lingonberry (Vacciniumvitis-idaea), and cloudberry (Rubus
chamaemorus) (3,6–8).
Anthocyanins are hydrophilic compounds, predictably unable to cross
the cell plasma membrane by passive diffusion (9). Therefore, without a hydrophilic
carrier, such as the NNS, which also have a hydrophobic, fatty acid
component that ‘melts’ into cell membranes to deposit the Bilberry
into the cancer cells, one could understand the poor
bioavailability of the anthocyanins (10,11). The
results obtained show that the bilberry extract does have an
increased anti-cancer effect when it is encapsulated compared to
the free compound. Experiments were performed to determine if there
was an effect, first of all, and then when that effect was
strongest. As shown in Fig. 4, the
inhibition at 72 h was much more pronounced than 48 h. However, at
96 h, the results were similar to the 24 h percentage inhibition
curve due to, in part, the metabolic destruction of the
anthocyanins and cell culture nutritional depletion.
Besides its role in fighting cancer, Bilberry is an
effective support against type 2 Diabetes (12–15),
vision improvement (16–18), obesity (19–21),
cancer prevention (22–24), ulcerative colitis (25), anti-inflammatory (26,27),
antioxidant (28,29), and gingival inflammation (30).
In conclusion, the direct cytotoxic effects of the
NNS Bilberry showed LD50 levels 8–40 times lower than
what is required for the Bilberry that is not encapsulated. The
increase in bioavailability with the Bilberry NNS and its water
solubility show the feasibility of using Bilberry NNS in cancer
patient clinical trials. Furthermore, the NNS Bilberry can be used
as a preventive supplement that is freely water soluble, consisting
of a micelle formulation that is 5.5 nm in diameter with a
well-defined dosage per NNS. The stability of this NNS preparation
enables its combination with other NNS supplements by simply adding
drops together in a beverage. Some of the NNS preparations that
have been successfully micellized using the NNS methodology include
Vitamins (C, B12, D3, E), CQ10, Curcumin,
Artemisinin, Frankincense, Riboside Nucleotide, Acemanan, among
others. Finally, all of our NNS preparations survive the stomach
and intestines and fuse with the intestinal cell walls and
deposited into the bloodstream with a bioavailability exceeding
90%.
Acknowledgements
The present study was conducted at the Cancer
Research Institute of West Tennessee, under the direction of Dr
Jerry Thornthwaite, who provided all of the materials and equipment
used. This research was supported in part by generous donations
from Mr. Henry Respess, The Shumard Foundation and the Carter
Family Trust. We thank Dr Carrie Schindler for the Malvern
Zetasizer Nano System analyses of the sizing of the
NutraNanoSpheres. We thank Dr Tony Kirk, and Bonita Thornthwaite
for reviewing this manuscript. Special thanks goes to Dr Lothar
Haegele of X-Labs (Singapore) for supplying the curcumin and
vitamin C NutraNanoSpheres™ preparations for this study.
References
|
1
|
Thomasset S, Berry DP, Cai H, West K,
Marczylo TH, Marsden D, Brown K, Dennison A, Garcea G, Miller A, et
al: Pilot study of oral anthocyanins for colorectal cancer
chemoprevention. Cancer Prev Res (Phila). 2:625–633. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sehitoglu MH, Farooqi AA, Qureshi MZ, Butt
G and Aras A: Anthocyanins: Targeting of signaling networks in
cancer cells. Asian Pac J Cancer Prev. 15:2379–2381. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alhosin M, León-González AJ, Dandache I,
Lelay A, Rashid SK, Kevers C, Pincemail J, Fornecker LM, Mauvieux
L, Herbrecht R and Schini-Kerth VB: Bilberry extract (Antho 50)
selectively induces redox-sensitive caspase 3-related apoptosis in
chronic lymphocytic leukemia cells by targeting the Bcl-2/Bad
pathway. Sci Rep. 5:89962015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kausar H, Jeyabalan J, Aqil F, Chabba D,
Sidana J, Singh IP and Gupta RC: Berry anthocyanidins
synergistically suppress growth and invasive potential of human
non-small-cell lung cancer cells. Cancer Lett. 325:54–62. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nguyen V, Tang J, Oroudjev E, Lee CJ,
Marasigan C, Wilson L and Ayoub G: Cytotoxic effects of bilberry
extract on MCF7-GFP-tubulin breast cancer cells. J Med Food.
13:278–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Misikangas M, Pajari AM, Päivärinta E,
Oikarinen SI, Rajakangas J, Marttinen M, Tanayama H, Törrönen R and
Mutanen M: Three nordic berries inhibit intestinal tumorigenesis in
Min/+ mice by modulating beta-catenin signaling in the tumorand
transcription in the mucosa1. J Nutr. 137:2285–2290.
2007.PubMed/NCBI
|
|
7
|
Mutanen M, Pajari AM, Paivarinta E,
Misikangas M, Rajakangas J, Marttinen M and Oikarinen S: Berries as
chemopreventive dietary constituents-a mechanistic approach with
the ApcMin/+ mouse. Asia Pac J Clin Nutr. 17 Suppl:S123–S125.
2008.
|
|
8
|
Wang Y, Zhao L, Lu F, Yang X, Deng Q, Ji B
and Huang F: Retinoprotective effects of bilberry anthocyanins via
antioxidant, anti-inflammatory and anti-apoptotic mechanisms in a
visible light-induced retinal degeneration model in pigmented
rabbits. Molecules. 20:22395–22410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walton MC, McGhie TK, Reynolds GW and
Hendriks WH: The flavonol quercetin-3-glucoside inhibits
cyanidin-3-glucoside absorption in vitro. J Agric Food Chem.
54:4913–4920. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakakibara H, Ichikawa Y, Tajima S, Makino
Y, Wakasugi Y, Shimoi K, Kobayashi S, Kumazawa S and Goda T:
Practical application of flavonoid-poor menu meals to the study of
the bioavailability of bilberry anthocyanins in human subjects.
Biosci Biotechnol Biochem. 78:1748–1752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Walle T: Methylation of dietary flavones
increases their metabolic stability and chemopreventive effects.
Int J Mol Sci. 10:5002–5019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim J, Kim CS, Lee YM, Sohn E, Jo K and
Kim JS: Vaccinium myrtillus extract prevents or delays the onset of
diabetes-induced blood-retinal barrier breakdown. Int J Food Sci
Nutr. 66:236–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh S, Netticadan T and Ramdath DD:
Expression of cardiac insulin signaling genes and proteins in rats
fed a high-sucrose diet: Effect of bilberry anthocyanin extract.
Genes Nutr. 11:82016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moshetova LK, Vorob'eva IV, Alekseev IB
and Mikhaleva LG: (Results of the use of antioxidant and
angioprotective agents in type 2 diabetes patients with diabetic
retinopathy and age-related macular degeneration). Vestn Oftalmol.
131:34–44. 2015.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asgary S, RafieianKopaei M, Sahebkar A,
Shamsi F and Goli-malekabadi N: Anti-hyperglycemic and
anti-hyperlipidemic effects of Vaccinium myrtillus fruit in
experimentally induced diabetes (antidiabetic effect of Vaccinium
myrtillus fruit). J Sci Food Agric. 96:764–768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng YP, Ke CY, Kuo CC and Lee YJ: Effect
of a complex lutein formula in an animal model for light-induced
retinal degeneration. Chin J Physiol. 59:202–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vorob'eva IV and Vorob'eva IV: Current
data on the role of anthocyanosides and flavonoids in the treatment
of eye diseases. Vestn Oftalmol. 131:104–110. 2015. View Article : Google Scholar
|
|
18
|
Ozawa Y, Kawashima M, Inoue S, Inagaki E,
Suzuki A, Ooe E, Kobayashi S and Tsubota K: Bilberry extract
supplementation for preventing eye fatigue in video display
terminal workers. J Nutr Health Aging. 19:548–554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van der Heijden RA, Morrison MC, Sheedfar
F, Mulder P, Schreurs M, Hommelberg PP, Hofker MH, Schalkwijk C,
Kleemann R, Tietge UJ, et al: Effects of anthocyanin and flavanol
compounds on lipid metabolism and adipose tissue associated
systemic inflammation in diet-induced obesity. Mediators Inflamm.
2016:20421072016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi A, Shimizu H, Okazaki Y,
Sakaguchi H, Taira T, Suzuki T and Chiji H: Anthocyanin-rich
phytochemicals from aronia fruits inhibit visceral fat accumulation
and hyperglycemia in high-fat diet-induced dietary obese rats. J
Oleo Sci. 64:1243–1250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taira T, Yamaguchi S, Takahashi A, Okazaki
Y, Yamaguchi A, Sakaguchi H and Chiji H: Dietary polyphenols
increase fecal mucin and immunoglobulin A and ameliorate the
disturbance in gut microbiota caused by a high fat diet. J Clin
Biochem Nutr. 57:212–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou DX: Potential mechanisms of cancer
chemoprevention by anthocyanins. Curr Mol Med. 3:149–159. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Popović D, Đukić D, Katić V, Jović Z,
Jović M, Lalić J, Golubović I, Stojanović S, Ulrih NP, Stanković M
and Sokolović D: Antioxidant and proapoptotic effects of
anthocyanins from bilberry extract in rats exposed to hepatotoxic
effects of carbon tetrachloride. Life Sci. 157:168–177. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aqil F, Jeyabalan J, Munagala R, Singh IP
and Gupta RC: Prevention of hormonal breast cancer by dietary
jamun. Mol Nutr Food Res. 60:1470–1481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roth S, Spalinger MR, Gottier C,
Biedermann L, Zeitz J, Lang S, Weber A, Rogler G and Scharl M:
Bilberry-derived anthocyanins modulate cytokine expression inthe
intestine of patients with ulcerative colitis. PLoS One.
11:e01548172016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nardi GM, Farias Januario AG, Freire CG,
Megiolaro F, Schneider K, Perazzoli MR, Do Nascimento SR, Gon AC,
Mariano LN, Wagner G, et al: Anti-inflammatory activity of berry
fruits in mice model of inflammation is basedon oxidative stress
modulation. Pharmacognosy Res. 8 Suppl 1:S42–S49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Colak N, Torun H, Gruz J, Strnad M,
Hermosín-Gutiérrez I, Hayirlioglu-Ayaz S and Ayaz FA: Bog bilberry
phenolics, antioxidant capacity and nutrient profile. Food Chem.
201:339–349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jakesevic M, Aaby K, Borge GI, Jeppsson B,
Ahrné S and Molin G: Antioxidative protection of dietary bilberry,
chokeberry and Lactobacillus plantarum HEAL19 in mice subjected to
intestinal oxidative stress by ischemia-reperfusion. BMC Complement
Altern Med. 11:82011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bornsek SM, Ziberna L, Polak T, Vanzo A,
Ulrih NP, Abram V, Tramer F and Passamonti S: Bilberry and
blueberry anthocyanins act as powerful intracellular antioxidants
in mammalian cells. Food Chem. 134:1878–1884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Widén C, Coleman M, Critén S,
Karlgren-Andersson P, Renvert S and Persson GR: Consumption of
bilberries controls gingival inflammation. Int J Mol Sci.
16:10665–10673. 2015. View Article : Google Scholar : PubMed/NCBI
|