Introduction
Osseous choristomas are rare benign lesions
characterized by ectopic bone formation in the soft tissue of the
head and neck region (1,2). They occur more frequently in the
lingual region, and there is a variety of the differential
diagnosis including the lesion of hard tissue in soft tissue
(3–7). Dermoscopy is a non-invasive diagnostic
method that allows an in vivo assessment of morphologic
features, which are not visible to the naked eye (8). This method can be regarded as a link
between clinical and histopathologic examination, and is
increasingly used in general dermatology (8). Vascular structures, color variegation,
follicular abnormalities, and specific features are the main
criteria to be considered (9).
Dermoscopy is a valuable tool that improves diagnostic accuracy
(9). It is commonly used for the
evaluation of pigmented skin lesions (10,11), and
also could be used for the evaluation of calcification under the
skin (12–14). In this paper, we report a case of
osseous choristoma arising in the tongue. We also examine the
dermoscopic features of osseous choristoma from surgical specimen
and evaluate its usefulness for the diagnosis of osseous
choristoma. Furthermore, we review the relevant literature and
discuss the pathophysiological mechanism responsible for
ossification in soft tissue.
Case report
A 7-year-old Japanese boy was referred in August
2012 to the Department of Dentistry and Oral Surgery, University of
Fukui Hospital for an evaluation of a mass in the tongue. The
patient's medical history revealed the presence of autism. Physical
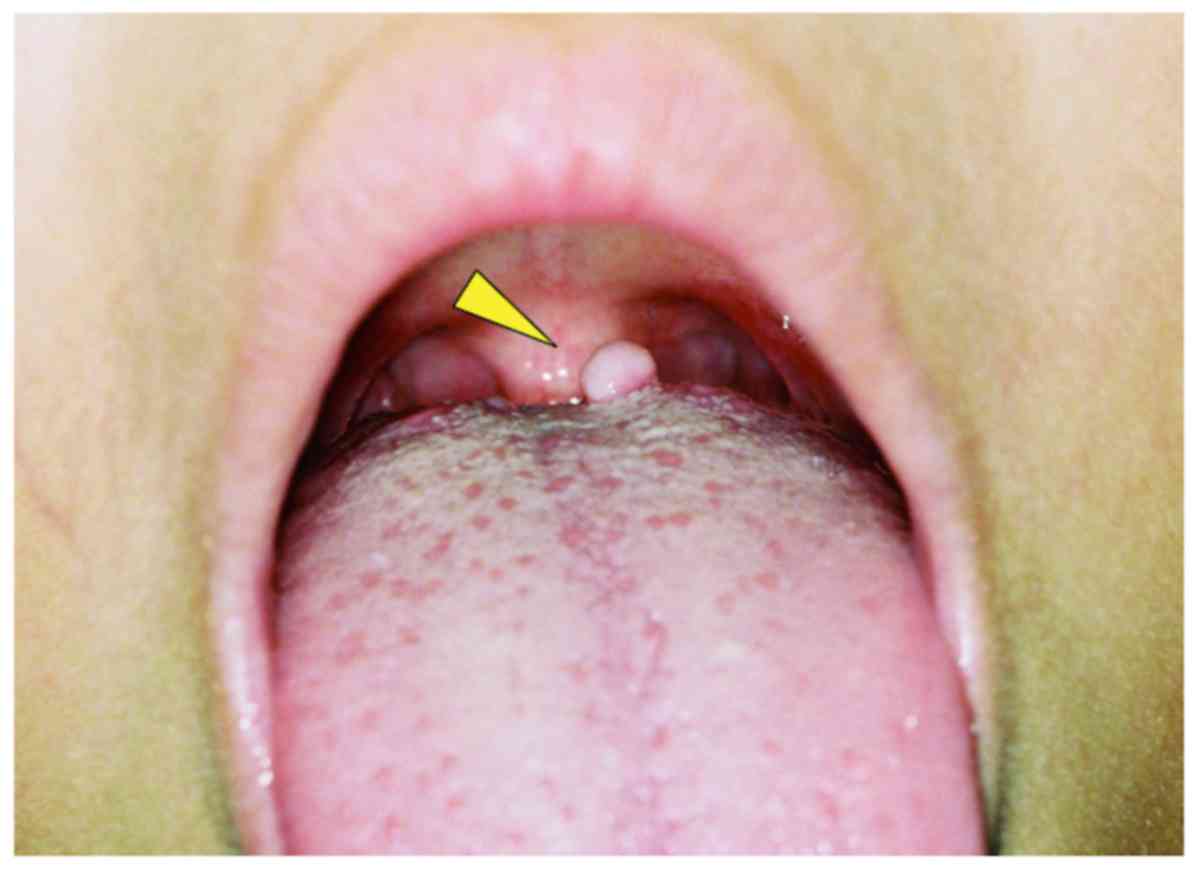
examination revealed a non-tender pedunculated mass covered with
normal mucosa in the posterior portion of the tongue (Fig. 1). The lesion was approximately 5 mm
in diameter. The mass was movable, and there was no evidence of
adhesion to the surrounding tissues. The patient had no history of
inflammation or trauma in the region. Sensory disturbance was not
evident. Magnetic resonance imaging (MRI) revealed a 5 mm,
well-circumscribed mass in the tongue region (Fig. 2). The mass exhibited homogeneous low
signal intensity on T1- and T2-weighted images. MRI also exhibited
a normal thyroid gland in shape and position. The clinical
diagnosis was a benign tumor in the tongue. The patient did not
complain of any symptoms and was placed under observation. The
lesion was slightly enlarged during 2 years of follow-up. Then, in
August 2014, the lesion was removed completely with the adjacent
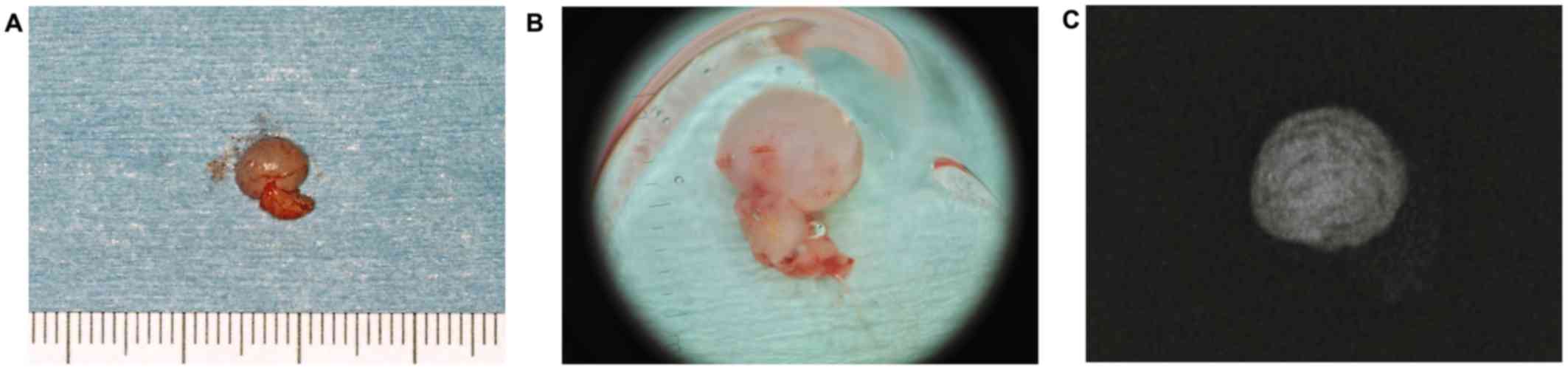
normal tissue under general anesthesia. The surgical specimen was
sized at 6 mm (Fig. 3A). Dermoscopy
of the surgical specimen revealed a hypovascular and homogeneous
pattern of the lesion with round extruded whitish material
(Fig. 3B). Based on dermoscopic
findings, the presence of calcified hard tissue in submucosa was
revealed by our dermatologist. Radiographic examination of the
surgical specimen showed the lesion containing a radiopaque
trabeculated mass (Fig. 3C).
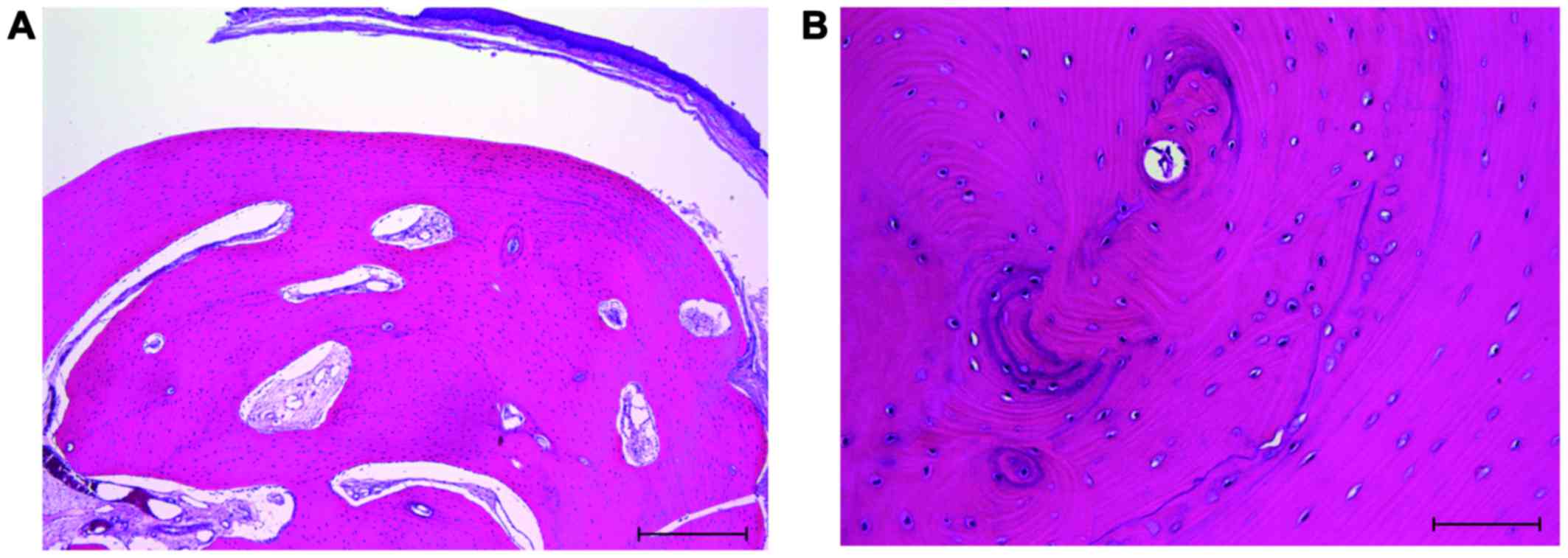
Histological examination revealed that the mass consisted of
well-circumscribed bone, and it was surrounded by stratified
squamous epithelium (Fig. 4A). The
bone tissue had lamellar structures. Osteoblasts and osteocytes
were found on the bone surface and in the bone lacunae, but
osteoclasts were not observed (Fig.
4B). There was no evidence of inflammation or malignancy in the
specimen. No thyroid tissue was found. The histological diagnosis
of an osseous choristoma was made after consideration of the
ectopic bony tissues that were localized away from the
maxillo-mandibular bone (3). The
postoperative course was uneventful. No signs of recurrence were
found during the 36 months of the follow-up examination. The
patient provided informed consent for the use of the data in this
study.
Discussion
The term of ‘choristoma’ defines a tumor-like lesion
that is composed of normal tissue in an abnormal region (1,2).
Choristomas are termed according to the tissues from which they are
derived; they have occurred from osseous, cartilaginous, lingual
thyroid, salivary gland, glial, gastric mucosal tissues and other
tissue (2). The term ‘osseous
choristoma’ was used by Krolls et al to define soft tissue
osteomas in the head and neck region (3).
To date, ninety-seven cases of osseous choristomas
of the oral and maxillofacial region have been reported in the
English-language literature. In our literature review, the mean
patient age was 32 years, ranging from 5 to 86 years. More than 70%
of these lesions have been reported in women. The size of the
lesions ranged from 5 to 50 mm in diameter. The location and
frequency of the lesions were as follows; tongue (76 cases: 78%),
buccal mucosa (14 cases: 15%), alveolar mucosa (2 cases: 2%),
submandibular region (2 cases: 2%), submental region (1 case: 1%),
masseter muscle (1 case: 1%), and hard plate (1 case: 1%). In the
case of the tongue, the most frequent affected region is the
posterior third of the tongue dorsum near the foramen caecum and
circumvallate papillae. A symptoms of a lump, dysphagia, pain,
gagging and nausea have been reported.
In clinical findings, osseous choristomas are
observed as either a sessile or a pedunculated mass (2). The clinical differential diagnosis
includes other tumor-like lesions, such as salivary gland tumors,
fibromas, lipomas, neural tumors, giant cell tumors, and soft
tissue cysts (4,5). If soft tissue calcification and bone
formation are observed, the differential diagnosis should include
teratomas, osteomas, calcified lymph nodes, osseous hamartoma,
calcified hamartomas and osteolipomas (6,7). When
the lesion is located close to the foramen caecum, the lingual
thyroid nodule should be considered. Osseous choristomas may also
occur in many pathological conditions, such as hyperparathyroidism
and skin inflammation (3).
The dermoscopy significantly improves the diagnostic
accuracy of skin lesions (14), and
in consequence, dermoscopy is now an integral part of the clinical
skin examination (13). Compared to
radiological and histological examinations, dermoscopy can detect
even small lesions without invasion or side effects, and it might
contribute to earlier treatment. This method is also effective for
evaluating the borders of pathologic lesions. This in turn might
improve the treatment outcomes. Dermoscopy could also be used for
the evaluation of calcification under the skin (12–14).
Recently, the usefulness of this technique for the evaluation of
oral soft tissue lesions, including those on the tongue, has been
reported (15–18). To identify the characteristics of the
lesion, we performed a dermoscopic study. In our case, dermoscopic
examination could not be performed before resection because the
instrument could not reach the lesion directly. The resected
specimen was evaluated, and a hypovascular and homogeneous pattern
of the lesion with round extruded whitish material was observed.
The feature was consistent with the previously described
dermoscopic pattern for calcification of skin lesion (12–14).
Based on dermoscopic findings, the presence of calcified hard
tissue in the submucosa was suggested by our dermatologist.
Actually, the calcified hard tissue was confirmed by radiographic
finding, and whitish structure in dermoscopic finding was
histopatologically corresponded to osseous choristoma. As far as we
know, this is the first report of dermoscopic evaluation of oral
calcified lesion in the soft tissue, and the usefulness of
dermoscopy could be confirmed. If lesions existed in the anterior
oral portion, dermoscopy might be beneficial in establishing
preoperative diagnosis of osseous choristoma. We have shown the
dermoscopic features of osseous choristoma, but this is a case
report and dermoscopic criteria of osseous choristoma has not been
established yet. Intraoral dermoscopy is still less investigated
and less popular among clinicians. There is a need to intensify
examination, which would result in creating clear-cut dermoscopic
criteria including other than this lesions. Dermoscopy might be
utilized as an adjunctive device, but a limiting factor is the
accessibility to the lesion. Previous reports of dermoscopical
evaluation were mostly performed in the lesion of tongue or lip.
The future technology would focus on developing a miniaturized,
flexible dermoscope that will allow detailed examination of the
whole oral cavity.
MRI is also one of the useful imaging modalities in
the head and neck region. To the best of our knowledge, only four
cases of osseous choristomas that were evaluated with MRI have been
reported (6,19–21). Lee
et al (19) indicated that
low signal intensity on both T1- and T2-weighted images without
enhancement with contrast medium reflects the ossific and calcific
nature of the lesion. If the existence of hard tissue is suspected,
X-ray examination and computed tomography (CT) are also useful, as
their results can yield further diagnostic confidence (19). In our case, CT examination was not
performed in consideration of the effect of radiation exposure.
However, X-ray examination of the surgical specimen could show the
radiopaque trabeculated mass in the lesion.
Histologically, the lesions are generally
constituted by a well-circumscribed, lamellated mass of dense,
vital bone with Haversian canals, circumscribed the fibrous
connective tissue (2). Osteocytes
are observed in the lacunae within the bony spherules (2). Occasionaly, the mass may contain blood
vessels lined with fibrous tissue or endothelium and several cells
that are similar to the osteoclasts (3). In our case, the lesion contained
well-circumscribed bone, and it was surrounded by stratified
squamous epithelium. Osteoblasts and osteocytes were observed on
the outer surface of bony trabeculae and in the lacunae of the
formed bone, respectively. Nevertheless, osteoclasts were not found
in the formed bone. In our case, a slight increase of the size was
observed during 2 years of follow-up. These results indicate that
bone formation slowly occurred in the lesion.
The pathogenesis of osseous choristomas remains
controversial. Various reports have suggested that the lesions
originate via the ossification of the branchial arch remnants
(22), epignathus formation and
degenerating ossifying fibroma (23), and metabolic alterations in
pluripotent mesenchymal cells as a response to an unknown stimulus
(24). In our previous report,
immunohistochemical examination of BMP-2 and −4 was performed to
investigate the molecular mediators of ectopic bone formation and
presented the evidence of the expression of BMP-2 and −4 in an
osseous choristoma (20). BMPs
belong to the transforming growth factor (TGF)-β superfamily and
are essentially involved in embryogenesis, skeletal formation,
hematopoiesis and neurogenesis (25). These proteins promote differentiation
of mesenchymal cells into osteoblasts and chondorocytes and play
critical roles in bone development and metabolism (25). Notably, BMP-2 and −4 have been
reported to play an important role in ectopic bone formation in an
experimental animal model and clinical research (25–28). Our
previous report indicates that the expression of BMP-2 and −4 is
associated with the ossification of osseous choristomas. Thus,
there is a possible mechanism of ectopic bone formation; BMPs
secreted from the lesion may trigger ectopic ossification in the
soft tissue by stimulating pluripotent mesenchymal progenitor cells
to differentiate into bone-forming cells.
Osseous choristomas are best treated by local
surgical excision (1). In our case,
no evidence of recurrence has appeared at 36 months
postoperatively.
Acknowledgements
The authors wish to thank Dr. Wataru Takashima,
Department of Dermatology, Unit of Sensory and Locomotor Medicine,
Division of Medicine, Faculty of Medical Sciences, University of
Fukui, for his support in dermoscopic diagnosis, and Dr. Minako
Shimada, Department of Dentistry and Oral Surgery, Unit of Sensory
and Locomotor Medicine, Division of Medicine, Faculty of Medical
Sciences, University of Fukui, Fukui, for her help in the
treatment.
References
|
1
|
Neville BW, Damm DD, Allen CM and Bouquot
JE: Oral and Maxillofacial Pathology. 3rd. Saunders Elsevier; St.
Louis: pp. 5522009
|
|
2
|
Chou LS, Hansen LS and Daniels TE:
Choristomas of the oral cavity: A review. Oral Surg Oral Med Oral
Pathol. 72:584–593. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krolls SO, Jacoway JR and Alexander WN:
Osseous choristomas (osteomas) of intraoral soft tissues. Oral Surg
Oral Med Oral Pathol. 32:588–595. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tohill MJ, Green JG and Cohen DM:
Intraoral osseous and cartilaginous choristomas: Report of three
cases and review of the literature. Oral Surg Oral Med Oral Pathol.
63:506–510. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Psimopoulou M and Antoniades K: Submental
osseous choristoma: A case report. J Oral Maxillofac Surg.
56:666–667. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dalkiz M, Hakan Yurdakul R, Pakdemirli E
and Beydemir B: Recurrent osseous choristoma of the masseter
muscle: Case report. J Oral Maxillofac Surg. 59:836–839. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamamoto N, Ishikawa A, Yamauchi K,
Miyamoto I, Tanaka T, Kito S, Matsuo K, Yamashita Y, Morimoto Y and
Takahashi T: Osteolipoma of the lower lip: A case report. Asian J
Oral Maxillofac Surg. 23:143–145. 2011. View Article : Google Scholar
|
|
8
|
Russo T, Piccolo V, Lallas A and
Argenziano G: Recent advances in dermoscopy. F1000Res.
5:1842016.
|
|
9
|
Lallas A, Zalaudek I, Argenziano G, Longo
C, Moscarella E, Di Lernia V, Al Jalbout S and Apalla Z: Dermoscopy
in general dermatology. Dermatol Clin. 31:679–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Giorgi V, Massi D and Carli P:
Dermoscopy in the management of pigmented lesions of the oral
mucosa. Oral Oncol. 39:534–535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olszewska M, Banka A, Gorska R and
Warszawik O: Dermoscopy of pigmented oral lesions. J Dermatol Case
Rep. 2:43–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Strumia R: Videodermatoscopy: A useful
tool for diagnosing cutaneous dystrophic calcifications. Dermatol
Online J. 11:282005.PubMed/NCBI
|
|
13
|
Lallas A, Moscarella E, Argenziano G,
Longo C, Apalla Z, Ferrara G, Piana S, Rosato S and Zalaudek I:
Dermoscopy of uncommon skin tumours. Australas J Dermatol.
55:53–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zaballos P, Llambrich A, Puig S and
Malvehy J: Dermoscopic findings of pilomatricomas. Dermatology.
217:225–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okamoto T, Sasaki R, Kataoka T, Kumasaka
A, Kaibuchi N, Naganawa T, Fukada K and Ando T: Dermoscopy imaging
findings in the normal Oral Mucosa. Oral Oncol. 51:e69–e70. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Warszawik-Hendzel O, Słowińska M,
Olszewska M and Rudnicka L: Melanoma of the oral cavity:
Pathogenesis, dermoscopy, clinical features, staging and
management. J Dermatol Case Rep. 8:60–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Güleç AT: Dermoscopic features of squamous
cell carcinoma of the tongue: It looks similar to cutaneous
squamous cell carcinoma. J Am Acad Dermatol. 75:e53–e54. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Drogoszewska B, Chomik P, Polcyn A and
Michcik A: Clinical diagnosis of oral erosive lichen planus by
direct oral microscopy. Postepy Dermatol Alergol. 31:222–228. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee DL, Wong KT, Mak SM, Soo G and Tong
MC: Lingual osteoma: Case report and literature review. Arch
Otolaryngol Head Neck Surg. 135:308–310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshimura H, Ohba S, Matsuda S, Kobayashi
J, Ishimaru K, Imamura Y and Sano K: Osseous choristoma of the
buccal mucosa: A case report with immunohistochemical study of bone
morphogenetic protein-2 and −4 and a review of the literature. J
Oral Maxillofac Surg Med Pathol. 26:351–355. 2014. View Article : Google Scholar
|
|
21
|
Yamamoto M, Migita M, Ogane S, Narita M,
Yamamoto N, Takaki T, Matsuzaka K and Shibahara T: Osseous
choristoma in child with strong vomiting reflex. Bull Tokyo Dent
Coll. 55:207–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Monserrat M: Ostéome de la langue. Bull
Soc Anat. 88:282–283. 1913.
|
|
23
|
Church LE: Osteoma of the tongue. Report
of a case. Oral Surg Oral Med Oral Pathol. 17:768–770. 1964.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roy JJ, Klein HZ and Tipton DL:
Osteochondroma of the tongue. Arch Pathol. 89:565–568.
1970.PubMed/NCBI
|
|
25
|
Xiao YT, Xiang LX and Shao JZ: Bone
morphogenetic protein. Biochem Biophys Res Commun. 362:550–553.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bragdon B, Moseychuk O, Saldanha S, King
D, Julian J and Nohe A: Bone morphogenetic proteins: A critical
review. Cell Signal. 23:609–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kusumoto K, Bessho K, Fujimura K, Akioka
J, Ogawa Y and Iizuka T: Comparison of ectopic osteoinduction in
vivo by recombinant human BMP-2 and recombinant Xenopus BMP-4/7
heterodimer. Biochem Biophys Res Commun. 239:575–579. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SY, Choi HY, Myung KB and Choi YW: The
expression of molecular mediators in the idiopathic cutaneous
calcification and ossification. J Cutan Pathol. 35:826–831. 2008.
View Article : Google Scholar : PubMed/NCBI
|