Introduction
The standard and most effective treatment for
thoracic esophageal cancer is currently esophagectomy with extended
three-field lymph node dissection, which eradicates a wide range of
clinically apparent and subclinical lymph node metastases in the
cervical, mediastinal and abdominal fields. Although this
state-of-the art surgical therapy has improved the prognosis of
patients to a certain extent, recurrence occurs in over half of the
patients who undergo curative resection (1,2). This
suggests that systemic micrometastases that cannot be eradicated by
surgery may exist in more than half of patients at the time of
surgery, and that multidisciplinary treatment is necessary for such
patients. The use of neoadjuvant chemotherapy (NAC) has increased
the hope of improvement in prognosis (3–7).
Several investigators have reported that responders
to NAC exhibit a better prognosis compared with non-responders
(8,9). This suggests that NAC may lead to
disease downstaging and increase the curability of subsequent
surgery in responders, whereas it may provide no clinical benefit,
or may even be harmful, to non-responders (5,8).
Although the precise assessment of the efficacy by NAC is crucial
for decision-making regarding subsequent treatment, conventional
imaging modalities, such as computed tomography (CT) and magnetic
resonance imaging (MRI), appear to be unsatisfactory, due to their
limited sensitivity and specificity.
Positron emission tomography with
18F-fluorodeoxyglucose (FDG-PET) is a metabolic imaging
modality that has recently been used for preoperative staging
(10–13) or for assessment of the efficacy of
NAC for esophageal cancer (14–17).
Specifically, combined PET/CT has been reported to be more
effective compared with PET alone in the preoperative diagnosis of
lymph node metastasis from thoracic esophageal cancer (18).
The present study was designed to evaluate the
potential benefits of PET/CT in the preoperative assessment of the
efficacy of NAC and prognostic prediction in patients with
esophageal cancer.
Patients and methods
Patients
Between January, 2007 and December, 2013, a total of
405 patients with thoracic esophageal cancer underwent surgery at
the Osaka Medical Center for Cancer and Cardiovascular Diseases
(Osaka, Japan). Among these, 157 patients were treated with NAC
followed by surgery. Of these 157 patients, 77 fulfilled the
following inclusion criteria: i) New diagnosis and no other
previous anticancer treatment; ii) ≤80 years of age; iii) Eastern
Cooperative Oncology Group performance status scores ≤3; iv) T1-T3;
v) any N (N0-N3); vi) no distant node metastasis or distant organ
metastasis except for supraclavicular nodes (M1LYM); vii)
evaluation by PET/CT both before and after NAC; viii) adequate bone
marrow function (leukocyte count >3,500 cells/mm3,
platelet count >100,000 cells/mm3); xi) normal renal
function (serum creatinine level <1.2 mg/dl or creatinine
clearance >50 ml/dl); and x) normal liver function (serum
transaminases <twice the upper limit of normal). The disease
stage was assigned according to the 7th edition of the Union for
International Cancer Control TNM classification (19). The T and N status of the disease was
diagnosed by chest and abdominal CT scans, esophagography and/or
bronchoscopy. The diagnostic criteria by CT scan for clinically
positive nodes included a round-shaped node measuring ≥10 mm in
diameter. MRI was used in certain cases to improve the accuracy of
the T4 diagnosis. Bronchoscopy was performed when tracheal invasion
was suspected on the basis of the CT scan. The study protocol was
approved by the Human Ethics Review Committee of Osaka Medical
Center for Cancer and Cardiovascular Diseases, and written informed
consent was obtained from each patient prior to inclusion.
Treatment regimen
In 47 patients, NAC consisted of a cisplatin,
adriamycin and 5-fluorouracil (5-FU) combination (FAP); 27 patients
were treated with 5-FU, cisplatin and docetaxel (DCF); the
remaining 3 patients received 5-FU plus cisplatin (FP). For the
administration of FAP, 5-FU was administered intravenously (i.v.)
at 750 mg/m2/day on days 1–7 in a continuous manner;
adriamycin was administered on day 1 at a dose of 30
mg/m2/day by i.v. injection; and cisplatin was
administered on day 1 at 70 mg/m2/day by drip infusion
for 2 h with sufficient pre- and post-treatment hydration to
prevent renal toxicity. For the administration of DCF, 5-FU was
administered i.v. at 700 mg/m2/day on days 1–5 in a
continuous manner, whereas docetaxel (70 mg/m2/day) and
cisplatin (70 mg/m2/day) were administered on day 1. For
the administration of FP, 5-FU (750 mg/m2/day) and
cisplatin (70 mg/m2/day) were administered on days 1–7
and on day 1, respectively. After a 2–3-week interval, the same
regimens were repeated.
Two weeks after completing NAC, the patients were
re-evaluated for their response to the abovementioned treatment
regimens. These examinations included observation of the main tumor
and metastatic lymph nodes by barium study, tissue biopsy obtained
by endoscopy, and chest and abdominal CT scans. The treatment
response was classified using general criteria that have been
previously described (20). Complete
response (CR) was defined as 100% regression of the disease.
Partial response (PR) was defined as regression of >50% of the
tumor and metastatic lymph nodes, as confirmed by esophagography
and CT scans. Progressive disease (PD) was defined as an increase
in the tumor mass and/or metastatic nodes, or the appearance of new
lesion(s). Patients who were not classified as CR, PR or PD were
defined as non-responders (NC). The patients were scheduled for
surgery ~4 weeks after the last day of chemotherapy. Histological
effectiveness was defined as follows: Grade 3, complete
disappearance of cancer cells; grade 2, >2/3 disappearance;
grade 1b, 1/3-2/3 disappearance; and grade 1a, <1/3
disappearance.
PET/CT imaging
All the patients received whole-body
18F-FDG-PET/CT scans prior to NAC. Additional PET scans
were performed 14–21 days after the completion of NAC. PET/CT scans
were performed as previously described (21). Briefly, patients were asked to fast,
except for glucose-free oral hydration, for at least 5 h prior to
the injection of 18F-FDG (3.5 MBq/kg body weight). After
injection of the tracer, the patients remained in a comfortable
position on the bed. Combined PET/CT scanning was performed 1 h
after the injection using either a dual-slice CT Biograph Duo
LSDPET-CT imaging system (Siemens-Asahi Medical Technologies,
Tokyo, Japan) or a 12-slice CT Discovery LS PET-CT imaging system
(Philips Medical Systems Inc., Cleveland, OH, USA) and covering the
area from the top of the brain to the upper thigh. Images were
reconstructed using an iterative procedure with an ordered subset
expectation maximization algorithm.
For the quantitative evaluation of regional
18F-FDG uptake, regions of interest (ROIs) were manually
placed over the primary tumor or the metastatic lymph nodes in
areas devoid of prominent artifacts and overlapping with organs
with increased FDG uptake. If no focal 18F-FDG uptake
was visible in the follow-up examinations, the ROI was placed in
the same location as the previously identified lesion using the
landmarks of the transmission images (apex of the lungs,
bifurcation of the trachea) as a reference. The standardized uptake
value (SUV) was measured for each ROI and was determined using the
whole-body attenuation-corrected image according to the following
equation: SUV=[regional activity (mCi/ml)]/[injected dose
(mCi)/body weight (g)]. SUVmax was adopted for analysis.
The reduction in tumor SUVmax was calculated as follows:
%SUVmax = 100 × (SUVmax after
NAC)/(SUVmax before NAC). When there were >2
PET-positive metastatic nodes, the lesion with the highest
SUVmax was used for response evaluation.
Surgical procedures
All 77 patients underwent subtotal esophagectomy
with two- or three-field lymph node dissection, according to the
procedures described by Akiyama et al (2). Three-field lymph node dissection was
performed for patients with upper or middle thoracic esophageal
cancer and for patients with supraclavicular and/or recurrent
laryngeal nerve node metastases.
Statistical methods
Statistical analyses were performed using StatView
5.0 J software (SAS Institute Japan, Tokyo, Japan). For ordered
categorical data, the Mann-Whitney U test was used for comparisons
among subgroups of patients for each clinicopathological factor.
Student's t-test was used to compare the number of lymph node
metastases. Survival time was calculated by the Kaplan-Meier method
and was statistically compared among patient subgroups by the
log-rank test. A two-sided P<0.05 was considered to indicate
statistically significant differences. All the statistically
significant variables identified in the univariate analysis were
included in the multivariate survival analysis using the Cox's
proportional hazards model.
Results
Patient and tumor characteristics
The clinicopathological characteristics of the 77
patients are summarized in Table I.
A total of 72 patients had squamous cell carcinoma and 67 patients
had clinically apparent lymph node metastases. All 5 cM1 cases were
supraclavicular node metastases. The mean SUVmax values
of the main tumor and the metastatic lymph nodes were 11.3±5.8 and
4.3±2.8, respectively.
 | Table I.Clinicopathological characteristics
of the enrolled patients (n=77). |
Table I.
Clinicopathological characteristics
of the enrolled patients (n=77).
|
Characteristics | Values |
|---|
| Age (years) | 64.7±7.1
(46–77) |
| Sex
(male/female) | 71/6 |
| Tumor location
(Ut/Mt/Lt/Ae) | 5/35/34/3 |
| Histology
(SCC/adeno/basaloid) | 72/4/1 |
| cT
(cT1/cT2/cT3/cT4) | 6/22/49/0 |
| cN
(cN0/cN1/cN2/cN3) | 10/41/25/1 |
| cM (cM0/cM1) | 72/5 |
| cStage
(IB/IIA/IIB/IIIA/IIIB/IIIC/IV) |
5/5/15/32/15/0/5 |
|
Pre-SUVmax-T | 11.3±5.8
(2.3–28.4) |
|
Pre-SUVmax-N | 4.3±2.8
(1.0–13.5) |
| Preoperative
chemotherapy (FAP/DCF/FP) | 47/27/3 |
Clinical and pathological responses to
NAC
Table II shows the
clinical and pathological responses to NAC in the 77 patients. The
clinical response was fairly good, with a major response rate of
72.7% (no CRs and 56 PRs). A pathological response of grade ≥2 was
observed in 20 patients (26.0%). A total of 24 patients (31.7%)
were pathologically node-negative. The %SUVmax of the
main tumors (T) and metastatic lymph nodes (N) was 49.0±35.1 and
67.0±39.6% of the pretreatment values, respectively. The
post-SUVmax-T and post-SUVmax-N were 5.1±4.8
and 2.5±1.9, respectively.
 | Table II.Efficacy of preoperative therapy and
pathological stage. |
Table II.
Efficacy of preoperative therapy and
pathological stage.
| Factors |
|
|---|
| Clinical effects
(CR/PR/NC/PD) | 0/56/18/3 |
| Pathological
effects (grade 3/2/1b/1a) | 5/15/16/41 |
| pT
(pCR/pT1/pT2/pT3/pT4) | 5/22/13/37/0 |
| pN
(pN0/pN1/pN2/pN3) | 24/26/17/10 |
| pM (pM0/pM1) | 71/6 |
| pStage
(pCR/IA/IB/IIA/IIB/IIIA/IIIB/IIIC/IV) |
3/9/3/9/18//14/8/7/6 |
|
%SUVmax-T | 49.0±35.1
(3.5–177.3) |
|
%SUVmax-N | 67.0±39.6
(15.0–190.3) |
|
Post-SUVmax-T | 5.1±4.8
(1.0–27.0) |
|
Post-SUVmax-N | 2.5±1.9
(1.0–10.6) |
Prognostic predictors among
preoperatively available factors
As previously reported by several investigators, we
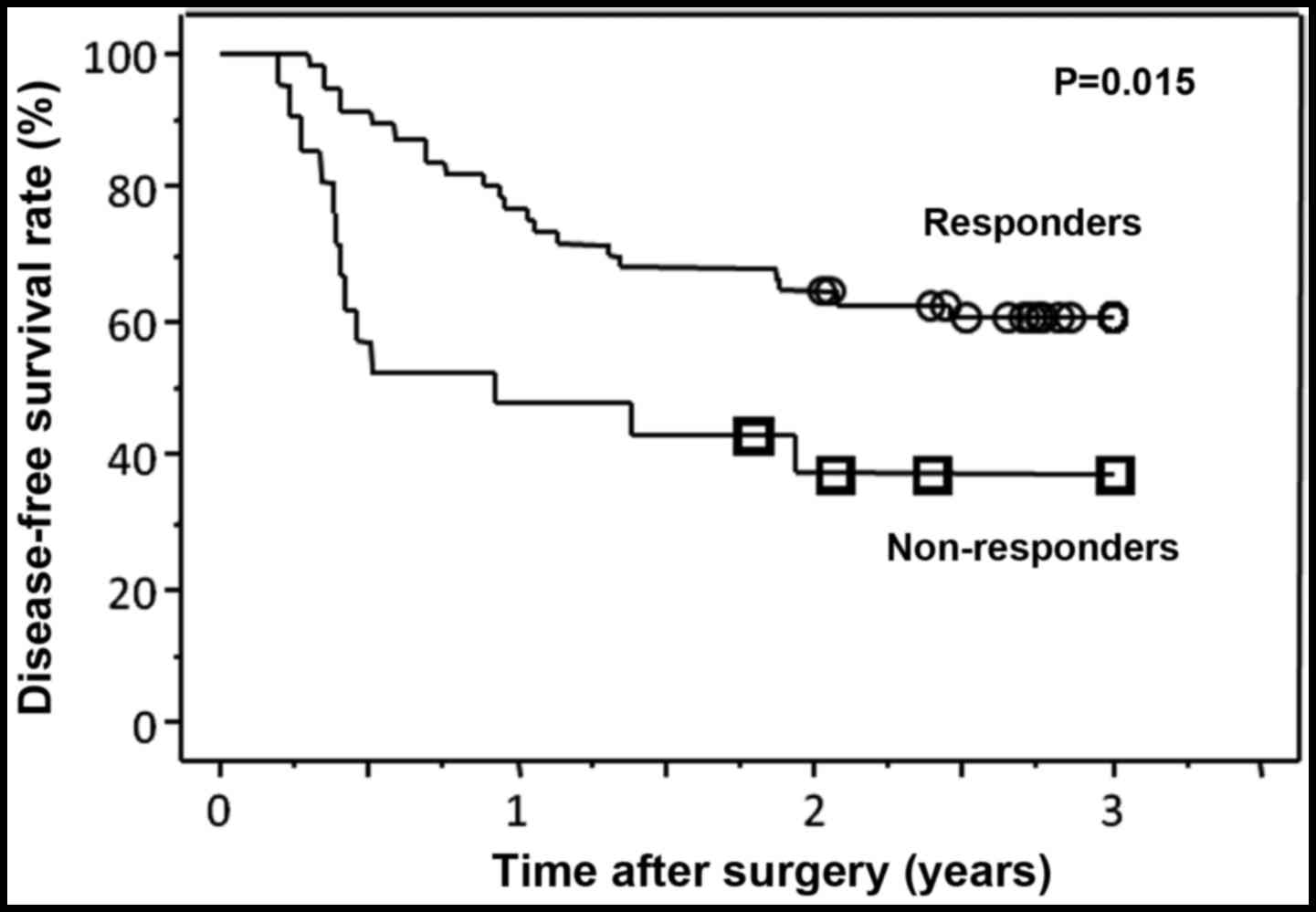
also observed that clinical responders exhibited a significantly
better DFS compared with non-responders (P=0.015, Fig. 1). We next examined which
preoperatively available clinical factors were significantly
associated with DFS by univariate analysis (Table III). Clinical non-responder
(P=0.0178), post-SUVmax-N (P=0.0001) and
%SUVmax-T (P=0.0031) were found to be significant
predictors of poor prognosis. We then incorporated 6 factors with
P-values in the univariate analysis of <0.1 into a multivariate
analysis. As shown in Table IV, the
post-SUVmax-N was identified as the only independently
significant prognostic factor (P=0.0254).
 | Table III.Univariate analysis of preoperative
prognostic factors. |
Table III.
Univariate analysis of preoperative
prognostic factors.
| Variables | HR | 95% CI | P-value |
|---|
| Age | 0.957 | 0.914–1.001 | 0.056 |
| Sex
(male/female) | 0.634 | 0.152–2.645 | 0.532 |
| Tumor location
(Ut/Mt vs. Lt/Ae) | 0.776 | 0.397–1.516 | 0.458 |
| cT (T1-2 vs.
T3) | 0.791 | 0.394–1.592 | 0.512 |
| cN (N0-1 vs.
N2-3) | 1.008 | 0.502–2.027 | 0.982 |
| cM (M0 vs. M1) | 0.646 | 0.198–2.110 | 0.469 |
| cStage (IB-IIB vs.
IIIA-IV) | 0.722 | 0.347–1.505 | 0.385 |
| Clinical response
(responder vs. non-responder) | 2.299 | 1.155–4.576 | 0.0178 |
|
Pre-SUVmax-T | 0.977 | 0.924–1.034 | 0.427 |
|
Pre-SUVmax-N | 1.102 | 0.994–1.221 | 0.064 |
|
Post-SUVmax-T | 1.053 | 0.996–1.114 | 0.071 |
|
Post-SUVmax-N | 1.317 | 1.143–1.516 | 0.0001 |
|
%SUVmax-T | 3.834 | 1.572–9.351 | 0.0031 |
|
%SUVmax-N | 1.623 | 0.709–3.717 | 0.252 |
 | Table IV.Multivariate analysis of preoperative
prognostic factors. |
Table IV.
Multivariate analysis of preoperative
prognostic factors.
| Variables | HR | 95% CI | P-value |
|---|
| Age | 0.970 | 0.928–1.014 | 0.181 |
| Clinical response
(responder vs. non-responder) | 1.331 | 0.592–2.995 | 0.490 |
|
Pre-SUVmax-N | 1.085 | 0.955–1.233 | 0.210 |
|
Post-SUVmax-T | 0.978 | 0.884–1.082 | 0.670 |
|
Post-SUVmax-N | 1.220 | 1.025–1.451 | 0.025 |
|
%SUVmax-T | 3.827 | 0.780–18.777 | 0.098 |
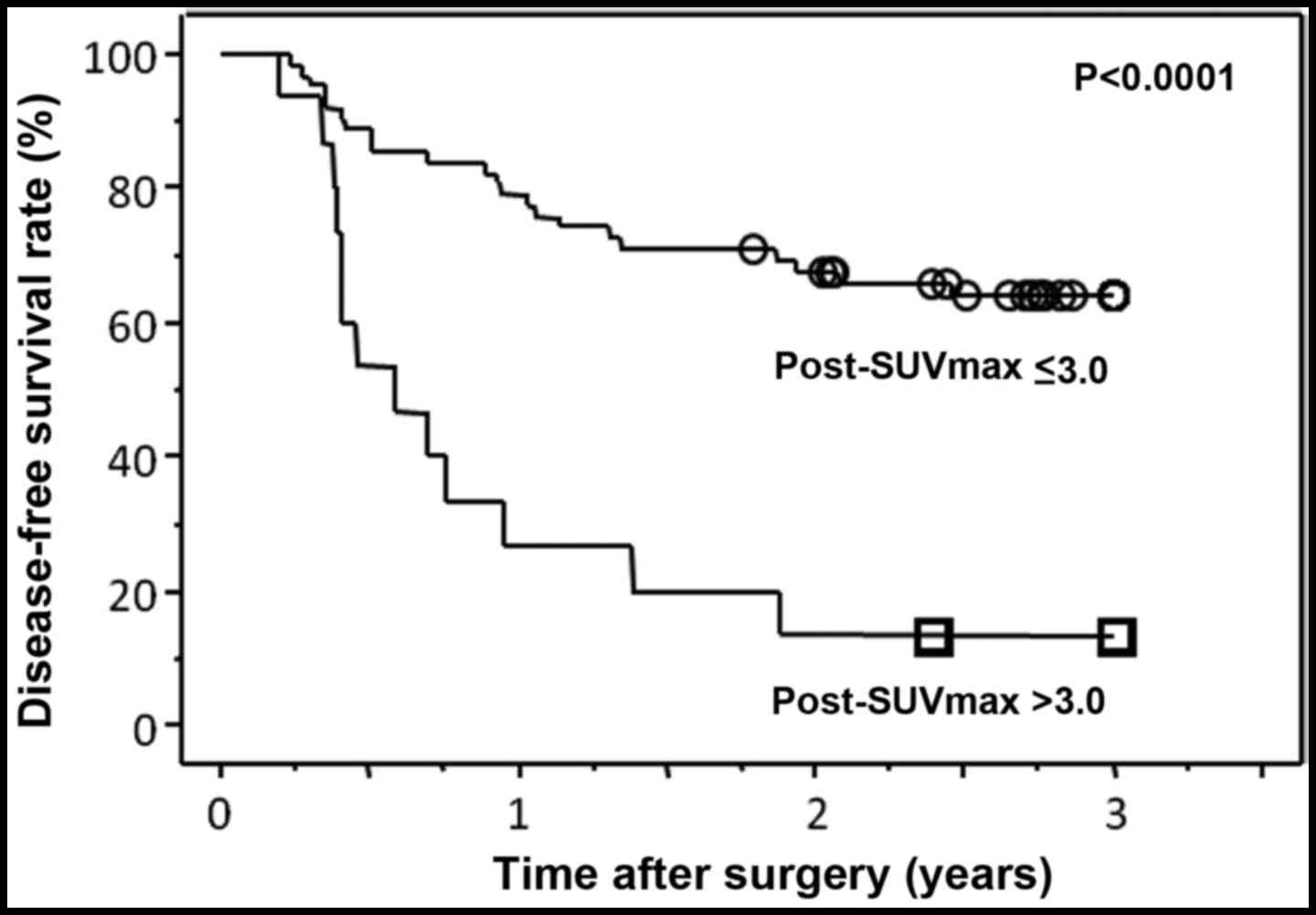
Finally, for a better prediction of prognosis, a
cut-off value was set for post-SUVmax-N. When the
patients were divided into two groups according to the cut-off
value of 3.0, the DFS curves were most clearly separated
(P<0.0001) (Fig. 2).
Association between pathological
findings and post-SUVmax-N
A comparison of the pathological findings between
the two groups classified by post-SUVmax-N (cut-off at
3.0) is shown in Table V. The group
of patients whose post-SUVmax-N was <3.0 exhibited
significantly fewer pathologically metastatic nodes 2.0±3.3 vs.
6.1±5.9 compared with the other group (P=0.0006).
 | Table V.Comparison of pathological findings
between the high and low post-SUVmax-N groups. |
Table V.
Comparison of pathological findings
between the high and low post-SUVmax-N groups.
| Findings |
Post-SUVmax-N ≥3.0 (n=62) |
Post-SUVmax-N <3.0
(n=15) | P-value |
|---|
| Pathological
effects (grade 3/2/1b/1a) | 4/14/12/32 | 1/1/4/9 | 0.414 |
| pT (CR/1/2/3) | 4/19/11/28 | 1/3/2/9 | 0.357 |
| pN
(N0/N1/N2/N3) | 23/21/14/4 | 1/5/3/6 | 0.002 |
| pStage
(CR/I/II/III/IV) | 3/12/21/22/4 | 0/0/6/7//2 | 0.063 |
| Number of
metastatic nodes (mean ± standard deviation) | 2.0±3.3 | 6.1± 5.9 | 0.0006 |
Discussion
This study investigated whether preoperative
evaluation by PET/CT performed before and after NAC may serve as a
useful predictor of prognosis in patients with locally advanced
esophageal cancer who had undergone NAC followed by surgery. The
results demonstrated that the post-SUVmax-N was the only
significant prognostic predictor among several preoperatively
available factors. The use of a cut-off value of 3.0 for the
post-SUVmax-N allowed the prediction of long-term DFS.
Patients with a post-SUVmax-N <3.0 had significantly
fewer pathologically metastatic nodes compared with patients with a
post-SUVmax-N >3.0. By contrast,
post-SUVmax-T, %SUVmax-T and
%SUVmax-N were not found to be associated with patient
prognosis.
Patients with lower post-SUVmax-N had a
better prognosis, partly due to those patients having fewer
metastatic nodes. The number of pathological lymph node metastases
is known to be the strongest prognostic predictor in patients with
esophageal cancer who have undergone surgical resection without
preoperative therapy (22–24). Recent studies have also demonstrated
that the number of pathological nodes is a strong prognostic factor
for patients who have undergone neoadjuvant therapy followed by
surgery (25,26). Although a precise preoperative
diagnosis of pathological lymph node status has thus far been
considered impossible by conventional imaging modalities, PET/CT
would be a useful tool to accurately assess pathological N status
following neoadjuvant therapy. Another possible explanation is that
the higher SUVmax of the lymph nodes reflects the larger
size of metastatic foci or higher malignant potential of cancer
cells. Several investigators have recently reported that the size
of the metastatic lymph node is one of the strongest prognostic
factors in esophageal cancer (27–29).
Moreover, higher SUVmax values indicate a higher
malignant potential of cancer cells through a variety of
mechanisms, such as cell proliferation, tissue hypoxia and
angiogenesis (30–33).
A number of investigators use the reduction rate in
SUVmax as a criterion for the assessment of the tumor
response to NAC (34,35), and several groups reported that a 50%
reduction in SUVmax following NAC is a more significant
predictor of DFS compared with pathological findings (36,37).
Roedl et al (38)
demonstrated that a reduction in tumor length between the pre- and
post-NAC PET scans is a better predictor of pathological
effectiveness and time-to-recurrence than a reduction in SUV. In
our study, neither a 50% reduction in SUVmax-T nor a 50%
reduction in SUVmax-N were found to be correlated with
DFS. The reason for this discordance between our results and
previously reported results is unknown. However, one possible
explanation is that, in this study, we only enrolled patients with
potentially resectable tumors and excluded patients with
unresectable, non-responding tumors from the analysis. Therefore,
downstaging of the T factor may not have a stronger impact on
prognosis than downstaging of the N factor and, as a result, the
effects of %SUVmax-T may have been underestimated
(34–37). Compared with post-SUVmax,
%SUVmax did not correlate well with prognosis. In a
clinical setting, in which patients with potentially resectable
cancers are treated with NAC followed by surgery, the finding that
the residual tumor volume within the metastatic node, as assessed
by PET/CT, becomes minimal just prior to surgery, is the most
important predictor for postoperative survival.
Neither pre-SUVmax-T nor
pre-SUVmax-N were found to be correlated with DFS, which
suggests that prognosis is not affected by the initial stage, but
by the post-treatment stage. In other words, even if the initial
stage is advanced, downstaged patients who respond more effectively
to the treatment have a more favorable prognosis compared with
non-responding patients. Recently, Suzuki et al (39) reported the significance of baseline
SUV in the prediction of prognosis in patients with esophageal and
gastroesophageal cancers who were treated with definitive
chemoradiotherapy. These authors demonstrated that a higher initial
SUV is significantly associated with higher T-stage, positive
N-stage, higher overall stage and poorer overall survival. As all
the patients in their study were treated with definitive
chemoradiotherapy and were not routinely treated by surgery, the CR
rate by chemoradiotherapy appears to be the most important
prognostic factor. Therefore, the initial tumor volume as assessed
by PET/CT may have directly affected the outcome. In general, the
larger the initial tumor mass, the lower the CR rate. By contrast,
in our study, all the patients received NAC followed by surgery. In
this situation, regardless of whether there are more or fewer
residual tumors, they may be easily eradicated by surgery.
Therefore, the preoperative tumor status may have less of an impact
on prognosis.
In conclusion, in patients with locally advanced
potentially resectable esophageal cancer who were treated with NAC
followed by surgery, post-SUVmax-N was significantly
correlated with the number of pathological lymph node metastases
and DFS. Furthermore, a SUVmax cut-off value of 3.0 for
the metastatic nodes clearly differentiated the patients with good
prognosis from those with poor prognosis. As this was a
retrospective study with a small number of patients, our results
require validation by a future prospective, large-scale study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Isono K, Sato H and Nakayama K: Results of
a nationwide study on the three-field lymph node dissection of
esophageal cancer. Oncology. 48:411–420. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akiyama H, Tsurumaru M, Udagawa H and
Kajiyama Y: Radical lymph node dissection for cancer of the
thoracic esophagus. Ann Surg. 220:364–372; discussion 372–373.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allum WH, Stenning SP, Bancewicz J, Clark
PI and Langley RE: Long-term results of a randomized trial of
surgery with or without preoperative chemotherapy in esophageal
cancer. J Clin Oncol. 27:5062–5067. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Eng J Med.
355:11–20. 2006. View Article : Google Scholar
|
|
5
|
Medical Research Council Oesophageal
Cancer Working Group, . Surgical resection with or without
preoperative chemotherapy in oesophageal cancer: A randomised
controlled trial. Lancet. 359:1727–1733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gebski V, Burmeister B, Smithers BM, Foo
K, Zalcberg J and Simes J; Australasian Gastro-Intestinal Trials
Group, : Survival benefits from neoadjuvant chemoradiotherapy or
chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet
Oncol. 8:226–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaklamanos IG, Walker GR, Ferry K,
Franceschi D and Livingstone AS: Neoadjuvant treatment for
resectable cancer of the esophagus and the gastroesophageal
junction: A meta-analysis of randomized clinical trials. Ann Surg
Oncol. 10:754–761. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Law S, Fok M, Chow S, Chu KM and Wong J:
Preoperative chemotherapy versus surgical therapy alone for
squamous cell carcinoma of the esophagus: A prospective randomized
trial. J Thorac Cardiovasc Surg. 114:210–217. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ancona E, Ruol A, Santi S, Merigliano S,
Sileni VC, Koussis H, Zaninotto G, Bonavina L and Peracchia A: Only
pathologic complete response to neoadjuvant chemotherapy improves
significantly the long term survival of patients with resectable
esophageal squamous cell carcinoma: Final report of a randomized,
controlled trial of preoperative chemotherapy versus surgery alone.
Cancer. 91:2165–2174. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Block MI, Patterson GA, Sundaresan RS,
Bailey MS, Flanagan FL, Dehdashti F, Siegel BA and Cooper JD:
Improvement in staging of esophageal cancer with the addition of
positron emission tomography. Ann Thorac Surg. 64:770–776;
discussion 776–777. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luketich JD, Schauer PR, Meltzer CC,
Landreneau RJ, Urso GK, Townsend DW, Ferson PF, Keenan RJ and
Belani CP: Role of positron emission tomography in staging
esophageal cancer. Ann Thorac Surg. 64:765–769. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flanagan FL, Dehdashti F, Siegel BA, Trask
DD, Sundaresan SR, Patterson GA and Cooper JD: Staging of
esophageal cancer with 18F-fluorodeoxyglucose positron
emission tomography. AJR Am J Roentgenol. 168:417–424. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Flamen P, Lerut A, Van Custem E, De Wever
W, Peeters M, Stroobants S, Dupont P, Bormans G, Hiele M, De Leyn
P, et al: Utility of positron emission tomography for the staging
of patients with potentially operable esophageal carcinoma. J Clin
Oncol. 18:3202–3210. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brücher BL, Weber W, Bauer M, Fink U,
Avril N, Stein HJ, Werner M, Zimmerman F, Siewert JR and Schwaiger
M: Neoadjuvant therapy of esophageal squamous cell carcinoma:
Response evaluation by positron emission tomography. Ann Surg.
233:300–309. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weber WA, Ott K, Becker K, Dittler HJ,
Helmberger H, Avril NE, Meisetschläger G, Busch R, Siewert JR,
Schwaiger M and Fink U: Prediction of response to preoperative
chemotherapy in adenocarcinomas of the esophagogastric junction by
metabolic imaging. J Clin Oncol. 19:3058–3065. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flamen P, Van Cutsem E, Lerut A, Cambier
JP, Haustermans K, Bormans G, De Leyn P, Van Raemdonck D, De Wever
W, Ectors N, et al: Positron emission tomography for assessment of
the response to induction radiochemotherapy in locally advanced
oesophageal cancer. Ann Oncol. 13:361–368. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higuchi I, Yasuda T, Yano M, Doki Y,
Miyata H, Tatsumi M, Fukunaga H, Takiguchi S, Fujiwara Y, Hatazawa
J and Monden M: Lack of fludeoxyglucose F 18 uptake in
posttreatment positron emission tomography as a significant
predictor of survival after subsequent surgery in multimodality
treatment for patients with locally advanced esophageal squamous
cell carcinoma. J Thorac Cardiovasc Surg. 136:205–212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan S, Yu Y, Chao KS, Fu Z, Yin Y, Liu T,
Chen S, Yang X, Yang G, Guo H and Yu J: Additional value of PET/CT
over PET in assessment of locoregional lymph nodes in thoracic
esophageal squamous cell cancer. J Nucl Med. 47:1255–1259.
2006.PubMed/NCBI
|
|
19
|
Sobin LH and Wittekind C: TNM
Classification of Malignant Tumors. 6th. Wiley; New York: 2002
|
|
20
|
Kelsen DP, Heelan R, Coonley C, Bains M,
Martini N, Hilaris B and Golbey RB: Clinical and pathological
evaluation of response to chemotherapy in patients with esophageal
carcinoma. Am J Clin Oncol. 6:539–546. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishihara R, Yamamoto S, Iishi H, Nagai K,
Matui F, Kawada N, Ohta T, Kanzaki H, Hanafusa M, Hanaoka N, et al:
Predicting the effects of chemoradiotherapy for squamous cell
carcinoma of the esophagus by induction chemotherapy response
assessed by positron emission tomography: Toward
PET-response-guided selection of chemoradiotherapy or
esophagectomy. Int J Clin Oncol. 17:225–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawahara K, Maekawa T, Okabayashi K,
Shiraishi T, Yoshinaga Y, Yoneda S, Hideshima T and Shirakusa T:
The number of lymph node metastases influences survival in
esophageal cancer. J Surg Oncol. 67:160–163. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tachibana M, Kinugasa S, Dhar DK, Kotoh T,
Shibakita M, Ohno S, Masunaga R, Kubota H, Kohno H and Nagasue N:
Prognostic factors after extended esophagectomy for squamous cell
carcinoma of the thoracic esophagus. J Surg Oncol. 72:88–93. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rizk N, Venkatraman E, Park B, Flores R,
Bains MS and Rusch V; American Joint Committee on Cancer staging
system, : The prognostic importance of the number of involved lymph
nodes in esophageal cancer: Implications for revisions of the
American Joint Committee on Cancer staging system. J Thorac
Cardiovasc Surg. 132:1374–1381. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akutsu Y, Shuto K, Kono T, Uesato M,
Hoshino I, Shiratori T, Isozaki Y, Akanuma N, Uno T and Matsubara
H: The number of pathologic lymph nodes involved is still a
significant prognostic factor even after neoadjuvant
chemoradiotherapy in esophageal squamous cell carcinoma. J Surg
Oncol. 105:756–760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gu Y, Swisher SG, Ajani JA, Correa AM,
Hofstetter WL, Liao Z, Komaki RR, Rashid A, Hamilton SR and Wu TT:
The number of lymph nodes with metastasis predicts survival in
patients with esophageal or esophagogastric junction adenocarcinoma
who receive preoperative chemoradiation. Cancer. 106:1017–1025.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhar DK, Tachibana M, Kinukawa N, Riruke
M, Kohno H, Little AG and Nagasue N: The prognostic significance of
lymph node size in patients with squamous esophageal cancer. Ann
Surg Oncol. 9:1010–1016. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Komori T, Doki Y, Kabuto T, Ishikawa O,
Hiratsuka M, Sasaki Y, Ohigashi H, Murata K, Yamada T, Miyashiro I,
et al: Prognostic significance of the size of cancer nests in
metastatic lymph nodes in human esophageal cancers. J Surg Oncol.
82:19–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Doi N, Imada T, Aoyama N, Kameda Y and
Koizumi H: Prognostic significance of the carcinoma area in the
thickest part of the lymph node. Hepatogastroenterology.
47:728–732. 2000.PubMed/NCBI
|
|
30
|
Vesselle H, Schmidt RA, Pugsley JM, Li M,
Kohlmyer SG, Vallires E and Wood DE: Lung cancer proliferation
correlates with [F-18]fluorodeoxyglucose uptake by positron
emission tomography. Clin Cancer Res. 6:3837–3844. 2000.PubMed/NCBI
|
|
31
|
Jacob R, Welkoborsky HJ, Mann WJ, Jauch M
and Amedee R: [Fluorine-18]fluorodeoxyglucose positron emission
tomography, DNA ploidy and growth fraction in squamous-cell
carcinomas of the head and neck. ORL J Otorhinolaryngol Relat Spec.
63:307–313. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Strauss LG, Koczan D, Klippel S, Pan L,
Cheng C, Willis S and Haberkorn U: Dimitrakopoulou-Strauss A:
Impact of angiogenesis-related gene expression on the tracer
kinetics of 18F-FDG in colorectal tumors. J Nucl Med.
49:1238–1244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaira K, Oriuchi N, Sunaga N, Ishizuka T,
Shimizu K and Yamamoto N: A systemic review of PET and biology in
lung cancer. Am J Transl Res. 3:383–391. 2011.PubMed/NCBI
|
|
34
|
Rebollo Aguirre AC, Ramos-Font C, Villegas
Portero R, Cook GJ, Llamas Elvira JM and Tabares AR:
18F-fluorodeoxyglucose positron emission tomography for
the evaluation of neoadjuvant therapy response in esophageal
cancer: Systematic review of the literature. Ann Surg. 250:247–254.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kwee RM: Prediction of tumor response to
neoadjuvant therapy in patients with esophageal cancer with use of
18F FDG PET: A systematic review. Radiology.
254:707–717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith JW, Moreira J, Abood G, Aranha GV,
Nagda S, Wagner RH and Shoup M: The influence of
(18)flourodeoxyglucose positron emission tomography on the
management of gastroesophageal junction carcinoma. Am J Surg.
197:308–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Port JL, Lee PC, Korst RJ, Liss Y,
Meherally D, Christos P, Mazumdar M and Altorki NK: Positron
emission tomographic scanning predicts survival after induction
chemotherapy for esophageal carcinoma. Ann Thorac Surg. 84:393–400;
discussion 400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Roedl JB, Harisinghani MG, Colen RR,
Fischman AJ, Blake MA, Mathisen DJ and Mueller PR: Assessment of
treatment response and recurrence in esophageal carcinoma based on
tumor length and standardized uptake value on positron emission
tomography-computed tomography. Ann Thorac Surg. 86:1131–1138.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Suzuki A, Xiao L, Hayashi Y, Macapinlac
HA, Welsh J, Lin SH, Lee JH, Bhutani MS, Maru DM, Hofstetter WL, et
al: Prognostic significance of baseline positron emission
tomography and importance of clinical complete response in patients
with esophageal or gastroesophageal junction cancer treated with
definitive chemoradiotherapy. Cancer. 117:4823–4833. 2011.
View Article : Google Scholar : PubMed/NCBI
|