Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, and the majority of the patients are at the
advanced stages of the disease at the time of diagnosis (1). Traditional chemotherapy regimens for
advanced-stage lung cancer have exhibited modest efficacy in
prolonging survival, and are associated with undesirable side
effects (2,3). Previously, therapy targeted towards the
epidermal growth factor receptor (EGFR) pathway has achieved great
success in the treatment of lung cancer. The EGFR pathway is an
attractive target for therapy, as EGFR signaling plays an important
role in the growth, proliferation and survival of several solid
tumors, including non-small-cell lung cancer (NSCLC) (4).
A subgroup of patients with NSCLC harbor specific
mutations in the tyrosine kinase domain of the EGFR gene,
which are correlated with favorable clinical responsiveness to EGFR
tyrosine kinase inhibitor (TKI) therapy (5). All mutations appear to be limited to
exons 18, 19, 20 and 21 of the EGFR gene (6), and are most frequently observed in lung
adenocarcinoma patients (7,8). Missense mutations in exon 21 (L858R)
and in-frame deletions in exon 19 are the most frequent
EGFR-TKI-sensitive mutations (80%) in NSCLC patients (9). Both the exon 19 deletion and the exon
21 missense mutation are common EGFR mutations that are
associated with a favorable response to first-line treatment with
gefitinib (10,11), as well as other EGFR-TKIs, including
erlotinib (12) and afatinib
(13), compared with standard
chemotherapy in NSCLC patients. In NSCLC patients with EGFR
mutations, the overall response rate (ORR) to first-line EGFR-TKI
therapy is 66.9–83%, with a progression-free survival (PFS) of
9.2–13.1 months (10–13). Despite the favorable response to
EGFR-TKIs in NSCLC patients with EGFR mutations, ~20–30% of
patients do not respond to EGFR-TKIs, and the clinical phenotypes
and survival-associated factors of these EGFR-TKI responders and
non-responders have not been previously described.
The aim of the present study was to elucidate the
clinical presentation and significance of EGFR-TKI responders and
non-responders in lung adenocarcinoma patients with common exon 21
and 19 EGFR activating mutations.
Patients and methods
Patients and study design
The present retrospective cohort study was approved
by the Institutional Review Board of the Chang Gung Memorial
Hospital. The cohort comprised 131 lung adenocarcinoma patients
from the Chang Gung Memorial Hospital, Chiayi Branch (Puzi, Taiwan)
(IRB No. 201600601B0), who had been diagnosed between December 2010
and January 2015. All participants were previously treatment-naive
advanced-stage (stage IIIB or IV) lung adenocarcinoma patients. The
EGFR mutation status at exons 18, 19, 20 and 21 of the
EGFR gene was determined by Sanger sequencing (8) or by using the Therascreen®
EGFR RGQ PCR kit (Qiagen, Manchester, UK) (14). All the patients received first-line
EGFR-TKI therapy (gefitinib, erlotinib or afatinib) and had
EGFR mutations at exon 19 or 21. Patients with combined exon
18 or 20 mutations were excluded from the study. Follow-up was
extended from the first diagnosis of advanced-stage lung cancer to
October 2016. The clinical phenotypes of these patients were
recorded and analyzed. The response of the lesions was evaluated by
chest computed tomography, brain magnetic resonance or bone scan,
according to the Response Evaluation Criteria in Solid Tumors 1.1
(15) at 3 months after the
initiation of treatment. EGFR-TKI responders were defined as
complete responders (CR) or partial responders (PR), while
non-responders were defined as those having stable disease (SD) or
progressive disease (PD) at 3 months after the initiation of
EGFR-TKI therapy. PFS is defined as the time from the first
treatment to PD or death. Overall survival (OS) is defined as the
time from diagnosis to death from any cause, or until the patients
were censored at the last follow-up.
Statistical analysis
The Pearson's χ2 test was used to
determine the correlations between the categorical variables in the
different groups. Survival analysis was performed using a
Kaplan-Meier analysis and log-rank test. Multivariate analysis was
performed by Cox proportional-hazards regression, and factors that
were determined as significant by the log-rank test were included
in the analysis. A P-value of <0.05 was considered as
statistically significant. All statistical tests were performed
using MedCalc software, version 15 (MedCalc Software, Ostend,
Belgium).
Results
Clinical characteristics common to all
first-line EGFR-TKI patients
In total, 131 patients were enrolled in the present
study (Table I). The median age was
70.0 years. The majority of the patients were female (n=72, 55%),
non-smokers (n=115, 87.8%), and had stage IV disease (n=121,
92.4%). Of the 131 patients, 59 (45%) had exon 19 deletions and 72
(55%) had exon 21 missense EGFR mutations. The EGFR-TKIs
gefitinib (n=99, 75.6%), erlotinib (n=27, 20.6%) or afatinib (n=5,
3.8%) were used as the first-line therapy in these patients. Three
months after EGFR-TKI treatment, the tumor response to treatment
was evaluated. PR was observed in 104 (79.3%), SD in 12 (9.2%), and
PD in 15 (11.5%) patients. There were no CR patients. The ORR to
EGFR-TKIs was 79.3%, and the disease control rate (DCR) was 88.5%.
The median PFS for all first-line EGFR-TKI patients was 12.7 months
(95% CI: 12.0–16.70 months), and the median OS was 32.7 months (95%
CI: 24.7–57.1 months).
 | Table I.Clinical characteristics of patients
treated with first-line EGFR-TKIs. |
Table I.
Clinical characteristics of patients
treated with first-line EGFR-TKIs.
|
| Total | Responders | Non-responders | P-value |
|---|
| Patients | 131 | 104 | 27 |
|
| Sex |
|
|
| 0.426 |
| Male | 59 (45.0) | 45 (43.2) | 14 (51.9) |
|
|
Female | 72 (55.0) | 59 (56.8) | 13 (48.1) |
|
| Smoking |
|
|
|
|
| Yes | 16 (12.2) | 12 (11.5) | 4 (14.8) | 0.645 |
| No | 115 (87.8) | 92 (88.5) | 23 (85.2) |
|
| Age
(years) | 70 | 70 | 74 | 0.219 |
| Age |
|
|
| 0.068 |
|
≥65 | 82 (62.6) | 61 (58.6) | 21 (77.8) |
|
|
<65 | 49 (37.4) | 43 (41.4) | 6 (22.2) |
|
| TKI |
|
|
| 0.157 |
|
Erlotinib | 27 (20.6) | 24 (23.0) | 3 (11.1) | 0.059 |
|
Gefitinib | 99 (75.6) | 75 (72.1) | 24 (88.9) |
|
|
Afatinib | 5 (3.8) | 5 (4.8) |
|
|
| Mutations |
| |
| 0.350 |
| Exon
19 | 59 (45.0) | 49 (47.1) | 10 (37.0) |
|
| Exon
21 | 72 (55.0) | 55 (52.9) | 17 (63.0) |
|
| Stage |
|
|
| 0.961 |
|
IIIb | 10 (7.6) | 8 (7.7) | 2 (7.4) |
|
| IV | 121 (92.4) | 96 (92.3) | 25 (92.6) |
|
| CEA
(ng/ml) | 95 | 79 | 156 | 0.325 |
| ECOG PS |
|
|
| 0.724 |
| ≤1 | 119 (90.8) | 94 (90.4) | 25 (92.6) |
|
| ≥2 | 12 (9.2) | 10 (9.6) | 2 (7.4) |
|
| Metastatic
sites |
|
|
|
|
|
Lung | 42 (32.1) | 36 (34.6) | 6 (22.2) | 0.221 |
|
Brain | 35 (26.7) | 33 (31.7) | 2 (7.4) | 0.011a |
|
Liver | 15 (11.4) | 12 (11.5) | 3 (11.1) | 0.951 |
|
Bone | 51 (38.9) | 37 (35.6) | 14 (51.9) | 0.124 |
|
Adrenal | 9 (6.9) | 8 (7.7) | 1 (3.7) | 0.467 |
Survival of first-line EGFR-TKI
responders and non-responders
EGFR-TKI responders and non-responders were
identified based on their response to treatment. A total of 104
(79.3%) EGFR-TKI responders (CR + PR) and 27 (20.7%) non-responders
(SD + PD) were identified. No significant differences were observed
between EGFR-TKI responders and non-responders in terms of sex,
smoking history, age, EGFR-TKI use, EGFR mutation status,
carcinoembryonic antigen, Eastern Cooperative Oncology Group (ECOG)
performance status (PS), and cancer stage (Table I). A significantly higher proportion
of brain metastasis was observed in EGFR-TKI responders compared
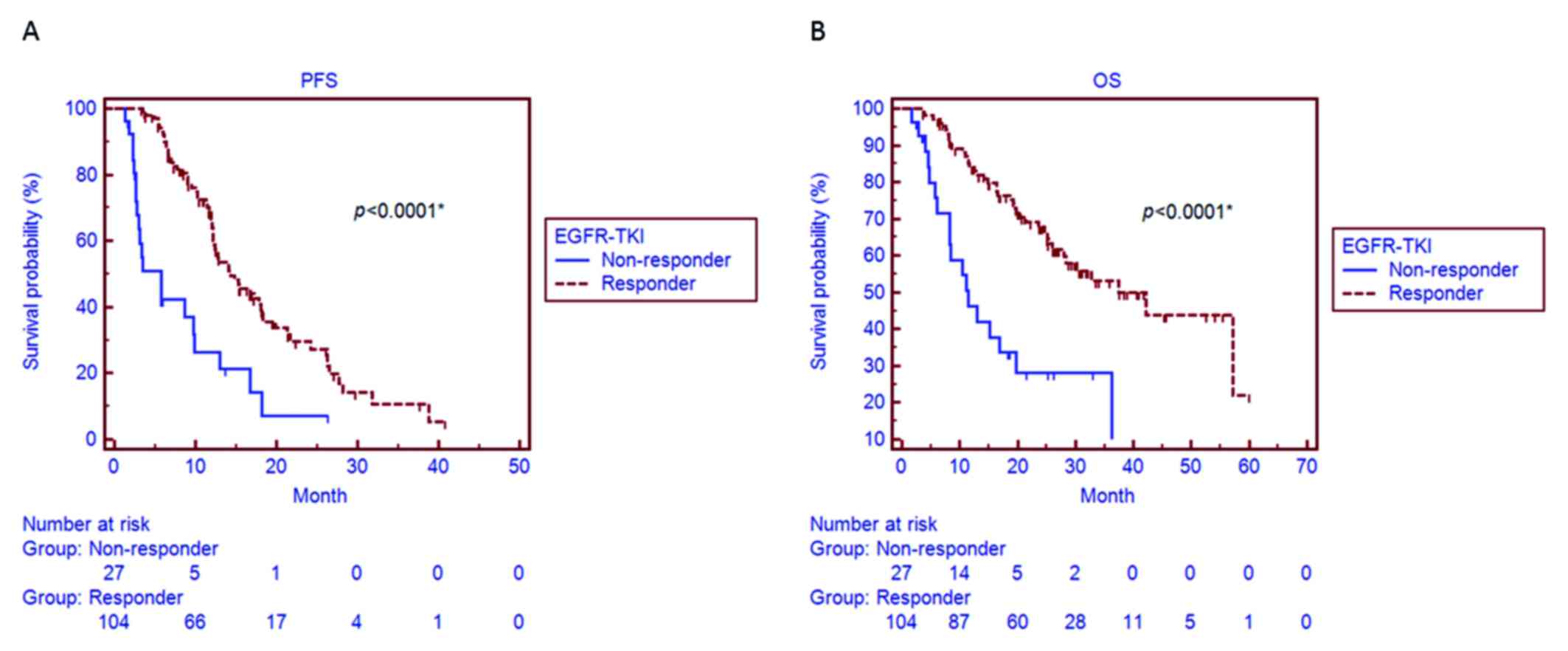
with non-responders (31.7 vs. 7.4%, respectively; P=0.011; Table I). A significantly longer median PFS
was observed in EGFR-TKI responders (14.3 months, 95% CI: 12.2–18.4
months) compared with that in non-responders (5.7 months, 95% CI:
2.7–9.9 months; P<0.001; Fig.
1A). We also observed a significantly longer median OS in
responders (42.2 months, 95% CI: 28.1–58.1 months) compared with
that in non-responders (11.5 months, 95% CI: 8.3–19.7 months;
P<0.001; Fig. 1B).
Characteristics of survival in
patients treated with first-line EGFR-TKIs
The associations between measured clinical variables
and survival were evaluated. According to the univariate analysis,
EGFR-TKI responder status (HR=0.33, 95% CI: 0.16–0.68, P<0.001)
and old age (>65 years) (HR=0.64, 95% CI: 0.41–1.00, P=0.038)
were significantly associated with a favorable PFS (Table II). Conversely, male sex (HR=1.66,
95% CI: 1.07–2.58, P=0.018), bone metastasis (HR=1.66, 95% CI:
1.07–2.58, P=0.024) and pleural metastasis (HR=1.62, 95% CI:
1.00–2.77, P=0.022) were significantly associated with an
unfavorable PFS (Table II).
According to the multivariate analysis, EGFR-TKI responder status
(HR=0.25, 95% CI: 0.15–0.42, P<0.001), old age (HR=0.58, 95% CI:
0.36–0.92, P=0.020) and male sex (HR=1.70, 95% CI: 1.07–2.67,
P=0.024) remained independent factors for PFS (Table II).
 | Table II.Clinical variables associated with
PFS in patients treated with first-line EGFR-TKIs. |
Table II.
Clinical variables associated with
PFS in patients treated with first-line EGFR-TKIs.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Covariates | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Male | 0.018a | 1.66 | 1.07–2.58 | 0.024a | 1.70 | 1.07–2.67 |
| Exon 19
mutationb | 0.090 | 0.69 | 0.45–1.06 |
|
|
|
| Smoking | 0.197 | 1.49 | 0.73–3.03 |
|
|
|
| Responder
status |
<0.001a | 0.33 | 0.16–0.68 |
<0.001a | 0.25 | 0.15–0.42 |
| Old age (≥65
years) | 0.038a | 0.64 | 0.41–1.00 | 0.020a | 0.58 | 0.36–0.92 |
| ECOG PS ≥2 | 0.502 | 1.30 | 0.55–3.09 |
|
|
|
| Gefitinib | 0.241 | 1.38 | 0.84–2.26 |
|
|
|
| Metastasis |
|
|
|
|
|
|
|
Lung | 0.515 | 1.16 | 0.73–1.86 |
|
|
|
|
Brain | 0.185 | 0.71 | 0.44–1.14 |
|
|
|
|
Liver | 0.074 | 1.70 | 0.83–3.49 |
|
|
|
|
Bone | 0.024a | 1.62 | 1.02–2.56 |
|
|
|
|
Adrenals | 0.540 | 1.27 | 0.54–2.99 |
|
|
|
|
Pleura | 0.022a | 1.67 | 1.00–2.77 |
|
|
|
According to the univariate analysis, exon 19
mutations (HR=0.55, 95% CI: 0.33–0.92, P=0.027) and EGFR-TKI
responder status (HR=0.30, 95% CI: 0.14–0.67, P<0.001) were
significantly associated with a favorable OS (Table III). By contrast, ECOG PS ≥2
(HR=2.21, 95% CI: 0.79–6.18, P=0.031) and bone metastasis (HR=1.79,
95% CI: 1.04–3.10, P=0.020) were significantly associated with an
unfavorable OS (Table III).
According to the multivariate analysis, EGFR-TKI responder status
(HR=0.30, 95% CI: 0.17–0.54, P<0.001), ECOG PS ≥2 (HR=2.70, 95%
CI: 1.25–5.86, P=0.012) and bone metastasis (HR=1.82, 95% CI:
1.06–3.14, P=0.030) remained independent factors for OS (Table III).
 | Table III.Clinical variables associated with OS
in patients treated with first-line EGFR-TKIs. |
Table III.
Clinical variables associated with OS
in patients treated with first-line EGFR-TKIs.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Covariates | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Male sex | 0.303 | 1.30 | 0.77–2.21 |
|
|
|
| Exon 19
mutationa | 0.027b | 0.55 | 0.33–0.92 |
|
|
|
| Smoking | 0.915 | 0.96 | 0.42–2.19 |
|
|
|
| Responder
status |
<0.001b | 0.30 | 0.14–0.67 |
<0.001b | 0.30 | 0.17–0.54 |
| Old age (≥65
years) | 0.300 | 1.33 | 0.79–2.25 |
|
|
|
| ECOG PS ≥2 | 0.031b | 2.21 | 0.79–6.18 | 0.012b | 2.70 | 1.25–5.86 |
| Gefitinib | 0.019b | 2.61 | 1.42–4.80 |
|
|
|
| Metastasis |
|
|
|
|
|
|
|
Lung | 0.798 | 1.07 | 0.61–1.87 |
|
|
|
|
Brain | 0.917 | 0.97 | 0.54–1.73 |
|
|
|
|
Liver | 0.314 | 1.49 | 0.59–3.76 |
|
|
|
|
Bone | 0.020b | 1.79 | 1.04–3.10 | 0.030b | 1.82 | 1.06–3.14 |
|
Adrenals | 0.080 | 0.20 | 0.08–0.53 |
|
|
|
|
Pleura | 0.434 | 1.24 | 0.70–2.20 |
|
|
|
Survival-associated factors in
first-line EGFR-TKI responders and non-responders
Since the initial response to EGFR-TKI treatment was
significantly associated with PFS and OS according to both
univariate and multivariate analysis, the characteristics of
EGFR-TKI responders and non-responders were then analyzed
separately.
According to the univariate analysis for EGFR-TKI
responders, old age (HR=0.56, 95% CI: 0.34–0.93, P=0.018) was
significantly associated with a favorable PFS, while pleural
metastasis (HR=2.01, 95% CI: 1.08–3.73, P=0.006) was significantly
associated with an unfavorable PFS (Table IV). According to the multivariate
analysis, bone metastasis (HR=1.87, 95% CI: 1.11–3.20, P=0.021) and
pleural metastasis (HR=2.40, 95% CI: 1.37–4.22, P=0.002) were
independent factors for PFS (Table
IV).
 | Table IV.Clinical variables associated with
PFS in first-line EGFR-TKI responders. |
Table IV.
Clinical variables associated with
PFS in first-line EGFR-TKI responders.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Covariates | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Male sex | 0.150 | 1.42 | 0.86–2.36 |
|
|
|
| Exon 19
mutationa | 0.189 | 0.72 | 0.44–1.19 |
|
|
|
| Smoking | 0.614 | 1.21 | 0.54–2.68 |
|
|
|
| Old age (≥65
years) | 0.018b | 0.56 | 0.34–0.93 |
|
|
|
| ECOG PS ≥2 | 0.657 | 1.23 | 0.45–3.33 |
|
|
|
| Gefitinib | 0.474 | 1.24 | 0.71–2.21 |
|
|
|
| Metastasis |
|
|
|
|
|
|
|
Lung | 0.395 | 1.25 | 0.73–2.13 |
|
|
|
|
Brain | 0.524 | 0.84 | 0.49–1.43 |
|
|
|
|
Liver | 0.081 | 1.80 | 0.78–4.18 |
|
|
|
|
Bone | 0.063 | 1.58 | 0.92–2.72 | 0.021b | 1.87 | 1.11–3.20 |
|
Adrenals | 0.512 | 1.32 | 0.51–3.39 |
|
|
|
|
Pleura | 0.006b | 2.01 | 1.08–3.73 | 0.002b | 2.40 | 1.37–4.22 |
Exon 19 mutations (HR=0.40, 95% CI: 0.22–0.74,
P=0.006) were significantly associated with a favorable OS, while
ECOG PS ≥2 (HR=2.61, 95% CI: 0.73–9.30, P=0.023) and bone
metastasis (HR=1.92, 95% CI: 0.98–3.77, P=0.030) were significantly
associated with an unfavorable OS (Table
V). Exon 19 mutations (HR=0.38, 95% CI: 0.19–0.76, P=0.006),
ECOG PS ≥2 (HR=3.53, 95% CI: 1.42–8.75, P=0.007) and bone
metastasis (HR=2.01, 95% CI: 1.05–3.85, P=0.034) remained
independent factors of OS according to the multivariate analysis
(Table V).
 | Table V.Clinical variables associated with OS
in first-line EGFR-TKI responders. |
Table V.
Clinical variables associated with OS
in first-line EGFR-TKI responders.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Covariates | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Male sex | 0.456 | 1.26 | 0.67–2.39 |
|
|
|
| Exon 19
mutationa | 0.006b | 0.40 | 0.22–0.74 | 0.006b | 0.38 | 0.19–0.76 |
| Smoking | 0.897 | 0.93 | 0.34–2.55 |
|
|
|
| Old age (≥65
years) | 0.844 | 1.06 | 0.57–1.99 |
|
|
|
| ECOG PS ≥2 | 0.023b | 2.61 | 0.73–9.30 | 0.007b | 3.53 | 1.42–8.75 |
| Gefitinib | 0.158 | 1.83 | 0.89–3.76 |
|
|
|
| Metastasis |
|
|
|
|
|
|
|
Lung | 0.206 | 1.49 | 0.76–2.90 |
|
|
|
|
Brain | 0.376 | 1.33 | 0.67–2.64 |
|
|
|
|
Liver | 0.081 | 2.11 | 0.66–6.72 |
|
|
|
|
Bone | 0.030b | 1.92 | 0.98–3.77 | 0.034b | 2.01 | 1.05–3.85 |
|
Adrenal | 0.235 | 0.32 | 0.10–1.05 |
|
|
|
|
Pleura | 0.875 | 1.06 | 0.53–2.08 |
|
|
|
Factors associated with EGFR-TKI non-responders were
also analyzed. According to the univariate analysis, male sex
(HR=2.24, 95% CI: 0.91–5.47, P=0.045) and smoking (HR=3.24, 95% CI:
0.59–17.70, P=0.020) were significantly associated with an
unfavorable PFS, and smoking (HR=3.97, 95% CI: 1.13–13.91, P=0.031)
remained an independent factor of PFS according to the multivariate
analysis (Table VI). No factor
analyzed in the present study was associated with OS in EGFR-TKI
non-responders (data not shown).
 | Table VI.Clinical variables associated with
PFS in first-line EGFR-TKI non-responders. |
Table VI.
Clinical variables associated with
PFS in first-line EGFR-TKI non-responders.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Covariates | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Male sex | 0.045a | 2.24 | 0.91–5.47 |
|
|
|
| Exon 19
mutationb | 0.610 | 0.79 | 0.31–1.97 |
|
|
|
| Smoking | 0.020a | 3.24 | 0.59–17.70 | 0.031a | 3.97 | 1.13–13.91 |
| Old age (≥65
years) | 0.053 | 0.41 | 0.12–1.40 |
|
|
|
| ECOG PS ≥2 | 0.277 | 2.17 | 0.28–16.56 |
|
|
|
| Gefitinib | 0.455 | 1.56 | 0.54–4.54 |
|
|
|
| Metastasis |
|
|
|
|
|
|
|
Lung | 0.717 | 1.20 | 0.41–3.47 |
|
|
|
|
Brain | 0.642 | 0.72 | 0.20–2.62 |
|
|
|
|
Liver | 0.718 | 1.25 | 0.33–4.72 |
|
|
|
|
Bone | 0.951 | 1.03 | 0.43–2.48 |
|
|
|
|
Adrenal | 0.077 | 4.92 | 0.07–356.78 |
|
|
|
|
Pleura | 0.552 | 0.77 | 0.32–1.87 |
|
|
|
Discussion
In the present study, 20.3% of lung adenocarcinoma
patients with common sensitizing exon 21 and exon 19 EGFR
mutations were EGFR-TKI non-responders. Our results are similar to
those of previous studies on first-line TKI treatment, in which
EGFR-TKI non-responders accounted for 20–30% of the study group
(10–13). In the present study, EGFR-TKI
non-responders had a poor prognosis. The clinical factors
associated with PFS and OS were also assessed and it was observed
that, in EGFR-TKI responders, bone and pleural metastasis were
independent factors for unfavorable PFS. Poor ECOG PS (≥2) and bone
metastasis were independent factors for unfavorable OS, and exon 19
deletions were an independent factor for favorable OS. In EGFR-TKI
non-responders, smoking was an independent factor for unfavorable
PFS.
PFS and OS were reduced in EGFR-TKI non-responders,
confirming the results of an earlier study, in which the median OS
was 21 months (95% CI: 26.1–30.4) in responders compared with 8
months (95% CI: 8.7–15.8) in non-responders (16). Based on the multivariate analysis,
EGFR-TKI non-responding status was found to be a strongly
unfavorable factor for both PFS and OS in patients receiving
first-line EGFR-TKI therapy. Rapid progression of lung cancer after
the initiation of EGFR-TKI therapy has been reported to be a poor
prognostic factor for survival outcomes (17). Our results further suggest that
EGFR-TKI non-responders are distinctly different from EGFR-TKI
responders. Since this group of patients had a worse prognosis, a
treatment strategy that overcomes primary resistance to EGFR-TKI is
urgently needed. Close monitoring of EGFR-TKI treatment response is
also mandatory for early detection of EGFR-TKI non-responders, so
that treatment may be adjusted accordingly.
Exon 19 deletions have been associated with better
outcomes compared with L858R mutations in EGFR-TKI patients as,
reported in several studies (18–20). In
the present study, exon 19 deletions were found to be an
independent predictor of outcome in first-line EGFR-TKI responders.
Exon 19 deletions have been previously associated with better
survival rates compared with exon 21 mutations in gefitinib-treated
NSCLC patients, due to the differential inhibition of downstream
signaling (21). Recently, exon 19
deletions were reported to be associated with a better outcome
after afatinib therapy, compared with that of the exon 21 L858R
mutation. Altogether, exon 19 deletions and L858R mutations
characterize two distinct groups of patients and, therefore,
different clinical treatment strategies for these patients should
be considered in the future. A reduced frequency of exon 19
deletions has also recently been reported in EGFR-TKI
non-responders (16). However, no
significant differences in the frequency of exon 19 deletions and
L858R mutations were observed, which may be due to the limited
number of patients in this cohort.
In the present study, poor baseline ECOG PS (≥2) was
associated with an unfavorable OS in EGFR-TKI responders, which is
similar to previously reported results (17). However, we did not observe a
significant effect of poor ECOG PS on PFS, which may indicate that
EGFR-TKIs are effective and well-tolerated in responders. In
addition, 35.6% of EGFR-TKI responders had developed bone
metastasis at the time of diagnosis of lung cancer, and the overall
incidence of bone metastasis in patients with EGFR mutations
was not higher compared with that reported previously (22). Bone metastasis was found to be
associated with unfavorable PFS and OS. EGFR-TKIs prolong survival
in patients with EGFR mutations and bone metastasis
(23). However, our results
highlight that management of bone metastasis should be a priority
in EGFR-TKI responders.
Smoking at the time of diagnosis of lung cancer was
associated with unfavorable PFS in EGFR-TKI non-responders. Indeed,
smoking for ≥30 pack-years is associated with a decreased ORR and
DCR in lung adenocarcinoma with activation EGFR mutations
(24). No clinical variables were
associated with OS in EGFR-TKI non-responders, which may be
attributed to the relatively short survival and small number of
these patients. In addition, non-response to EGFR-TKIs (primary
resistance) may be associated with underlying genetics or molecular
mechanisms in lung cancer cells. Several mechanisms for primary
EGFR-TKI resistance have been proposed, including v-Ki-ras2 Kirsten
rat sarcoma viral oncogene homolog mutations (25), phosphoinositide-3-kinase catalytic
subunit α mutation (26), de novo
MET amplification (27,28), Bim deletion polymorphisms
(29,30), and phosphatase and tensin homolog
loss (31). De novo T790M
mutations of the EGFR gene have also been reported to be
associated with poorer response to first-line EGFR-TKI treatment
(32). Since patients with de
novo T790M mutations were excluded from the present study, we
hypothesized that other genetic or molecular changes may be
implicated in EGFR-TKI resistance in EGFR-TKI non-responders in the
present study, and these changes warrant further investigation.
Although this study was limited by the small cohort
size and limited number of EGFR mutations, the results may
help elucidate the clinical presentation of the EGFR-TKI response,
and contribute to the development of novel treatment strategies for
lung adenocarcinoma patients with common EGFR mutations.
In summary, it was demonstrated that different
prognosis and survival-associated factors are observed in EGFR-TKI
responders and non-responders. These groups of patients should
therefore be considered as two distinct groups, and novel treatment
strategies should be developed and applied to EGFR-TKI
non-responders.
Acknowledgements
The present study was supported by grants
CORPG6B0353 (to YHT and MSH), CORPG6F0041 (to MSH, YCL and YHF) and
CORPG6B0363 (to YCL and MSH) in Chang Gung Memorial Hospital,
Chiayi, Taiwan.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Breathnach OS, Freidlin B, Conley B, Green
MR, Johnson DH, Gandara DR, O'Connell M, Shepherd FA and Johnson
BE: Twenty-two years of phase III trials for patients with advanced
non-small-cell lung cancer: Sobering results. J Clin Oncol.
19:1734–1742. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goulart BH, Martins RG and Lynch TJ:
Twenty-two years of phase III trials for patients with advanced
non-small-cell lung cancer: Sobering results. J Clin Oncol.
19:40892001.PubMed/NCBI
|
|
3
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arteaga CL: Epidermal growth factor
receptor dependence in human tumors: More than just expression?
Oncologist. 7(Suppl 4): S31–S39. 2002. View Article : Google Scholar
|
|
5
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu CQ, da Cunha Santos G, Ding K,
Sakurada A, Cutz JC, Liu N, Zhang T, Marrano P, Whitehead M, Squire
JA, et al: Role of KRAS and EGFR as biomarkers of response to
erlotinib in National Cancer Institute of Canada Clinical Trials
Group Study BR.21. J Clin Oncol. 26:4268–4275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang SF, Liu HP, Li LH, Ku YC, Fu YN,
Tsai HY, Chen YT, Lin YF, Chang WC, Kuo HP, et al: High frequency
of epidermal growth factor receptor mutations with complex patterns
in non-small cell lung cancers related to gefitinib responsiveness
in Taiwan. Clin Cancer Res. 10:8195–8203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gazdar AF, Shigematsu H, Herz J and Minna
JD: Mutations and addiction to EGFR: The Achilles ‘heal’ of lung
cancers? Trends Mol Med. 10:481–486. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang
Y, Li W, Hou M, Shi JH, Lee KY, et al: Afatinib versus cisplatin
plus gemcitabine for first-line treatment of Asian patients with
advanced non-small-cell lung cancer harbouring EGFR mutations
(LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet
Oncol. 15:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vallée A, Le Loupp AG and Denis MG:
Efficiency of the Therascreen® RGQ PCR kit for the
detection of EGFR mutations in non-small cell lung carcinomas. Clin
Chim Acta. 429:8–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim GW, Song JS, Choi CM, Rho JK, Kim SY,
Jang SJ, Park YS, Chun SM, Kim WS, Lee JS, et al: Multiple
resistant factors in lung cancer with primary resistance to EGFR-TK
inhibitors confer poor survival. Lung cancer. 88:139–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cha YK, Lee HY, Ahn MJ, Choi YL, Lee JH,
Park K and Lee KS: Survival outcome assessed according to tumor
burden and progression patterns in patients with epidermal growth
factor receptor mutant lung adenocarcinoma undergoing epidermal
growth factor receptor tyrosine kinase inhibitor therapy. Clin Lung
Cancer. 16:228–236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Riely GJ, Pao W, Pham D, Li AR, Rizvi N,
Venkatraman ES, Zakowski MF, Kris MG, Ladanyi M and Miller VA:
Clinical course of patients with non-small cell lung cancer and
epidermal growth factor receptor exon 19 and exon 21 mutations
treated with gefitinib or erlotinib. Clin Cancer Res. 12:839–844.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosell R, Moran T, Queralt C, Porta R,
Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M,
et al: Screening for epidermal growth factor receptor mutations in
lung cancer. N Engl J Med. 361:958–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jackman DM, Miller VA, Cioffredi LA, Yeap
BY, Jänne PA, Riely GJ, Ruiz MG, Giaccone G, Sequist LV and Johnson
BE: Impact of epidermal growth factor receptor and KRAS mutations
on clinical outcomes in previously untreated non-small cell lung
cancer patients: Results of an online tumor registry of clinical
trials. Clin Cancer Res. 15:5267–5273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu JQ, Zhong WZ, Zhang GC, Li R, Zhang
XC, Guo AL, Zhang YF, An SJ, Mok TS and Wu YL: Better survival with
EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small
cell lung cancer patients is due to differential inhibition of
downstream signals. Cancer Lett. 265:307–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bury T, Barreto A, Daenen F, Barthelemy N,
Ghaye B and Rigo P: Fluorine-18 deoxyglucose positron emission
tomography for the detection of bone metastases in patients with
non-small cell lung cancer. Eur J Nucl Med. 25:1244–1247. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hong SH, Kim YS, Lee JE, Kim IH, Kim SJ,
Han D, Yoo Ie R, Chung YG, Kim YH, Lee KY and Kang JH: Clinical
characteristics and continued epidermal growth factor receptor
tyrosine kinase inhibitor administration in EGFR-mutated non-small
cell lung cancer with skeletal metastasis. Cancer Res Treat.
48:1110–1119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim MH, Kim HR, Cho BC, Bae MK, Kim EY,
Lee CY, Lee JS, Kang DR and Kim JH: Impact of cigarette smoking on
response to epidermal growth factor receptor (EGFR)-tyrosine kinase
inhibitors in lung adenocarcinoma with activating EGFR mutations.
Lung cancer. 84:196–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takeda M, Okamoto I, Fujita Y, Arao T, Ito
H, Fukuoka M, Nishio K and Nakagawa K: De novo resistance to
epidermal growth factor receptor-tyrosine kinase inhibitors in EGFR
mutation-positive patients with non-small cell lung cancer. J
Thorac Oncol. 5:399–400. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ludovini V, Bianconi F, Pistola L, Chiari
R, Minotti V, Colella R, Giuffrida D, Tofanetti FR, Siggillino A,
Flacco A, et al: Phosphoinositide-3-kinase catalytic alpha and KRAS
mutations are important predictors of resistance to therapy with
epidermal growth factor receptor tyrosine kinase inhibitors in
patients with advanced non-small cell lung cancer. J Thorac Oncol.
6:707–715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cappuzzo F, Jänne PA, Skokan M,
Finocchiaro G, Rossi E, Ligorio C, Zucali PA, Terracciano L, Toschi
L, Roncalli M, et al: MET increased gene copy number and primary
resistance to gefitinib therapy in non-small-cell lung cancer
patients. Ann Oncol. 20:298–304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanaka A, Sueoka-Aragane N, Nakamura T,
Takeda Y, Mitsuoka M, Yamasaki F, Hayashi S, Sueoka E and Kimura S:
Co-existence of positive MET FISH status with EGFR mutations
signifies poor prognosis in lung adenocarcinoma patients. Lung
cancer. 75:89–94. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ying HQ, Chen J, He BS, Pan YQ, Wang F,
Deng QW, Sun HL, Liu X and Wang SK: The effect of BIM deletion
polymorphism on intrinsic resistance and clinical outcome of cancer
patient with kinase inhibitor therapy. Sci Rep. 5:113482015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JY, Ku BM, Lim SH, Lee MY, Kim H, Kim
M, Kim S, Jung HA, Sun JM, Ahn JS, et al: The BIM deletion
polymorphism and its clinical implication in patients with
EGFR-mutant non-small-cell lung cancer treated with EGFR tyrosine
kinase inhibitors. J Thorac Oncol. 10:903–909. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sos ML, Koker M, Weir BA, Heynck S,
Rabinovsky R, Zander T, Seeger JM, Weiss J, Fischer F, Frommolt P,
et al: PTEN loss contributes to erlotinib resistance in EGFR-mutant
lung cancer by activation of Akt and EGFR. Cancer Res.
69:3256–3261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee Y, Lee GK, Hwang JA, Yun T, Kim HT and
Lee JS: Clinical likelihood of sporadic primary EGFR T790M mutation
in EGFR-mutant lung cancer. Clin Lung Cancer. 16:46–50. 2015.
View Article : Google Scholar : PubMed/NCBI
|