Introduction
The most recently reported estimate of the annual
global incidence of endometrial carcinoma (EC) is 320,000 new cases
(1), making it the most common
gynecological malignancy (2), with
an estimated annual global mortality of 76,000 (1). The surgical management and prediction
of outcome for patients with EC are guided by the International
Federation of Gynecology and Obstetrics (FIGO) staging system
(3). However, patients with the same
disease stage may experience very different clinical courses
(1,4). To elucidate the reasons for these
differences, several investigators have evaluated the effect of
various tumor attributes on outcomes to delineate prognostic
factors, such as histological subtype and FIGO stage (4–6).
Unfortunately, preoperative evaluations generally require invasive,
costly and time-consuming procedures, such as fractional curettage,
transvaginal ultrasonography, magnetic resonance imaging, or
hysteroscopic assessment (1,7–9). Early
detection and improvements in surgical techniques and chemotherapy
have contributed to improvements in prognosis. However, precise
predictions of prognosis remain elusive, although they are crucial
for optimal treatment decisions.
Previous studies of various cancer types have
reported that cancers associated with a systemic inflammatory
response are associated with poorer prognosis (10–12). In
particular, the Glasgow prognostic score (GPS) is useful for
predicting prognosis in a number of cancer types (13–16). The
GPS is derived from an inflammation-based prognostic scoring
system, including serum C-reactive protein (CRP) and albumin
levels. A high pretreatment GPS has been reported to be a poor
prognostic factor for patients with cervical and ovarian carcinomas
(17–20), but it has not been evaluated
thoroughly in EC. Although the prognosis of EC is generally
favorable (21), it is poor for a
proportion of the patients. Therefore, the aim of the present study
was to elucidate the clinical impact of a high pretreatment GPS in
patients with EC.
Patients and methods
Patients
The present study was approved by the Ethics
Committee of Shimane Medical University. A total of 118 patients
with EC who underwent surgery at the University Hospital of Shimane
(Izumo, Japan) between January 1997 and December 2013 were
investigated. Patients with insufficient data, non-surgical
treatment, secondary malignancies and hematological diseases were
excluded.
Diagnosis was based on conventional morphological
examinations of hematoxylin and eosin-stained sections, and tumors
were classified according to the World Health Organization
classification (22). Tumor staging
and grading were performed according to the FIGO classification.
All patients underwent surgery (total abdominal hysterectomy and
bilateral salpingo-oophorectomy), and those with a cancer stage
>1a and grade 1 underwent pelvic lymph node dissection and
adjuvant platinum and taxane chemotherapy. Pelvic lymph node
dissection and adjuvant chemotherapy were omitted in EC patients
with stage 1a and grade 1 disease.
Measurement of GPS
Pretreatment serum CRP and albumin levels were
measured 4 weeks prior to surgery. The pretreatment GPS was
classified as follows: Patients with both a high CRP level (>1.0
mg/dl) and hypoalbuminemia (<3.5 g/dl) were assigned a score of
2, those with only one of these biochemical abnormalities were
assigned a score of 1, and those with neither of these
abnormalities were assigned a score of 0 (23).
Statistical analysis
Statistical analyses were conducted with SPSS
software for Windows, version 19.0 (SPSS Inc., Chicago, IL, USA).
Binomial logistic regression analysis was used for univariate
analysis in case of ordered categorical variables. The following
clinical factors were used for modeling: Patient age at diagnosis
(<60 vs. ≥60 years), stage (I/II vs. III/IV), histological type
(endometrioid vs. others), tumor grade (1 vs. 2/3), pretreatment
GPS (0 vs. 1 vs. 2), carbohydrate antigen (CA) 19-9 level (<37
vs. ≥37 U/ml), and carcinoembryonic antigen (CEA) level (<5 vs.
≥5 ng/ml). Progression-free survival (PFS) and overall survival
(OS) were the endpoints of the analysis. PFS was defined as the
date from initial diagnosis to initial recurrence of disease.
Patients with no recurrence at their last follow-up visit were
censored at that time. OS was defined as the date from initial
diagnosis to death. Patients alive at their last follow-up visit
were censored at that time. Kaplan-Meier curves and log-rank tests
were used to plot the survival data and determine the statistical
significance of survival differences. Variables that were
significant (P<0.05) in the univariate analysis were entered
into the multivariate analysis. The Cox's proportional hazards
model was used for the prognostic analysis. Data of patients who
were lost to follow-up were censored. All reported P-values were
two-sided, and P<0.05 was considered to indicate statistically
significant differences.
Results
Patient and clinical
characteristics
A total of 118 patients with EC were enrolled. Their
clinicopathological characteristics are summarized in Table I.
 | Table I.Clinical characteristics of the
patient population (n=118). |
Table I.
Clinical characteristics of the
patient population (n=118).
| Characteristics | No. of patients
(%) |
|---|
| Age at diagnosis,
years |
|
|
<60 | 55 (47) |
| ≥60 | 63 (53) |
| FIGO stage |
|
| I,
II | 79 (67) |
| III,
IV | 39 (33) |
| Histology |
|
|
Endometrioid | 99 (84) |
|
Other | 19 (16) |
| Grade |
|
| G1 | 53 (45) |
| G2,
G3 | 65 (55) |
| Pretreatment GPS |
|
| 0 | 91 (77) |
| 1 | 19 (16) |
| 2 | 8 (7) |
| CA19-9, U/ml |
|
|
<37 | 93 (79) |
| ≥37 | 25 (21) |
| CEA, ng/ml |
|
|
<5.0 | 97 (82) |
| ≥5.0 | 21 (18) |
Associations between
clinicopathological parameters and preoperative GPS in patients
with EC
Binomial logistic regression analyses (Table II) were used to evaluate the
associations between patient clinicopathological factors and
pretreatment GPS. Clinical stage (P<0.001), histological type
(P=0.007) and tumor grade (P=0.006) were found to be significantly
associated with pretreatment GPS.
 | Table II.Association of clinicopathological
parameters with pretreatment Glasgow prognostic score. |
Table II.
Association of clinicopathological
parameters with pretreatment Glasgow prognostic score.
|
|
| Pretreatment GPS |
|
|---|
|
|
|
|
|
|---|
| Variables | No. of patients | 0 | 1 | 2 | P-value |
|---|
| Age at diagnosis,
years |
|
|
|
| 0.348 |
|
<60 | 55 | 45 | 6 | 4 |
|
| ≥60 | 63 | 46 | 13 | 4 |
|
| FIGO stage |
|
|
|
| <0.001 |
| I,
II | 79 | 70 | 8 | 1 |
|
| III,
IV | 39 | 21 | 11 | 7 |
|
| Histology |
|
|
|
| 0.007 |
|
Endometrioid | 99 | 82 | 12 | 5 |
|
|
Other | 19 | 9 | 7 | 3 |
|
| Grade |
|
|
|
| 0.006 |
| G1 | 53 | 47 | 6 | 0 |
|
| G2,
G3 | 65 | 44 | 13 | 8 |
|
| CA19-9, U/ml |
|
|
|
| 0.116 |
|
<37 | 93 | 76 | 13 | 4 |
|
|
≥37 | 25 | 16 | 5 | 4 |
|
| CEA, ng/ml |
|
|
|
| 0.567 |
|
<5.0 | 97 | 79 | 11 | 7 |
|
|
≥5.0 | 21 | 15 | 4 | 1 |
|
Univariate and multivariate analyses
of prognostic factors in patients with EC
Age, clinical stage, histological type, grade,
pretreatment GPS, CA19-9, and CEA levels were investigated in
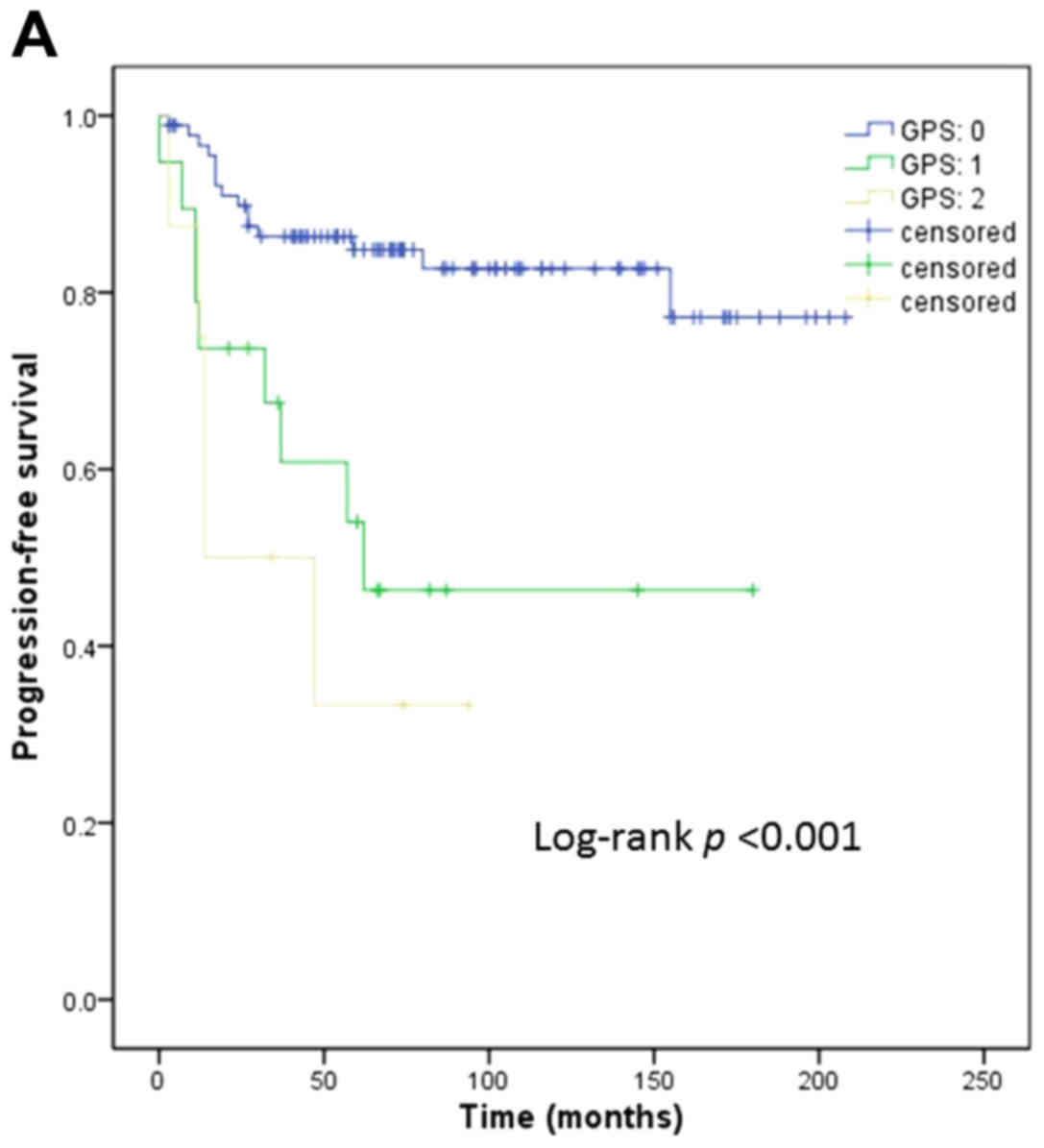
univariate analyses of survival. As regards PFS, age (P=0.024),
stage (P<0.001), histological type (P<0.001), grade (P=0.004)
and pretreatment GPS (P<0.001; Fig.
1A) were found to be significant predictors. Multivariate
analysis revealed that advanced stage (III/IV) [hazard ratio
(HR)=3.981; 95% confidence interval (CI): 1.712–9.256; P=0.001],
histological type (except for endometrioid; HR=2.794; 95% CI:
1.249–6.248; P=0.012), and high pretreatment GPS (HR=1.792; 95% CI:
1.076–2.985; P=0.025) were independent negative predictors for PFS
(Table III). As regards OS, as
shown in Table IV, stage
(P<0.001), histological type (P<0.001), grade (P=0.039),
pretreatment GPS (P<0.001; Fig.
1B) and CEA level (P=0.013) were found to be significant
predictors. The multivariate analysis revealed that advanced stage
(stage III/IV) (HR=5.469; 95% CI: 1.649–18.137; P=0.005),
histological type (except for endometrioid; HR=4.214; 95% CI:
1.346–13.197; P=0.014), high pretreatment GPS (HR=2.126; 95% CI:
1.021–4.428; P=0.044) and CEA level (≥5.0 ng/ml; HR=4.110; 95% CI:
1.246–13.553; P=0.020) were significant independent negative
predictors for OS (Table IV).
 | Table III.Univariate and multivariate analyses
of prognostic factors for progression-free survival. |
Table III.
Univariate and multivariate analyses
of prognostic factors for progression-free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age at diagnosis,
years | 2.559 | 1.132–5.784 | 0.024 | 2.090 | 0.899–4.862 | 0.087 |
| FIGO stage | 5.271 | 2.376–11.694 | <0.001 | 3.981 | 1.712–9.256 | 0.001 |
| Histology | 4.787 | 2.268–10.162 | <0.001 | 2.794 | 1.249–6.248 | 0.012 |
| Grade | 4.778 | 1.662–13.738 | 0.004 | N/A | N/A | N/A |
| Pretreatment
GPS | 2.618 | 1.654–4.154 | <0.001 | 1.792 | 1.076–2.985 | 0.025 |
| CA19-9, U/ml | 0.943 | 0.381–2.332 | 0.899 | – | – | – |
| CEA, ng/ml | 1.978 | 0.727–5.380 | 0.181 | – | – | – |
 | Table IV.Univariate and multivariate analyses
of prognostic factors for overall survival. |
Table IV.
Univariate and multivariate analyses
of prognostic factors for overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age at diagnosis,
years | 1.976 | 0.740–5.275 | 0.174 | – | – | – |
| FIGO stage | 8.658 | 2.802–26.754 | <0.001 | 5.469 | 1.649–18.137 | 0.005 |
| Histology | 5.447 | 2.129–13.940 | <0.001 | 4.214 | 1.346–13.197 | 0.014 |
| Grade | 3.698 | 1.070–12.782 | 0.039 | N/A | N/A | N/A |
| Pretreatment
GPS | 2.583 | 1.454–4.588 | <0.001 | 2.126 | 1.021–4.428 | 0.044 |
| CA19-9, U/ml | 1.392 | 0.493–3.927 | 0.532 | – | – | – |
| CEA, ng/ml | 4.030 | 1.342–12.101 | 0.013 | 4.110 | 1.246–13.553 | 0.020 |
Discussion
To the best of our knowledge, this is the first
report to demonstrate that a high preoperative GPS is a negative
prognostic factor in patients with EC. Using Cox's regression
analysis, it was revealed that a high pretreatment GPS is an
independent prognosticator in EC, which is consistent with its
prognostic ability in other malignancies.
The mechanisms of the correlation between an
elevated GPS and poor prognosis remain unclear. As GPS is
determined by CRP and albumin levels, proposed explanations include
the following: i) Circulating CRP levels may indicate inflammatory
status in the tumor microenvironment (23). Tumor growth, invasion and metastasis
may be stimulated by inflammation in the tumor microenvironment
(20,24). ii) CRP production is stimulated by
proinflammatory cytokines, such as interleukin (IL)-6 and tumor
necrosis factor (TNF)-α, which may promote tumor cell survival,
growth and migration (25–27); elevated CRP levels may reflect
increased levels of these cytokines. iii) CRP may protect tumor
cells from apoptosis. Human CRP binds Fc gamma receptors, and by
activating the phosphatidylinositol 3-kinase/Akt, nuclear factor-κB
and extracellular signal-regulated kinase pathways, prevents
chemotherapy-induced apoptosis of myeloma cells (28). CRP also increases the secretion of
IL-6 and exerts a synergistic effect to protect myeloma cells from
chemotherapy-induced apoptosis (25); if these effects occur in EC, CRP may
become a treatment target. iv) In addition, serum albumin levels
may be decreased by proinflammatory cytokines, such as IL-6 and
TNF, via inhibition of albumin production in hepatic cells
(10,26); thus, the possible mechanisms
underlying the association between serum albumin concentration and
survival are similar to those for CRP. Furthermore, since albumin
is a marker of nutritional status, pretreatment hypoalbuminemia may
indicate pre-existing malnutrition or poor health status (29).
There were certain limitations to the present study,
such as its retrospective design. In addition, the GPS was measured
only once, as part of a routine examination prior to treatment. The
GPS has been shown to be a clinical biomarker that potentially
reflects aggressive tumor biology. Therefore, longitudinal studies
with continuous GPS determinations over different treatment periods
are required to elucidate the association between GPS and
prognosis, and the mechanisms underlying this association.
In conclusion, a high pretreatment GPS was found to
be correlated with poor surgical outcomes in patients with EC. The
pretreatment GPS may represent a cost-effective and convenient
predictive marker for the identification of high-risk populations,
which may guide clinical decision-making and improve patient
outcomes.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
SGO Clinical Practice Endometrial Cancer
Working Group, ; Burke WM, Orr J, Leitao M, Salom E, Gehrig P,
Olawaiye AB, Brewer M, Boruta D, Villella J, et al: Endometrial
cancer: A review and current management strategies: Part I. Gynecol
Oncol. 134:385–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ho CM, Chien TY, Huang SH, Wu CJ, Shih BY
and Chang SC: Multivariate analysis of the prognostic factors and
outcomes in early cervical cancer patients undergoing radical
hysterectomy. Gynecol Oncol. 93:458–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamura T, Tsukamoto N, Tsuruchi N, Saito
T, Matsuyama T, Akazawa K and Nakano H: Multivariate analysis of
the histopathologic prognostic factors of cervical cancer in
patients undergoing radical hysterectomy. Cancer. 69:181–186. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takeda N, Sakuragi N, Takeda M, Okamoto K,
Kuwabara M, Negishi H, Oikawa M, Yamamoto R, Yamada H and Fujimoto
S: Multivariate analysis of histopathologic prognostic factors for
invasive cervical cancer treated with radical hysterectomy and
systematic retroperitoneal lymphadenectomy. Acta Obstet Gynecol
Scand. 81:1144–1151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fotiou S, Vlahos N, Kondi-Pafiti A,
Zarganis P, Papakonstantinou K and Creatsas G: Intraoperative gross
assessment of myometrial invasion and cervical involvement in
endometrial cancer: Role of tumor grade and size. Gynecol Oncol.
112:517–520. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lo KW, Cheung TH, Yim SF and Chung TK:
Preoperative hysteroscopic assessment of cervical invasion by
endometrial carcinoma: A retrospective study. Gynecol Oncol.
82:279–282. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haldorsen IS, Berg A, Werner HM, Magnussen
IJ, Helland H, Salvesen OO, Trovik J and Salvesen HB: Magnetic
resonance imaging performs better than endocervical curettage for
preoperative prediction of cervical stromal invasion in endometrial
carcinomas. Gynecol Oncol. 126:413–418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guthrie GJ, Charles KA, Roxburgh CS,
Horgan PG, McMillan DC and Clarke SJ: The systemic
inflammation-based neutrophil-lymphocyte ratio: Experience in
patients with cancer. Crit Rev Oncol Hematol. 88:218–230. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McMillan DC: Systemic inflammation,
nutritional status and survival in patients with cancer. Curr Opin
Clin Nutr Metab Care. 12:223–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McMillan DC, Elahi MM, Sattar N, Angerson
WJ, Johnstone J and McArdle CS: Measurement of the systemic
inflammatory response predicts cancer-specific and non-cancer
survival in patients with cancer. Nutr Cancer. 41:64–69. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ, Dagg K and Scott HR: A prospective longitudinal study
of performance status, an inflammation-based score (GPS) and
survival in patients with inoperable non-small-cell lung cancer. Br
J Cancer. 92:1834–1836. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brown DJ, Milroy R, Preston T and McMillan
DC: The relationship between an inflammation-based prognostic score
(Glasgow Prognostic Score) and changes in serum biochemical
variables in patients with advanced lung and gastrointestinal
cancer. J Clin Pathol. 60:705–708. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Glen P, Jamieson NB, McMillan DC, Carter
R, Imrie CW and McKay CJ: Evaluation of an inflammation-based
prognostic score in patients with inoperable pancreatic cancer.
Pancreatology. 6:450–453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ramsey S, Lamb GW, Aitchison M, Graham J
and McMillan DC: Evaluation of an inflammation-based prognostic
score in patients with metastatic renal cancer. Cancer.
109:205–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Omichi C, Nakamura K, Haraga J, Masuyama H
and Hiramatsu Y: Glasgow prognostic score is an independent marker
for poor prognosis with all cases of epithelial ovarian cancer.
Cancer Med. 5:1074–1080. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu J, Wang H, Liu CC, Lu Y and Tang H:
The Glasgow Prognostic Score (GPS) is a novel prognostic indicator
in advanced epithelial ovarian cancer: A multicenter retrospective
study. J Cancer Res Clin Oncol. 142:2339–2345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishida T, Nakamura K, Haraga J, Ogawa C,
Kusumoto T, Seki N, Masuyama H, Katayama N, Kanazawa S and
Hiramatsu Y: The Glasgow prognostic score determined during
concurrent chemoradiotherapy is an independent predictor of
survival for cervical cancer. Int J Gynecol Cancer. 25:1306–1314.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Polterauer S, Grimm C, Seebacher V, Rahhal
J, Tempfer C, Reinthaller A and Hefler L: The inflammation-based
Glasgow prognostic score predicts survival in patients with
cervical cancer. Int J Gynecol Cancer. 20:1052–1057. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO Classification of Tumors of Female Reproductive
Organs. Fourth. Lyon, France: IARC Press; pp. 62014
|
|
23
|
Marnell L, Mold C and Du Clos TW:
C-reactive protein: Ligands, receptors and role in inflammation.
Clin Immunol. 117:104–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ and Dunlop DJ: Evaluation of cumulative prognostic
scores based on the systemic inflammatory response in patients with
inoperable non-small-cell lung cancer. Br J Cancer. 89:1028–1030.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Candido J and Hagemann T: Cancer-related
inflammation. J Clin Immunol. 33 Suppl 1:S79–S84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang J, Wezeman M, Zhang X, Lin P, Wang M,
Qian J, Wan B, Kwak LW, Yu L and Yi Q: Human C-reactive protein
binds activating Fcgamma receptors and protects myeloma tumor cells
from apoptosis. Cancer Cell. 12:252–265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gupta D and Lis CG: Pretreatment serum
albumin as a predictor of cancer survival: A systematic review of
the epidemiological literature. Nutr J. 9:692010. View Article : Google Scholar : PubMed/NCBI
|