Introduction
Renal cell carcinoma (RCC) is clinically
characterized by late recurrence and metastasis, and McNichols
et al defined late RCC recurrence as occurrence more than 10
years after nephrectomy (1).
Duodenal metastasis from RCC is rare (2,3). Herein,
we report a very rare case of RCC in a man for whom we performed
imaging studies to evaluate the clinical stage of newly developed
diffuse large B-cell lymphoma (DLBCL), and were incidentally able
to find the ectopic recurrence of RCC in the duodenum/pancreatic
head 25 years after its curative resection. Two different
malignancies occurred simultaneously in the small intestine
(duodenum and ileum), highlighting the need for careful
differential diagnosis.
Case report
A 64-year-old Japanese man with systemic lymph nodes
swelling who had undergone left nephrectomy for RCC 25 years
previously was admitted to our hospital. The patient complained of
abdominal pain, night-time fever, anorexia, and weight loss (−7 kg
in 2 months), and the performance status was 1. Blood test
examinations on admission revealed mild anemia (hemoglobin 12.4:
normal range 14.0–18.0 g/dl), decreased total protein (6.2: 6.7–8.3
g/dl) and albumin (3.4: 3.8–5.3 g/dl), and increased lactate
dehydrogenase (LDH 798: 120–245 U/l) and C-reactive protein (CRP
4.16: <0.30 mg/dl). The soluble interleukin-2 receptor level had
risen to 7,597 (sIL-2R: 121–613 U/ml) and it further increased to
9,300 within 2 weeks. Inguinal lymph nodes biopsy was performed,
leading to a diagnosis of DLBCL. The Ki-67 labeling (MIB1) index
was approximately 70%.
We performed imaging studies to evaluate the
clinical stage. fluorine-18-fluorodeoxy-glucose
(18F-FDG)-positron emission tomography (PET)/computed
tomography (CT) showed multiple lymph nodes involving the cervical
region, an abdominal bulky mass, spleen, and ileocecal lesions. CT
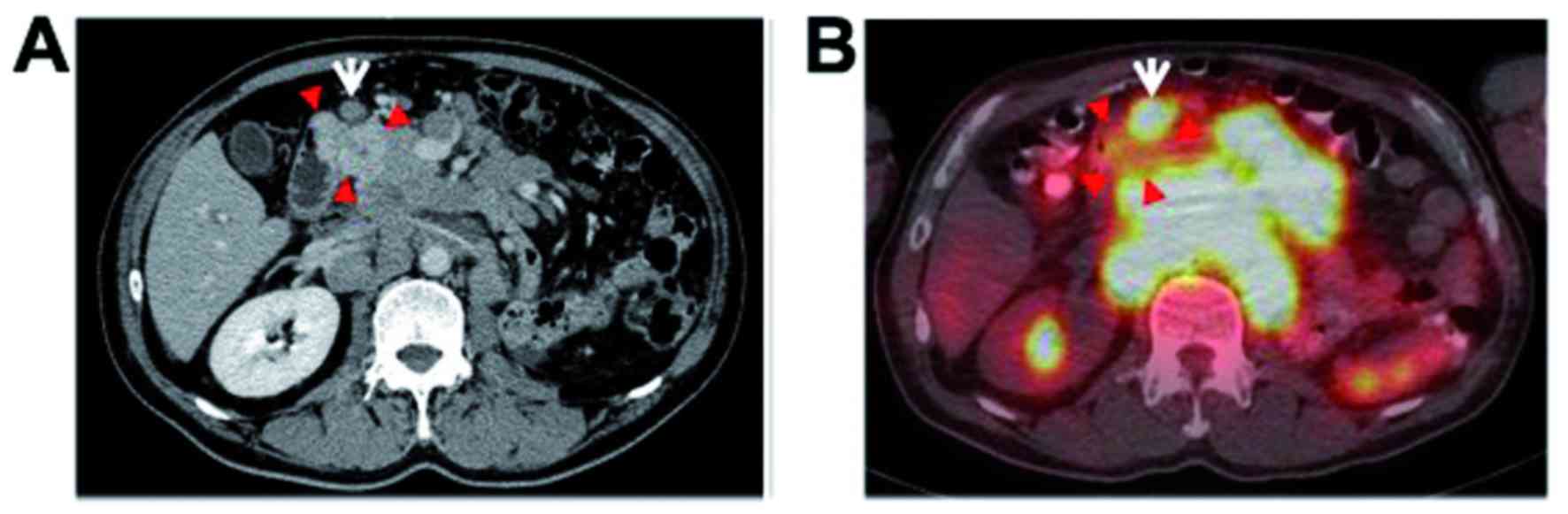
revealed an obvious hypervascular tumor involving the
duodenum/pancreatic head (Fig. 1A),
and small nodules up to 1 cm in diameter were scattered throughout
both lung fields, but these tumors demonstrated no uptake on
18F-FDG-PET/CT (Fig. 1B).
The right kidney exhibited no abnormalities. On
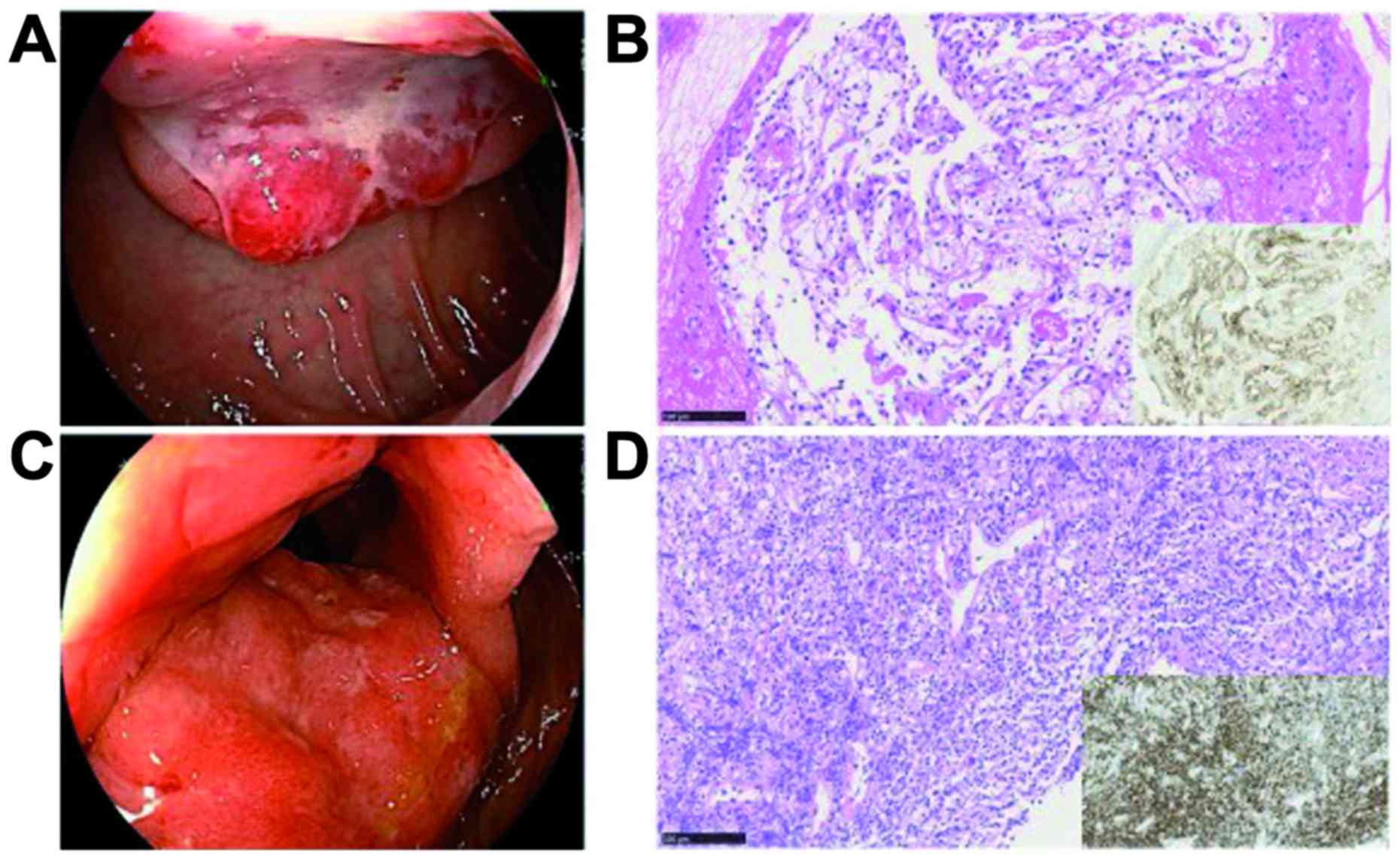
esophagogastroduodenoscopy (EGD), blood refluxed from the duodenum
was seen retained in the stomach. The tumor was detected in the
descending portion of the duodenum apart from the periampullary
region, and was seen consistent with submucosal tumor with central
ulcer resembled those of ulcer-forming DLBCL (Fig. 2A). Biopsy was performed carefully,
and this tumor was pathologically diagnosed with a clear cell
RCC-derived metastatic cancer immunohistochemically positive for
CD10 (Fig. 2B). Ileocolonoscopy
showed mucosal thickening of the terminal ileum including ileocecal
valve (Fig. 2C), and biopsy led to a
diagnosis of DLBCL infiltration immunohistochemically positive for
CD20 (Fig. 2D).
Based on imaging studies and pathological findings,
the patient was diagnosed with coexistence of metastatic
duodenal/pancreatic cancer from RCC and DLBCL (Ann Arbor stage IV),
and the latter was classified as high-intermediate risk with the
international prognostic index (IPI). We then began treatment for
DLBCL because of its tumor volume. The patient received five
courses of chemotherapy, including rituximab (RTX) + EPOCH regimen
(etoposide, prednisone, vincristine, cyclophosphamide, and
doxorubicin) and RTX + GDP regimen (gemcitabine, cisplatin, and
dexamethasone). As a result, the sIL-2R level decreased to 903
U/ml, and the patient achieved complete remission (assessed by
PET). Although the tumor in the duodenum/pancreatic head slightly
decreased in size (from 3.6 to 3.0 cm) in the past 4 months, a
tumor of about 1 cm in diameter appeared in the pancreatic body,
and some of the nodules scattered in both lung fields grew and
increased slightly. As these findings suggested that the metastatic
cancer from RCC had started to grow mainly in the pancreas and
lungs, the patient was transferred to a designated cancer hospital
to receive treatment for these lesions. Subsequent oral pazopanib
treatment was successful for this patient, and the treatment is
being continued as the outpatient. Written informed consent was
obtained from the patient for publication of this case report.
Discussion
RCC has a potential to metastasize to almost any
site. The most common sites of metastasis are the lung, liver, and
bones (4). Clinically evident
gastrointestinal involvement of RCC, especially solitary duodenal
metastasis from RCC is rare and most frequently involves the
periampullary region or the duodenal bulb (5,6).
According to recent report (7), of
the 3637 patients diagnosed with RCC, 15 patients (0.4%) with 19
gastrointestinal lesions were identified, and duodenum involvement
was 6 lesions.
Because coexistence of two different malignancies,
metastatic cancer from RCC and malignant lymphoma (DLBCL), in the
small intestine simultaneously is extremely rare, it was necessary
to make a differential diagnosis carefully with imaging studies.
Metastatic RCC is frequently hypervascular as with primary tumors
(8). In gastrointestinal metastasis
from RCC, intraluminal polypoid masses (63.2%) with
hyperenhancement (78.9%) and heterogeneous enhancement (63.2%) were
the most common CT findings (7),
especially, than in lymphoma, lymphadenopathy has been reported to
be much less prominent, the involved bowel segment shorter, and
multi-focality less common (9). RCC
(especially clear cell carcinoma) commonly exhibits a low
18F-FDG uptake, and FDG-PET has a high false-negative
rate (68.5%) for detecting primary lesions (10). Aide et al reported that the
sensitivity of FDG-PET for RCC was 47% (11). Comparing the sensitivity and
specificity of FDG-PET and CT for primary and metastatic lesions in
66 patients with RCC, Kang et al also reported that although
the sensitivity of FDG-PET (primary 60%, metastasis 75.0–77.3%) was
lower than that of CT (primary 91.7%, metastasis 91.1–93.8%), the
specificity of FDG-PET (primary 100%, metastasis 97.1–100%) was
higher than that of CT (primary 100%, metastasis 73.1–98.1%)
(12). Thus, the role of
18F-FDG-PET in the detection of RCC is limited by low
sensitivity (10–12). On the other hand, FDG-PET has emerged
as a powerful functional imaging tool for staging, restaging, and
is essential for the post-treatment assessment of DLBCL (13). On endoscopy, the metastatic duodenal
cancer can be seen as a submucosal mass with ulceration of the tip,
multiple nodules of varying sizes or raised plaques (14). Endoscopic findings of ulcer-forming
lymphoma are the submucosal tumor with central ulcer (15), and resemble those of metastatic
intestinal cancer. In our case, FDG-PET/CT revealed a high uptake
in DLBCL lesions, whereas it was false-negative for the metastatic
duodenal RCC lesion. In addition, on CT, the metastatic duodenal
RCC lesion demonstrated hyper-enhancement more clearly than the
DLBCL lesions. These findings led to a differential diagnosis by
imaging, and endoscopic biopsy confirmed the diagnosis. CD10
immunostaining is helpful in separating metastatic RCC from other
cancers (16).
According to the review of Rustagi et al
(17), the mean duration post
nephrectomy to diagnosis of solitary duodenal metastases was
7.9±4.7 years (median 8 years). RCC can metastasize for a long
period of disease latency after nephrectomy, via the lymphatic or
hematogenous route, as well as by peritoneal dissemination or
direct invasion into adjacent anatomic structures (18). Our case is rare in that
‘late’-recurring RCC, so long 25 years after nephrectomy,
metastasized to the duodenum/pancreatic head. This tumor was
thought to be a slow-growing and direct duodenal invasion from an
adjacent recurrent/metastatic lesion of the pancreatic head. On the
other hand, DLBCL is considered to progress monthly (19). In our patient, the tumor volume of
DLBCL observed on imaging was much greater than that of the
metastatic cancer from RCC. IL-2R sharply increased in this
patient, revealing that progression was rapid and the current
pathology may have been completed within 0.5–1 year after the newly
development. As DLBCL was considered to determine the prognosis of
this patient, we prioritized its treatment, and the patient
achieved CR. However, during the treatment period, the metastatic
cancer from RCC spread, mainly to the pancreas and lungs.
Generally, an immune cell-inhibiting mechanism is present in the
microcirculatory environment of tumors (20). A trace number of cancer cells of RCC
were latently present under the control of the immunological
surveillance mechanism, but it may have manifested because the
DLBCL tumor volume rapidly increased and inhibited immunity, and
the subsequent growth of metastatic cancer cells from RCC may have
been slightly rapid, unlike the slow growth previously reported
(1,6,17).
To the best of our knowledge, this is the first case
report of the coexistence of metastatic cancer from RCC and
malignant lymphoma in the small intestine simultaneously. It was
necessary to make a careful differential diagnosis in the imaging
studies.
References
|
1
|
McNichols DW, Segura JW and DeWeerd JH:
Renal cell carcinoma: Long-term survival and late recurrence. J
Urol. 126:17–23. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhatia A, Das A, Kumar Y and Kochhar R:
Renal cell carcinoma metastasizing to duodenum: A rare occurrence.
Diagn Pathol. 1:292006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brener ZZ, Zhuravenko I, Jacob CE and
Bergman M: An unusual presentation of renal cell carcinoma with
late metastases to the small intestine, thyroid gland, nose and
skull base. Nephrol Dial Transplant. 22:930–932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murphy WM, Beckwith JB and Farrow GM:
Atlas of tumor pathology, 3rd series, fascicle 11. Tumors of the
kidney, bladder and related urinary structures. Armed Forces
Institute of Pathology; Washington: pp. 1281994
|
|
5
|
Pavlakis GM, Sakorafas GH and
Anagnostopoulos GK: Intestinal metastases from renal cell
carcinoma: A rare cause of intestinal obstruction and bleeding. Mt
Sinai J Med. 71:127–130. 2004.PubMed/NCBI
|
|
6
|
Featherstone JM, Bass P, Cumming J and
Smart CJ: Solitary, late metastatic recurrence of renal cell
carcinoma: Two extraordinary cases. Int J Urol. 13:1525–1527. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park HJ, Kim HJ, Park SH, Lee JS, Kim AY
and Ha HK: Gastrointestinal involvement of recurrent renal cell
carcinoma: CT findings and clinicopathologic features. Korean J
Radiol. 18:452–460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sheth S, Scatarige JC, Horton KM, Corl FM
and Fishman EK: Current concepts in the diagnosis and management of
renal cell carcinoma: Role of multidetector ct and
three-dimensional CT. Radiographics. 21:S237–S254. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Byun JH, Ha HK, Kim AY, Kim TK, Ko EY, Lee
JK, Yu ES, Myung SJ, Yang SK, Jung HY, et al: CT findings in
peripheral T-cell lymphoma involving the gastrointestinal tract.
Radiology. 227:59–67. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyakita H, Tokunaga M, Onda H, Usui Y,
Kinoshita H, Kawamura N and Yasuda S: Significance of
18F-fluorodeoxyglucose positron emission tomography (FDG-PET) for
detection of renal cell carcinoma and immunohistochemical glucose
transporter 1 (GLUT-1) expression in the cancer. Int J Urol.
9:15–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aide N, Cappele O, Bottet P, Bensadoun H,
Regeasse A, Comoz F, Sobrio F, Bouvard G and Agostini D: Efficiency
of [(18)F]FDG PET in characterising renal cancer and detecting
distant metastases: A comparison with CT. Eur J Nucl Med Mol
Imaging. 30:1236–1245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang DE, White RL Jr, Zuger JH, Sasser HC
and Teigland CM: Clinical use of fluorodeoxyglucose F 18 positron
emission tomography for detection of renal cell carcinoma. J Urol.
171:1806–1809. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheson BD, Pfistner B, Juweid ME, Gascoyne
RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca
E, et al: Revised response criteria for malignant lymphoma. J Clin
Oncol. 25:579–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu CC, Chen JJ and Changchien CS:
Endoscopic features of metastatic tumors in the upper
gastrointestinal tract. Endoscopy. 28:249–253. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al Mofleh IA: Endoscopic features of
primary upper gastrointestinal lymphoma. J Clin Gastroenterol.
19:69–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Avery AK, Beckstead J, Renshaw AA and
Corless CL: Use of antibodies to RCC and CD10 in the differential
diagnosis of renal neoplasms. Am J Surg Pathol. 24:203–210. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rustagi T, Rangasamy P and Versland M:
Duodenal bleeding from metastatic renal cell carcinoma. Case Rep
Gastroenterol. 5:249–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang WT, Chai CY and Lee KT: Unusual
upper gastrointestinal bleeding due to late metastasis from renal
cell carcinoma: A case report. Kaohsiung J Med Sci. 20:137–141.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Armitage JO and Weisenburger DD: New
approach to classifying non-Hodgkin's lymphomas: Clinical features
of the major histologic subtypes. Non-Hodgkin's lymphoma
classification project. J Clin Oncol. 16:2780–2795. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mailloux AW and Young MR: Regulatory
T-cell trafficking: From thymic development to tumor-induced immune
suppression. Crit Rev Immunol. 30:435–447. 2010. View Article : Google Scholar : PubMed/NCBI
|