Introduction
Epstein-Barr virus (EBV)-associated gastric
carcinoma (GC) is defined by the presence of EBV within tumor cells
(1–3). Clonal EBV is present in ~10% of GCs and
is associated with distinct clinicopathological features. The
typical characteristics of EBV-positive GC are male predominance,
upper- and middle-third anatomical location, poorly differentiated
carcinoma with dense infiltration of lymphocytes (1–3), and
lymph node metastasis is rare in the EBV-positive than in
EBV-negative GC. (4). On endoscopy,
the informative features have been suggested to be superficial,
depressed, ulcerated (5) or
‘saucer-like’ tumor appearance, accompanied by submucosal nodules
of carcinoma with lymphoid stroma (1).
The present study reports a case of unusual growth
of EBV-associated early-stage GC, which exhibited a polypoid form
and appeared to be differentiated on histopathology. In addition,
the clinicopathological features of 25 EBV-associated GCs from 20
patients treated in our hospital between 2005 and 2014 were
reviewed for comparison with the current case.
Fujita Health University School of Medicine approved
the study protocol, and written informed consent was obtained from
all participants.
Case report
In June 2014, A 72-year-old male was receiving
treatment for a cerebral infarction in the left medulla oblongata
in the Fujita Health University Hospital. He underwent
esophagogastroduodenoscopy (EGD) due to suspicion of
gastrointestinal bleeding following the appearance of blood in the
nasogastric tube aspirate. Physical examination revealed left
hemiparesis. Laboratory evaluation did not show any abnormal
findings, including red blood cell count and levels of hemoglobin,
hematocrit and tumor markers (carcinoembryonic antigen and
carbohydrate antigen 19-9).
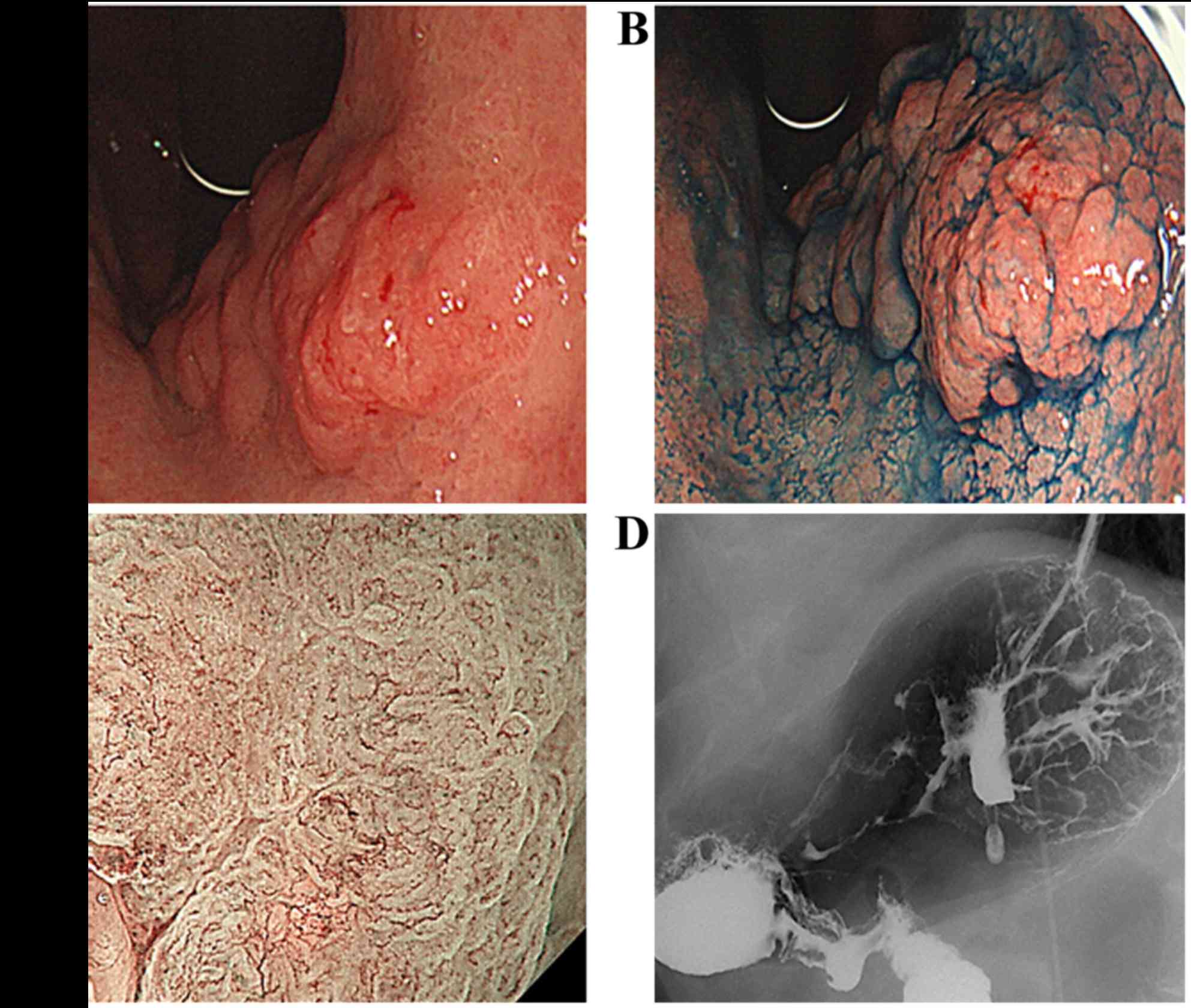
EGD revealed an uneven, type 0–I protruding lesion
measuring ~4.0 cm, which was located in the lesser curvature of the
upper gastric corpus (Fig. 1A and
B). Magnifying endoscopy with narrow-band imaging revealed
irregular microvascular and microsurface patterns with clear
demarcation, suggesting that the lesion was a carcinoma (Fig. 1C). Double-contrast upper
gastrointestinal barium X-ray radiography also showed an uneven,
type 0–I protruding lesion in the same location in the stomach,
consistent with the EGD findings (Fig.
1D).
Biopsy specimens taken from the protruding lesion
during EGD indicated a well-differentiated adenocarcinoma. Although
no distant or lymph node metastases were observed on computed
tomography, submucosal invasion was suspected due to the marked
thickness, uneven form, and 4-cm diameter of the tumor. Based on
its macroscopic appearance, laparoscopic gastrectomy with D1
lymphadenectomy was performed.
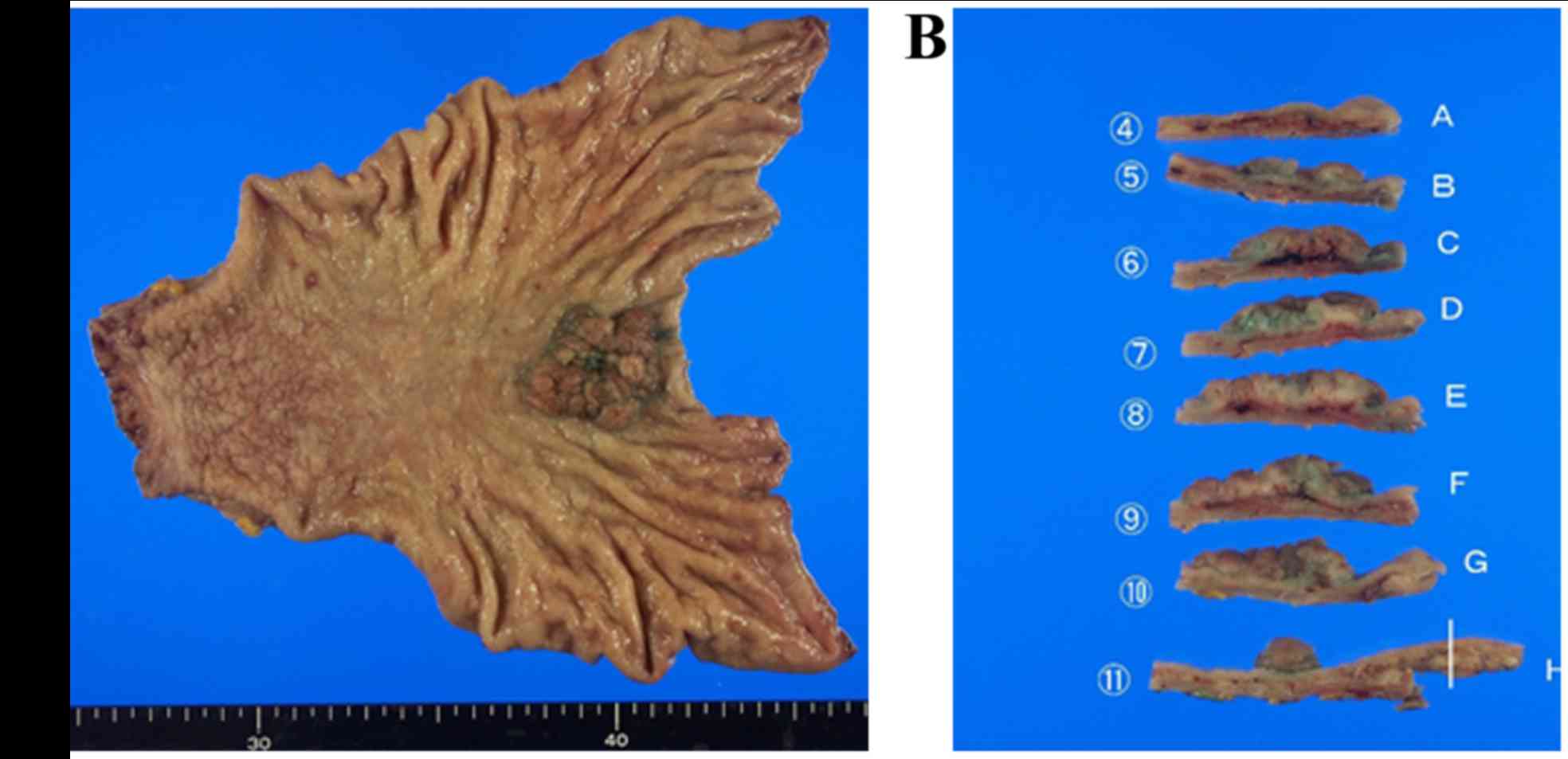
The resected tumor was 3.8×3.5×1.0 cm in size within
the resected surgical specimen. The tumor had an elastic
consistency, and its surface was uneven and irregular (Fig. 2A). The specimen was then cut into 11
pieces for histological assessment (Fig.
2B).
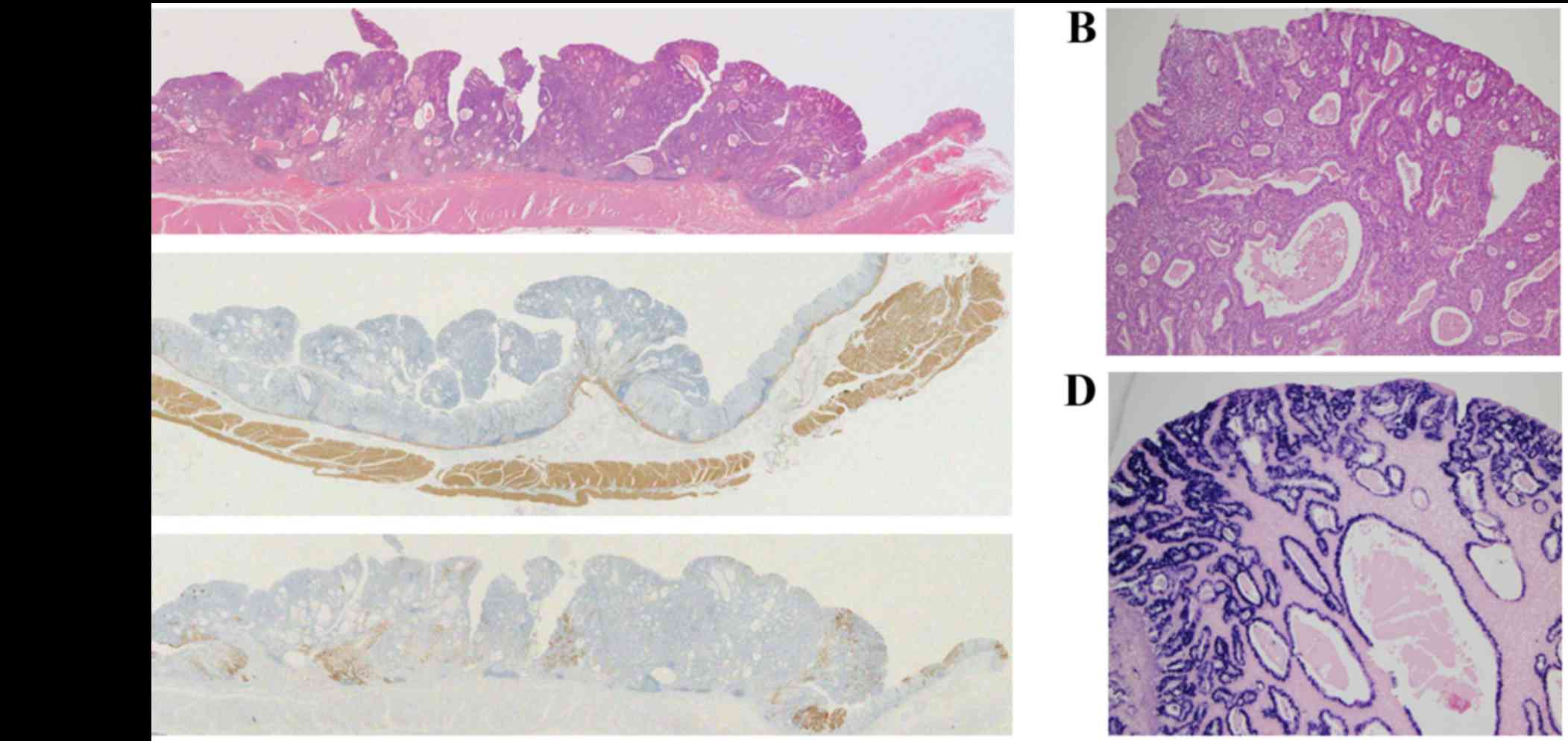
Histological assessment using the hematoxylin and
eosin staining revealed polypoid growth of a well-differentiated
tubular or papillary adenocarcinoma, with dense infiltration of
lymphocytes through the entire layer of the tumor (Fig. 3A and B). Marked cystic dilatation and
rounded expansion of the malignant glands with rich vascularization
was also observed (Fig. 3B). The
tumor cells were localized within the mucosal layer of the stomach,
and an absence of submucosal invasion was confirmed using desmin
immunohistochemistry (Fig. 3C). The
carcinoma crypts, but not the infiltrating lymphocytes were
EBV-positive on in situ hybridization (Fig. 3D). Immunohistochemistry also revealed
that the tumor cells were partially positive for mucin (MUC) 5AC
(Fig. 3E), but not for MUC6 or MUC1
(data not shown). No lymph node metastasis was observed in the
resected lymph nodes.
Discussion
EBV-positive GCs are characterized by distinctive
clinicopathological features (1–4). TP53
mutations are rare in the EBV-positive than in EBV-negative GC
(1). The typical endoscopic findings
of EBV-associated GC are reported to be superficial, depressed,
ulcerated (5) or ‘saucer-like’
(surrounding elevation) appearance of the tumor (1). When reviewing the clinicopathological
features of 25 EBV-associated GCs from 20 patients treated in our
hospital between 2005 and 2014 (Table
I), all tumors except that in the current case appeared as
shallow, depressed lesions (0–IIc, 0–IIc+III or 0–IIa+IIc) in the
early stages and as ulcerative lesions (type 2 or type 3) in the
advanced stages, according to the Japanese classification (6). Therefore, the current case, which
showed polypoid growth of a well-differentiated tubular or
papillary adenocarcinoma, with dense infiltration of lymphocytes
through the entire layer of the tumor, seemed to be an unusual
morphological appearance.
 | Table I.Clinicopathological features of 25
Epstein-Barr virus-associated gastric cancers from 20 patients. |
Table I.
Clinicopathological features of 25
Epstein-Barr virus-associated gastric cancers from 20 patients.
| Patient no. | Age, years | Sex | H. pylori | Location | Multifocal | Morphology | Pathology | Lymphocytic
infiltration | Depth | Node stage | Met. stage | Stage |
|---|
| 1 | 52 | Male | + | Upper | No | 0–IIc | Poor | + | SM2 | 0 | 0 | 1A |
| 2 | 49 | Male | − | Upper | No | 0–IIc+III | Well | − | M | 0 | 0 | 1A |
| 3 | 75 | Male | − | Middle | No | 0–IIc | Poor | − | M | 0 | 0 | 1A |
| 4 | 40 | Male | + | Upper | No | 0–IIa+IIc | Mod | + | SM1 | 0 | 0 | 1A |
| 5 | 48 | Male | − | Upper | No | 0–IIa+IIc | Poor | + | SM2 | 0 | 0 | 1A |
| 6 | 62 | Male | − | Middle | Yes | Type 3 | Poor | + | SE | 2 | 0 | 3B |
|
|
|
| − | Middle | Yes | Type 3 | Poor | + | SE | 2 | 0 | 3B |
| 7 | 74 | Male | + | Upper | No | Type 2 | Poor | + | SS | 2 | 0 | 3A |
| 8 | 63 | Male | + | Middle | No | Type 3 | Poor | + | SE | 2 | 0 | 4 |
| 9 | 55 | Male | + | Upper | Yes | Type 3 | Poor | + | SE | 2 | 0 | 4 |
|
|
|
| + | Upper | Yes | Type 3 | Poor | + | SE | 2 | 0 | 4 |
|
|
|
| + | Upper | Yes | Type 3 | Poor | + | SE | 2 | 0 | 4 |
| 10 | 72 | Female | + | Upper | Yes | Type 3 | Poor | + | SE | 1 | 0 | 3A |
|
|
|
| + | Lower | Yes | Type 2 | Poor | + | MP | 1 | 0 | 3A |
| 11 | 57 | Male | ND | Upper | No | Type 2 | Poor | + | SE | 0 | 0 | 2B |
| 12 | 64 | Male | − | Upper | Yes | 0–IIc | Mod | + | SM1 | 0 | 0 | 1A |
|
|
|
| − | Middle | Yes | 0–IIc | Mod | + | M | 0 | 0 | 1A |
| 13 | 70 | Male | − | Upper | No | Type 2 | Mod | + | SS | 2 | 0 | 3A |
| 14 | 61 | Male | − | Upper | No | Type 2 | Poor | + | SE | 1 | 0 | 3A |
| 15 | 73 | Male | ND | Upper | No | Type 2 | Poor | + | SM2 | 0 | 0 | 1A |
| 16 | 72 | Male | ND | Upper | No | 0–IIa | Well | − | M | 0 | 0 | 1A |
| 17 | 47 | Male | ND | Upper | No | Type 3 | Poor | + | SS | 0 | 0 | 2A |
| 18 | 75 | Male | − | Middle | No | Type 2 | Poor | + | MP | 0 | 0 | 1B |
| 19 | 55 | Male | + | Upper | No | 0–IIc | Poor | + | SM2 | 0 | 0 | 1A |
| 20 | 72 | Male | ND | Upper | No | 0–I | Well | − | M | 0 | 0 | 1A |
The reasons underlying the unusual morphological
appearance of the present case of EBV-associated GC are not clear.
The background gastric mucosa in this case showed severe gastric
atrophy, suggesting severe hypochlorhydria. Such a pathological
state of the gastric mucosa may be associated with polypoid growth
of well-differentiated tubular or papillary adenocarcinoma, even if
it is EBV-associated. The typical histological features of
EBV-associated GC include poorly differentiated carcinoma with
dense infiltration of lymphocytes (1–3), while
well or moderately differentiated histopathology may also be
observed (1).
In the cases reviewed from our hospital, 5 out of 6
superficial cancers (M or SM1), including the current case,
exhibited a differentiated histological type (well or moderately
differentiated adenocarcinoma), suggesting that a considerable
portion of the EBV-associated cases may have originally developed
with a differentiated histopathology and then become poorly
differentiated in the more advanced stages.
Dense infiltration of lymphocytes is a typical
pathological feature of EBV-associated GC (1) (Table I),
which was also observed in the current case (Fig. 3A and B). Furthermore, marked cystic
dilatation and rounded expansion of the malignant glands with rich
vascularization was also shown in this case (Fig. 3B); this may also explain the
thickness of the lesion, which led to difficultly in predicting
tumor depth. Although laparoscopic distal gastrectomy was performed
in the present case, considering the increased risk of gastric
surgery due to cerebral infarction, endoscopic submucosal
dissection could be chosen with extended indication as the
minimally invasive and curative treatment in this case.
Furthermore, EBV infects B-, T- and NK cells and has been
associated with a wide range of lymphoid malignancies as well as
autoimmune diseases, such as lupus erythematosus, rheumatoid
arthritis and particularly multiple sclerosis. Therefore, a
vaccination strategy may be considered (7).
In conclusion, the present study reports a case of
unusual growth of EBV-associated early-stage GC, which exhibited a
polypoid form and appeared to be differentiated on histopathology.
We hope the current report provide useful information for the
physician treating the GC.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Authors' contributions
NH, TT, TK and MO performed data collection,
analyzed data. NH and TT wrote manuscript.TI, NN, YN and MN,
advised about the data analyzing. MN and TT performed histological
examination. TS and N supervised throughout the study.
Ethics approval and consent to
participate
Fujita Health University School of Medicine approved
the protocol, and written informed consent was obtained from all
participating subjects.
Consent for publication
Written informed consent was obtained from all
participants of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fukayama M, Hino R and Uozaki H:
Epstein-Barr virus and gastric carcinoma: Virus-hostinteractions
leading to carcinoma. Cancer Sci. 99:1726–1733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akiba S, Koriyama C, Herrera-Goepfert R
and Eizuru Y: Epstein-Barr virus associated gastric carcinoma:
Epidemiological and clinicopathological features. Cancer Sci.
99:195–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uozaki H and Fukayama M: Epstein-Barr
virus and gastric carcinoma-viral carcinogenesis through epigenetic
mechanisms. Int J Clin Exp Pathol. 1:198–216. 2008.PubMed/NCBI
|
|
4
|
van Beek J, zur Hausen A, Kranenbarg Klein
E, van de Velde CJ, Middeldorp JM, van den Brule AJ, Meijer CJ and
Bloemena E: EBV-positive gastric adenocarcinomas: A distinct
clinicopathologic entity with a low frequency of lymph node
involvement. J Clin Oncol. 22:664–670. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yanai H, Nishikawa J, Mizugaki Y, Shimizu
N, Takada K, Matsusaki K, Toda T, Matsumoto Y, Tada M and Okita K:
Endoscopic and pathologic features of Epstein-Barr virus-associated
gastric carcinoma. Gastrointest Endosc. 45:236–242. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma: III English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Capone G, Fasano C, Lucchese G, Calabrò M
and Kanduc D: EBV-Associated Cancer and Autoimmunity: Searching for
Therapies. Vaccines. 3:74–89. 2015. View Article : Google Scholar : PubMed/NCBI
|