Introduction
Non-muscle invasive bladder cancer (NMIBC) is
subdivided into recurrence/progression risk groups according to
various clinical and pathological characteristics. Urologists
choose adjuvant or therapeutic intravesical instillation after
transurethral resection of bladder tumor (TURBT) or radical
cystectomy by reference to these risk classifications. Generally,
the criteria for lowest risk tumors are restricted to primary,
solitary, small, and histologically low-grade (LG) Ta tumors.
Low-risk NMIBC is defined as primary, solitary, Ta, LG/G1, size
<3 cm, no carcinoma in situ (CIS) by European Association
of Urology (EAU) (1); as LG,
solitary, Ta, ≤3 cm by American Urological Association
(AUA)/Society of Urologic Oncology (SUO) (2); and as solitary, primary LG Ta by
International Bladder Cancer Group (IBCG) (3). Single instillation of chemotherapeutic
agent is recommended as postoperative adjuvant therapy in these
low-risk NMIBC patients; however, the definitions of low-risk NMIBC
are not consistent among the guidelines.
Most primary and solitary LG Ta tumors are
relatively small. So, it is unclear whether the generally adopted
cutoff size of 3 cm is really appropriate in these tumors, because
this cutoff value was derived from randomized controlled trials
(RCTs) involving NMIBC patients who had diverse clinical and
pathological characteristics including biologically more aggressive
tumors such as recurrent and/or high-grade tumors. Similarly, the
cutoff value of tumor number to appropriately predict the risk is
not clear in these populations.
In the current study, we analyzed patients with only
primary LG Ta tumors and examined the cutoff values of tumor size
and tumor number to appropriately select low-risk patients.
Patients and methods
Patients
We reviewed the clinical and pathological records of
consecutive patients who underwent TURBT for primary bladder cancer
from January 2010 to June 2015, and who were histologically
diagnosed as LG Ta UC at Kyushu University Hospital and Harasanshin
Hospital. Patients with prior and/or concurrent history of upper
urinary tract UC and those lacking records of clinical data were
excluded. A total of 245 patients were included in the final
analysis. Histological diagnoses were based on both the WHO
classification 2004 (4) and WHO
classification 1973 (5). This was an
institutional review boards-approved study, and recruitment and
protection of patient data were performed according to the approved
protocols.
Follow-up evaluations consisted of cystoscopy and
urine cytology performed 3 months after TURBT. If no recurrence was
seen, the same evaluations were performed every 3 months for 2–3
years, and every 6 months thereafter.
The relationships between clinicopathological
characteristics, especially cutoff value of tumor size and tumor
number, and clinical outcome in terms of recurrence-free survival
(RFS) were examined. Tumor recurrence was defined as identification
of a new tumor in the bladder that was confirmed by histological
examination of consequent TURBT. Concerning progression-free
survival (PFS), only one patient experienced tumor progression,
defined as intravesical recurrence with confirmed histological
proper muscle invasion or detectable distant metastasis, thus we
did not analyze the relationship between tumor progression and
clinicopathological features.
Statistical analysis
Statistical analyses were performed with JMP Pro
version 12 (SAS Institute, Tokyo, Japan). Actuarial RFS and PFS
were calculated by Kaplan-Meier analysis, and univariate
comparisons between groups were assessed by log-rank tests.
Univariate and multivariate analysis were performed using a Cox
proportional hazards model to identify the variables that predict
prognostic outcomes. Values of P<0.05 were considered to be
statistically significant.
Results
Patient characteristics
Patient characteristics are shown in Table I. All bladder tumors were
histologically diagnosed as LG UC according to the 2004 WHO
classification (4), and 91 (37.1%)
were G1 and 154 (62.9%) were G2 according to the 1973 WHO
classification (5). Tumor number was
distributed as follows: single tumor in 153 patients (62.5%); 2–7
tumors in 78 patients (31.8%); and 8 or more tumors in 14 patients
(5.7%). Median size of maximum tumor was 1.4 cm in diameter (range,
0.2–6.0 cm), and 45 patients (18.4%) had tumors ≥3.0 cm in
diameter. A total of 107 patients (43.7%) received induction
intravesical chemotherapy postoperatively. Chemotherapeutic agents
used were either epirubicin (Epi-ADM) or a combination of mitomycin
C (MMC) and cytarabine (Ara-C), as chosen by the urologist in
charge. No patients received Bacille de Calmette et Guérin (BCG)
instillation therapy.
 | Table I.Patient and tumor characteristics. |
Table I.
Patient and tumor characteristics.
|
| No. of cases | % |
|---|
| Cases | 245 | – |
| Median age (year,
range) | 69 (37–90) | – |
| Sex |
|
|
| Male | 200 | 81.6 |
|
Female | 45 | 18.4 |
| No. of tumors |
|
|
| 1 | 153 | 62.5 |
| 2–7 | 78 | 31.8 |
|
>8 | 14 | 5.7 |
| Grade (WHO 1973) |
|
|
| G1 | 91 | 37.1 |
| G2 | 154 | 62.9 |
| Median tumor size
(cm, range) | 1.4 (0.2–6.0) |
|
| Tumor size |
|
|
| ≥1.0 | 188 | 76.7 |
| ≥1.5 | 121 | 49.4 |
| ≥2.0 | 99 | 40.4 |
| ≥3.0 | 45 | 18.4 |
| Introduction
intravesical chemotherapy |
|
|
| Done | 107 | 43.7 |
| Not
done | 138 | 56.3 |
| Median follow-up
(month, range) | 34 (3–73) | – |
Recurrence-free survival analysis
Forty-nine patients (24.1%) experienced intravesical
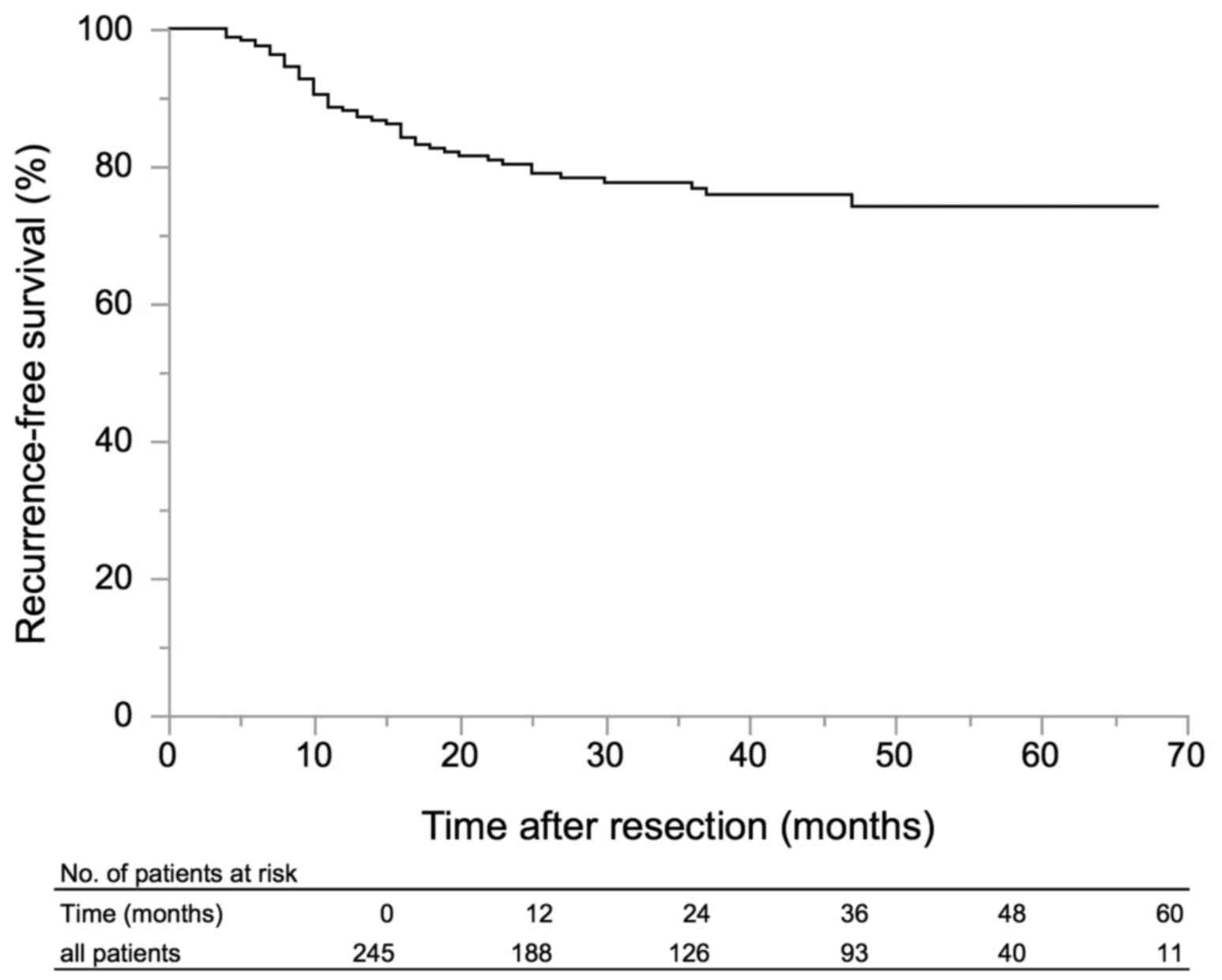
recurrence in the follow-up period. The RFS of all patients is
shown in Fig. 1. Kaplan-Meyer
analysis revealed RFS of 88.1% at 1 year, 80.3% at 2 years, and
76.7% at 3 years. On univariate analyses, tumor number ≥8 (P=0.03),
tumor size ≥1.0 cm (P=0.01), tumor size ≥1.5 cm (P<0.0001),
tumor size ≥2.0 cm (P<0.0001), and tumor size ≥3.0 cm (P=0.006)
were significantly associated with shorter RFS (Table II). On multivariate models, RFS was
shorter in patients with tumor size ≥1.5 cm [hazard ratio (HR)
4.12, 95% confidence interval (CI) 2.11–8.81, P<0.001; Table II]. When the cutoff of tumor size
was changed from 1.5 to 1.0 cm, 2.0 or 3.0 cm, all of the cutoff
sizes were found to predictors of shorter RFS (tumor size ≥1.0 cm:
HR 2.77, 95% CI 1.20–8.03, P=0.014; tumor size ≥2.0 cm: HR 4.01,
95% CI 2.18–7.79, P<0.0001; tumor size ≥3.0 cm: HR 2.16, 95% CI
1.13–3.97, P=0.02; data not shown). However, the HR was highest for
tumor size ≥1.5 cm. Patients with tumor number ≥8 also tended to
have shorter RFS, but this was not statistically significant (HR
2.67, 95% CI 0.94–6.58, P=0.06; Table
II).
 | Table II.Univariate and multivariate analyses
for intravesical recurrence. |
Table II.
Univariate and multivariate analyses
for intravesical recurrence.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
|
| ≤69
(reference) | 1 |
|
| 1 |
|
|
| ≥70 | 0.62 | 0.31–1.14 | 0.12 | 0.67 | 0.34–1.23 | 0.2 |
| Sex |
|
|
|
|
|
|
| Male
(reference) | 1 |
|
| – | – | – |
|
Female | 0.86 | 0.37–1.74 | 0.7 | – | – | – |
| Grade |
|
|
|
|
|
|
| G1
(reference) | 1 |
|
| 1 |
|
|
| G2 | 1.02 | 0.58–1.85 | 0.93 | 1.06 | 0.59–1.95 | 0.85 |
| Tumor numbler |
|
|
|
|
|
|
| Single
(reference) | 1 |
|
| – | – | – |
|
Multiple | 1.61 | 0.91–2.82 | 0.1 | – | – | – |
| Tumor numbler |
|
|
|
|
|
|
| 1
(reference) | 1 |
|
| 1 |
|
|
| 2–7 | 1.38 | 0.73–2.52 | 0.31 | 1.36 | 0.69–2.62 | 0.37 |
| 8- | 3.05 | 1.14–6.94 | 0.03 | 2.67 | 0.94–6.58 | 0.06 |
| Tumor size
(cm) |
|
|
|
|
|
|
| ≤0.9
(reference) | 1 |
|
| – | – | – |
|
≥1.0 | 2.82 | 1.23–8.15 | 0.01 | – | – | – |
| Tumor size
(cm) |
|
|
|
|
|
|
| ≤1.4
(reference) | 1 |
|
| 1 |
|
|
|
≥1.5 | 4.28 | 2.22–9.07 | <0.0001 | 4.12 | 2.11–8.81 | <0.001 |
| Tumor size
(cm) |
|
|
|
|
|
|
| ≤1.9
(reference) | 1 |
|
| – | – | – |
|
≥2.0 | 4.04 | 2.22–7.77 | <0.0001 | – | – | – |
| Tumor size
(cm) |
|
|
|
|
|
|
| ≤2.9
(reference) | 1 |
|
| – | – | – |
|
≥3.0 | 2.43 | 1.30–4.35 | 0.006 | – | – | – |
| Induction
intravesical chemotherapy |
|
|
|
|
|
|
| Not
done (reference) | 1 |
|
| 1 |
|
|
|
Done | 1.48 | 0.85–2.63 | 0.17 | 0.9 | 0.47–1.74 | 0.75 |
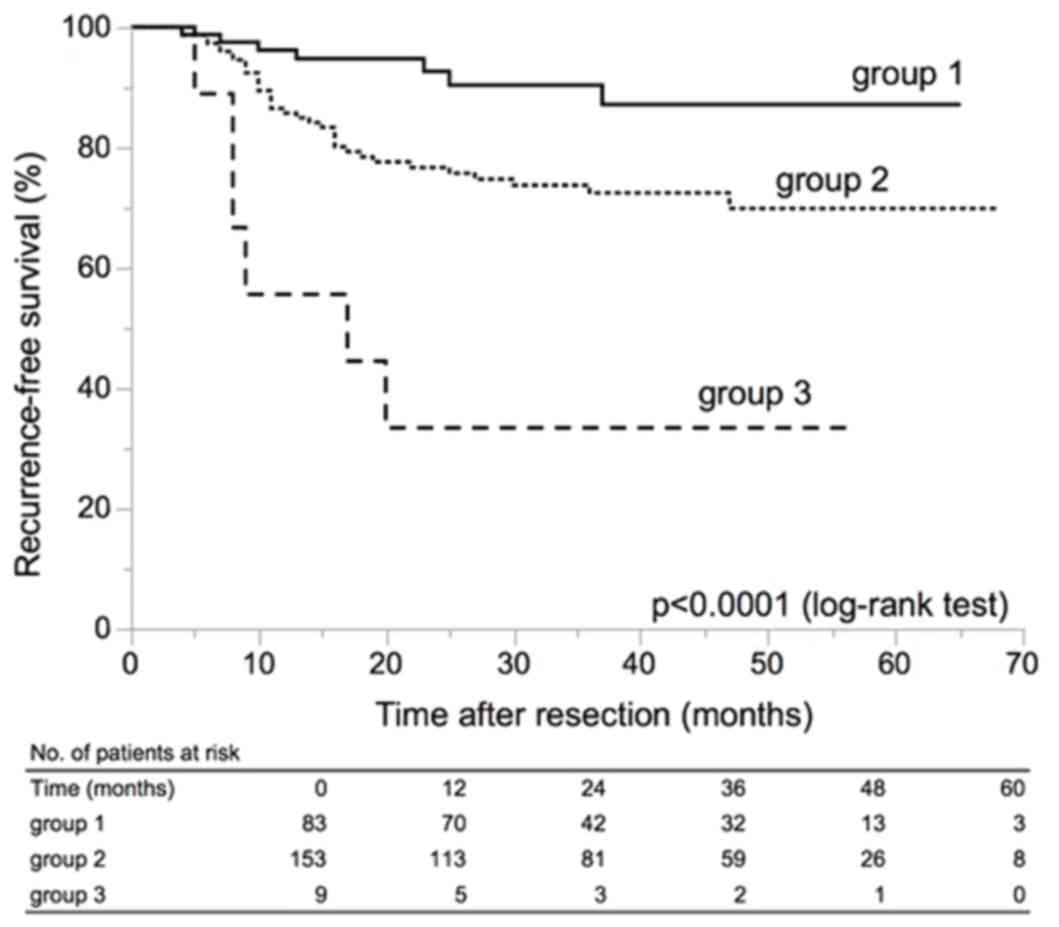
Among the above clinicopathological variables, we
selected two variables for risk stratification in patients with
primary LG Ta UC: tumor number ≥8 and tumor size ≥1.5 cm based on
the results of multivariate analyses. The patients were classified
into three groups as follows: Group 1, patients with a single tumor
and maximum tumor diameter less than 1.5 cm; group 3, patients with
8 or more tumors and maximum tumor diameter 1.5 cm or larger; group
2, patients who did not belong to group 1 or group 3. These three
groups showed significantly different RFS (Fig. 2) (P<0.0001).
Discussion
Most of the guidelines for NMIBC are based on
evidence from many kinds of clinical trials. For example, EAU
guidelines for NMIBC are derived from evidence concerning cutoff
values of tumor size and tumor number from seven RCTs that compared
prophylactic treatments after TURBT in stage Ta, T1, and Tis
bladder cancer patients carried out by the European Organization
for Research and Treatment of Cancer (EORTC) (1,6–12). The seven RCTs consisted of 2,596
NMIBC patients who had diverse clinicopathological characteristics
composed of not only solitary small-sized low-grade Ta tumors but
also multiple large-sized high-grade T1 tumors. In AUA/SUO
guidelines, risk categories are not based on a meta-analysis or
original studies but represent the panel's consensus regarding the
likelihood of recurrence and progression (2); however, the background seems to be
based on literature for NMIBC patients with various risks for
recurrence and progression.
We formed a hypothesis that data collected from only
NMIBC patients with lower risk for recurrence and progression would
classify the risk differently from analyses of all NMIBC patients.
In the current study, all cutoff points of tumor size: 1.0, 1.5,
2.0 and 3.0 cm, were significant predictors for shorter RFS,
however, the cutoff point of 1.5 cm showed the highest risk (HR
4.12, 95% CI 2.11–8.81, P<0.001). In addition, as the median
tumor size of the current study was 1.4 cm it is meaningful to use
a cutoff point for tumor size of 1.5 cm in NMIBC patients with
lower risk.
Golabesk et al analyzed 704 cases of primary
bladder UC with G1-2 Ta/T1 disease. In this case series, 414
patients (58.9%) had tumors >1.5 cm and 290 (41.1%) had tumors
≤1.5 cm; those with tumor >1.5 cm had a significantly higher
recurrence rate (66.7% vs. 53.6%, P=0.001) during a median
follow-up period of 64.9 months (13). These results suggest that tumor size
of 1.5 cm could be an appropriate cutoff in patients with primary
LG Ta bladder UC.
Regarding the tumor number, we did not find a
significant difference in intravesical RFS between patients with
single tumor and those with multiple tumors; however, in a
comparison among patients with single tumor, 2–7 tumors, and 8 or
more tumors in a similar manner to the EORTC risk table (6), those with 8 or more tumors seemed to
have a tendency for shorter intravesical RFS than those with single
tumor. Thus, we inferred that tumor multiplicity is likely to have
an impact to intravesical recurrence, even in the restricted to
patients with primary LG Ta tumors.
In the current study, we did not find a significant
difference in intravesical RFS according to histological grade (WHO
1973 G1 vs. G2). There is no discussion about the difference
between G1 and G2 in the EORTC report (6). In the newest WHO classification (WHO
2016), the authors emphasized the substantial advantage of
eliminating the ambiguity of the grading system in WHO 1973
(14). Therefore, we consider that
there is no need to re-classify LG tumors into G1 or G2 according
to the WHO 1973 system.
The National Comprehensive Cancer Network (NCCN)
guideline of bladder cancer classifies risk category by only
histopathological factors, such as LG Ta, HG Ta, LG T1, HG T1 and
CIS, and does not consider clinical factors such as past bladder
cancer history, tumor size, or tumor number (15). In a recent report, Klaassen et
al proposed that LG Ta bladder cancer should not be classified
into an intermediate risk group because of its very low risk of
progression, and proposed that the criterion of low-risk NMIBC
should be ‘all LG Ta (regardless of size, multifocal, recurrence)’
(16). As mentioned above, there are
some classifications that do not include recurrence, tumor number,
and tumor size in the risk criteria. However, it is clear that
there is a statistically significant difference in RFS when primary
LG Ta cancer is classified by tumor size and number, as shown in
Fig. 2. Similarly, IBCG classified
patients with multiple and/or recurrent LG Ta tumors (intermediate
risk group) into groups with different recommendations for
intravesical adjuvant therapy using several factors composed of
number (greater than 1) and size (greater than 3 cm) of tumors and
timing (recurrence within 1 year) and frequency (more than 1 per
year) of recurrence (17). Thus,
size and numbers of tumors are such major risk factors that it is
important to develop a strategy according to these factors.
In the current study, we did not analyze tumor
progression because only one patient showed progression to
muscle-invasive disease within a median follow-up period of 34
months. Mariappan and Smith reported that there were no cases that
progressed to muscle-invasive disease among 115 cases with primary
G1 Ta bladder cancer in a mean follow-up of 19.4 years, although 14
cases (12%) progressed to G2 or Tis/T1 tumors (18). Similarly, Rieken reported that among
1,436 patients with G1 Ta tumors (601 low-risk patients and 835
intermediate-risk patients), 613 patients (42.7%) experienced at
least one disease recurrence within a median follow-up of 33.5
months, and 68 (4.7%) showed progression to muscle-invasive disease
within a median follow-up of 67.2 months (19). In the recent study of Golabesk et
al, among 704 patients with primary G1-2 Ta/G1-2 T1 tumors, 284
patients (40.3%) had recurrence but only 8 (1.1%) progressed to
muscle-invasive disease within a median follow-up of 64.9 months
(13). Thus, patients with primary
LG Ta bladder cancer rarely show progression to muscle-invasive
disease even during a long follow-up period. Consequently, we
should understand the characteristics of primary LG Ta bladder
cancer, i.e., not always low risk for recurrence but always low
risk for progression.
There are several limitations in the current study.
First, the analysis was performed retrospectively and the cohort
size is not sufficiently large. Second, we did not perform central
pathology analyses. Third, there were no definite criteria for
performing induction intravesical chemotherapy. Indication of
additional induction therapy was individually decided by each
urologist in charge according to patients and tumor
characteristics.
These limitations might lead to some selection bias,
however, we showed the prognostic significance of tumor size, in
particular a cutoff size of 1.5 cm. Among patients with primary LG
Ta bladder cancer, patients with single tumor and tumor smaller
than 1.5 cm have a far lower risk for recurrence, thus
postoperative single instillation of chemotherapeutic agents is
enough to prevent recurrence. On the other hand, patients with
tumors ≥1.5 cm have such a significantly high recurrence risk;
thus, another prophylactic treatment should be considered to
decrease the recurrence risk.
Conclusion
We described the criteria for selection of the
lowest risk patients among those with low-grade (LG) Ta bladder
urothelial carcinoma (UC). If we consider only the lower risk NMIBC
patients, the appropriate cutoff value of tumor size to predict
intravesical recurrence might be 1.5 cm, which is smaller than 3.0
cm generally adopted in major NMIBC guidelines. On the other hand,
the tumor number was not independent recurrence predictor, however,
patients with tumor number ≥8 tended to have shorter RFS in these
lower risk NMIBC patients. Our findings suggest the need for
rational risk assessment with consideration of the diversity of
NMIBC.
Acknowledgements
The authors would like to thank Dr Mary Derry for
editing a draft of this manuscript.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
MA, KK and AYo designed the study, and MA wrote the
initial draft of the manuscript. KS, HK, AT and MS contributed to
data collection and interpretation. JI, KT, AYa and ME contributed
to analysis and interpretation of data. AYa and ME critically
reviewed the manuscript. All authors read and approved the final
manuscripts.
Ethics approval
This study was institutional review boards-approved,
and recruitment and protection of patient data were performed
according to the approved protocols.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Babjuk M, Böhle A, Burger M, Capoun O,
Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M,
et al: EAU guidelines on non-muscle-invasive urothelial carcinoma
of the bladder: Update 2016. Eur Urol. 71:447–461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang SS, Boorjian SA, Chou R, Clark PE,
Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD,
et al: Diagnosis and treatment of non-muscle invasive bladder
cancer: AUA/SUO Gudeline. J Urol. 196:1021–1029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brausi M, Witjes JA, Lamm D, Persad R,
Palou J, Colombel M, Buckley R, Soloway M, Akaza H and Böhle A: A
review of current guidelines and best practice recommendations for
the management of nonmuscle invasive bladder cancer by the
International Bladder Cancer Group. J Urol. 186:2158–2167. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eble JN, Sauter G, Epstein JI and
Sesterhenn IA: World health organization classification of
tumoursPathology and Genetics of Tumours of the Urinary System and
Male Genital Organs. IARC Press; Lyon: 2004
|
|
5
|
Mostofi FK, Sobin LH and Torloni H:
Histological typing of urinary bladder tumoursInternational
Classification of Tumours 10. World Health Organization; Geneva:
1973
|
|
6
|
Sylvester RJ, van der Meijden AP,
Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW and
Kurth K: Predicting recurrence and progression in individual
patients with stage Ta T1 bladder cancer using EORTC risk tables: A
combined analysis of 2596 patients from seven EORTC trials. Eur
Urol. 49:466–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Newling DW, Robinson MR, Smith PH, Byar D,
Lockwood R, Stevens I, De Pauw M and Sylvester R: Tryptophan
metabolites, pyridoxine (vitamin B6) and their influence on the
recurrence rate of superficial bladder cancer. Eur Urol.
27:110–116. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bouffioux CH, Denis L, Oosterlinck W,
Viggiano G, Vergison B, Keuppens F, De Pauw M, Sylvester R and
Cheuvart B: Adjuvant chemotherapy of recurrent superficial
transitional cell carcinoma: Results of a European Organization for
Research on Treatment of Cancer randomized trial comparing
intravesical instillation of thiotepa, doxorubicin and cisplatin. J
Urol. 148:297–301. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurth K, Tunn U, Ay R, Schröder FH,
Pavone-Macaluso M, Debruyne F, ten Kate F, de Pauw M and Sylvester
R: Adjuvant chemotherapy for superficial transitional cell bladder
carcinoma: Long-term results of a European Organization for
Research and Treatment of Cancer randomized trial comparing
doxorubicin, ethoglucid and transurethral resection alone. J Urol.
158:378–384. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bouffioux CH, Kurth KH, Bono A,
Oosterlinck W, Kruger CB, De Pauw M and Sylvester R: Intravesical
adjuvant chemotherapy for superficial transitional cell bladder
carcinoma: Results of 2 European Organization for Research and
Treatment of Cancer randomized trials with mitomycin C and
doxorubicin comparing early versus delayed instillations and
short-term versus long-term treatment. J Urol. 153:934–941. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Witjes JA, v d Meijden AP, Collette L,
Sylvester R, Debruyne FM, van Aubel A and Witjes WP: Long-term
follow-up of an EORTC randomized prospective trial comparing
intravesical bacille Calmette-Guerin-RIVM and mitomycin C in
superficial bladder cancer. Urol. 52:403–410. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oosterlinck W, Kurth KH, Schröder F,
Bultinck J, Hammond B and Sylvester R: A prospective European
Organization for Research and Treatment of Cancer Genitourinary
Group randomized trial comparing transurethral resection followed
by a single intravesical instillation of epirubicin or water in
single stage Ta, T1 papillary carcinoma of the bladder. J Urol.
149:749–752. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Golabesk T, Palou J, Rodriguez O, Parada
R, Skrobot S, Peña JA and Villavicencio H: Long-term bladder and
upper urinary tract follow-up recurrence and progression rates of
G1-2 non-muscle-invasive urothelial carcinoma of the bladder.
Urology. 100:145–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moch H, Humphrey PA, Ulbright TM and
Reuter VE: WHO Classification of Tumours of the Urinary System and
Male Genital OrgansInternational Agency for Research on Cancer.
Lyon: 2016, View Article : Google Scholar
|
|
15
|
National Comprehensive Cancer Network:
Clinical Practice Guidelines in Oncology: Bladder Cancer. Version
5. 2017.National Comprehensive Cancer Network 2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#bladderNovember
8–2017
|
|
16
|
Klaassen Z and Soloway MS: European
association of urology and American urological association/Society
of urologic oncology guidelines on risk categories for
non-muscle-invasive bladder cancer may lead to overtreatment for
low-grade Ta bladder tumors. Urology. 105:14–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamat AM, Witjes JA, Brausi M, Soloway M,
Lamm D, Persad R, Buckley R, Böhle A, Colombel M and Palou J:
Defining and treating the spectrum of intermediate risk nonmuscle
invasive bladder cancer. J Urol. 192:305–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mariappan P and Smith G: A Surveillance
schedule for G1Ta bladder cancer allowing efficient use of check
cystoscopy and safe discharge at 5 years based on 1 25-year
prospective database. J Urol. 173:1108–1111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rieken M, Xylinas E, Kluth L, Crivelli JJ,
Chrystal J, Faison T, Lotan Y, Karakiewicz PI, Holmäng S, Babjuk M,
et al: Long-term cancer-specific outcomes of TaG1 urothelial
carcinoma of the bladder. Eur Urol. 65:201–249. 2014. View Article : Google Scholar : PubMed/NCBI
|