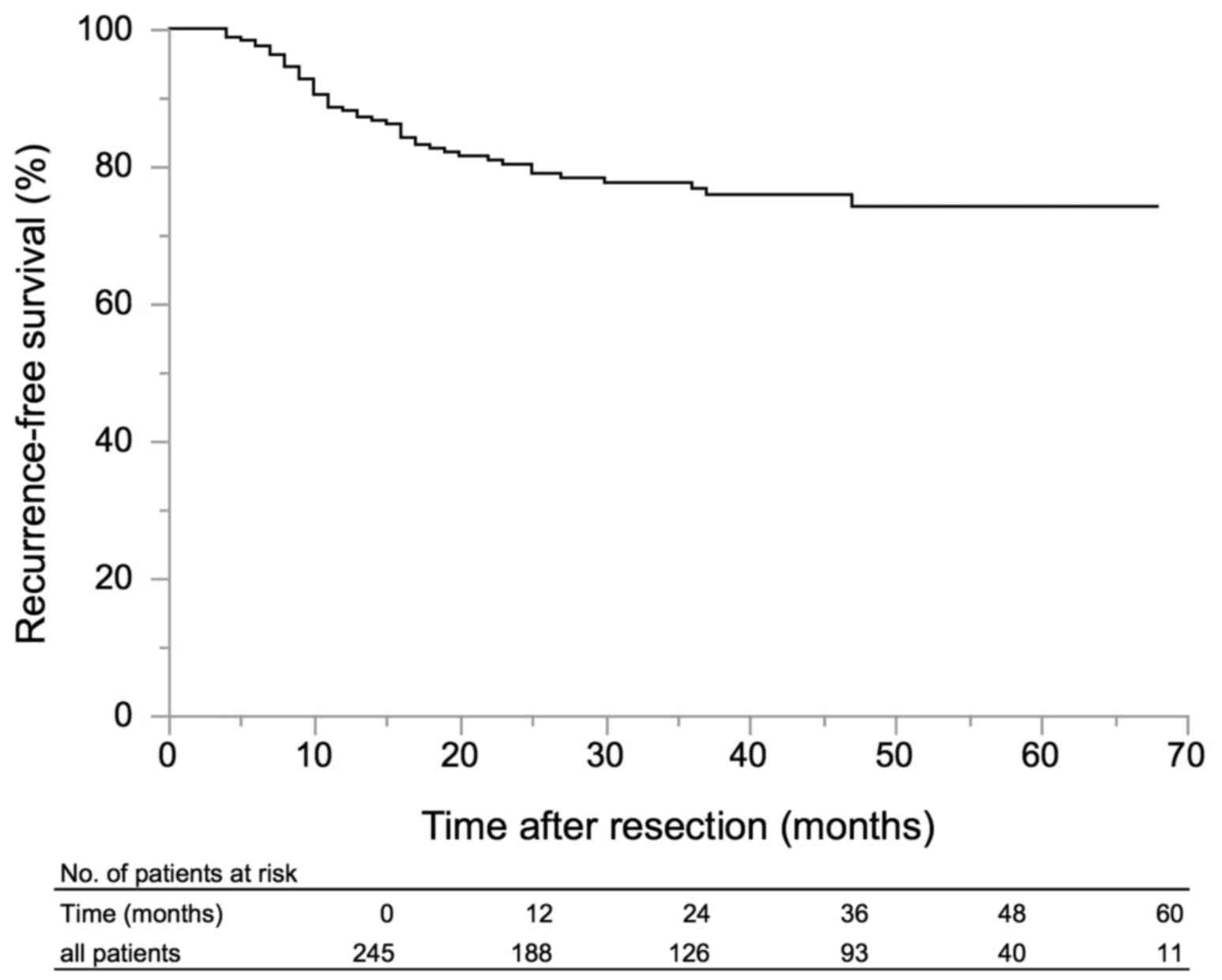
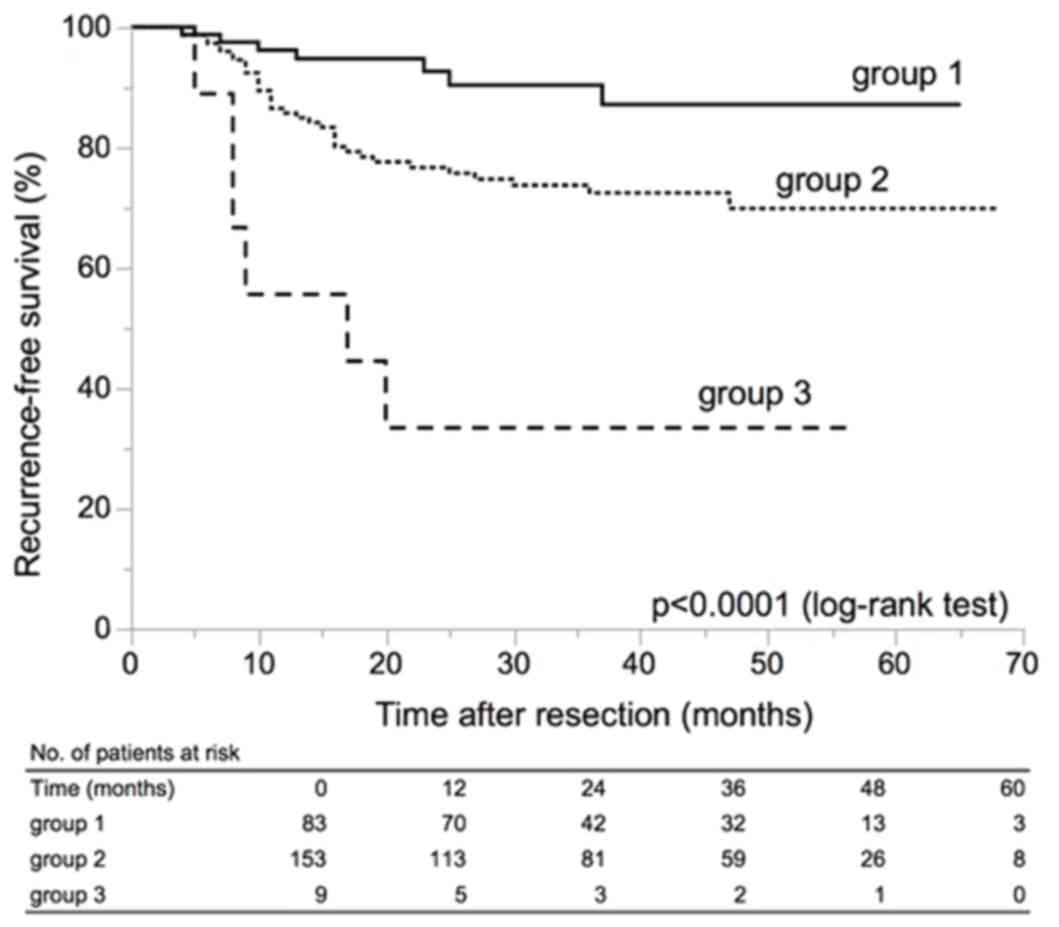
|
1
|
Babjuk M, Böhle A, Burger M, Capoun O,
Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M,
et al: EAU guidelines on non-muscle-invasive urothelial carcinoma
of the bladder: Update 2016. Eur Urol. 71:447–461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang SS, Boorjian SA, Chou R, Clark PE,
Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD,
et al: Diagnosis and treatment of non-muscle invasive bladder
cancer: AUA/SUO Gudeline. J Urol. 196:1021–1029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brausi M, Witjes JA, Lamm D, Persad R,
Palou J, Colombel M, Buckley R, Soloway M, Akaza H and Böhle A: A
review of current guidelines and best practice recommendations for
the management of nonmuscle invasive bladder cancer by the
International Bladder Cancer Group. J Urol. 186:2158–2167. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eble JN, Sauter G, Epstein JI and
Sesterhenn IA: World health organization classification of
tumoursPathology and Genetics of Tumours of the Urinary System and
Male Genital Organs. IARC Press; Lyon: 2004
|
|
5
|
Mostofi FK, Sobin LH and Torloni H:
Histological typing of urinary bladder tumoursInternational
Classification of Tumours 10. World Health Organization; Geneva:
1973
|
|
6
|
Sylvester RJ, van der Meijden AP,
Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW and
Kurth K: Predicting recurrence and progression in individual
patients with stage Ta T1 bladder cancer using EORTC risk tables: A
combined analysis of 2596 patients from seven EORTC trials. Eur
Urol. 49:466–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Newling DW, Robinson MR, Smith PH, Byar D,
Lockwood R, Stevens I, De Pauw M and Sylvester R: Tryptophan
metabolites, pyridoxine (vitamin B6) and their influence on the
recurrence rate of superficial bladder cancer. Eur Urol.
27:110–116. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bouffioux CH, Denis L, Oosterlinck W,
Viggiano G, Vergison B, Keuppens F, De Pauw M, Sylvester R and
Cheuvart B: Adjuvant chemotherapy of recurrent superficial
transitional cell carcinoma: Results of a European Organization for
Research on Treatment of Cancer randomized trial comparing
intravesical instillation of thiotepa, doxorubicin and cisplatin. J
Urol. 148:297–301. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurth K, Tunn U, Ay R, Schröder FH,
Pavone-Macaluso M, Debruyne F, ten Kate F, de Pauw M and Sylvester
R: Adjuvant chemotherapy for superficial transitional cell bladder
carcinoma: Long-term results of a European Organization for
Research and Treatment of Cancer randomized trial comparing
doxorubicin, ethoglucid and transurethral resection alone. J Urol.
158:378–384. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bouffioux CH, Kurth KH, Bono A,
Oosterlinck W, Kruger CB, De Pauw M and Sylvester R: Intravesical
adjuvant chemotherapy for superficial transitional cell bladder
carcinoma: Results of 2 European Organization for Research and
Treatment of Cancer randomized trials with mitomycin C and
doxorubicin comparing early versus delayed instillations and
short-term versus long-term treatment. J Urol. 153:934–941. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Witjes JA, v d Meijden AP, Collette L,
Sylvester R, Debruyne FM, van Aubel A and Witjes WP: Long-term
follow-up of an EORTC randomized prospective trial comparing
intravesical bacille Calmette-Guerin-RIVM and mitomycin C in
superficial bladder cancer. Urol. 52:403–410. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oosterlinck W, Kurth KH, Schröder F,
Bultinck J, Hammond B and Sylvester R: A prospective European
Organization for Research and Treatment of Cancer Genitourinary
Group randomized trial comparing transurethral resection followed
by a single intravesical instillation of epirubicin or water in
single stage Ta, T1 papillary carcinoma of the bladder. J Urol.
149:749–752. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Golabesk T, Palou J, Rodriguez O, Parada
R, Skrobot S, Peña JA and Villavicencio H: Long-term bladder and
upper urinary tract follow-up recurrence and progression rates of
G1-2 non-muscle-invasive urothelial carcinoma of the bladder.
Urology. 100:145–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moch H, Humphrey PA, Ulbright TM and
Reuter VE: WHO Classification of Tumours of the Urinary System and
Male Genital OrgansInternational Agency for Research on Cancer.
Lyon: 2016, View Article : Google Scholar
|
|
15
|
National Comprehensive Cancer Network:
Clinical Practice Guidelines in Oncology: Bladder Cancer. Version
5. 2017.National Comprehensive Cancer Network 2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#bladderNovember
8–2017
|
|
16
|
Klaassen Z and Soloway MS: European
association of urology and American urological association/Society
of urologic oncology guidelines on risk categories for
non-muscle-invasive bladder cancer may lead to overtreatment for
low-grade Ta bladder tumors. Urology. 105:14–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamat AM, Witjes JA, Brausi M, Soloway M,
Lamm D, Persad R, Buckley R, Böhle A, Colombel M and Palou J:
Defining and treating the spectrum of intermediate risk nonmuscle
invasive bladder cancer. J Urol. 192:305–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mariappan P and Smith G: A Surveillance
schedule for G1Ta bladder cancer allowing efficient use of check
cystoscopy and safe discharge at 5 years based on 1 25-year
prospective database. J Urol. 173:1108–1111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rieken M, Xylinas E, Kluth L, Crivelli JJ,
Chrystal J, Faison T, Lotan Y, Karakiewicz PI, Holmäng S, Babjuk M,
et al: Long-term cancer-specific outcomes of TaG1 urothelial
carcinoma of the bladder. Eur Urol. 65:201–249. 2014. View Article : Google Scholar : PubMed/NCBI
|