Introduction
Carcinoma of the pancreas (CAP) portends a poor
prognosis. In fact, at diagnosis, the disease is generally
advanced, with frequent presence of epigastric and back pain
(1). Radiation therapy (RT) is
effective for pain palliation, even with short-term and
involved-field radiotherapy (2).
The standard treatment technique of CAP is
three-dimensional conformal radiotherapy (3D-CRT), intensity
modulated radiotherapy, and stereotactic radiotherapy (3). These techniques however, are not
available in several centers, especially in developing countries,
where the only available technique is standard two-dimensional (2D)
technique, often based on a cobalt unit (4–6). For 2D
technique, guidelines for adjuvant or radical treatments involving
the prophylactic irradiation of regional lymph nodes are available
(7). Conversely, guidelines for
palliative RT with standard 2D technique are lacking.
Therefore, the purpose of this study is to provide
guidelines for palliative treatment of advanced CAP with a 2D
technique.
Patients and methods
Fifteen patients with locally advanced CAP
consecutively treated with RT in our center were identified.
Patients underwent computed tomography (CT) simulation in supine
position. Prior to CT-simulation, 100 cc of contrast medium
(Gastrografin) were administered orally in order to visualize the
stomach and duodenum. Scans were performed at an interval of 5 mm,
from T9 to L5.
The definition of the Clinical Target Volume (CTV)
included the head and body of the pancreas irrespective of tumor
stage and tumor site of the individual patient. In the CTV, the
retropancreatic space was included, from the posterior surface of
the gland to the middle of the aorta and inferior vena cava. All
contours were verified by an experienced radiation oncologist (GM)
and by a senior consultant (AGM). All patients enrolled in the
study signed a consent form for the use of their data and CT images
for performing this analysis. The study was approved by the
institutional board of Research and Care Foundation ‘Giovanni Paolo
II’.
The contours of the following Organs at Risk (OARs)
were defined: spinal cord, liver, kidneys, small bowel and
duodenum. An Internal Margin of 10 mm in cranio-caudal direction
and 4 mm in the radial direction was considered (8). A Set-Up Margin of 10 mm in all
directions was also considered. The Planning Target Volume (PTV)
was defined by adding a margin of 14 mm to the CTV in cranio-caudal
direction and 11 mm in radial direction.
For each patient, 3 treatment plans were calculated
using: A cobalt source, 6 MV photons and 15 MV photons,
respectively. Treatment plans based on the box technique were
generated, with a pair of anterior-posterior and postero-anterior
(AP-PA) and with a pair of lateral beams (LL). A fixed Source-Axis
Distance (SAD) technique was used. The SAD was 100 cm for photon
beams and 80 cm for the cobalt unit. The beams weights were: 20%
AP, 30% PA, and 25% for L-L beams, in order to reduce the dose to
liver and kidneys.
Beams were drawn using the primary collimators
(without using multileaf collimators). Primary collimators were
initially placed at 5 mm distance with respect to the PTV margins.
Then the minimum dose (Dmin) was evaluated. Fields sizes
were gradually increased, in steps of 2–3 mm in order to respect
the minimum dose (Dmin >90%) constraint. This
progressive optimization was analyzed with an iterative procedure
assessing the three-dimensional dose distribution. In this way, it
was possible to identify the fields sizes to be enlarged on the
basis of ‘cold spots’ sites.
Once the final plan was achieved, distances of the
fields edges from a set of reference points were measured. In this
way 15 AP-PA beams and 15 pairs of LL beams were defined for the
different patients. Finally, minimal individual field margins for
AP-PA and LL beams, able to encompass the entire different 15 sets
were defined.
Results
Fifteen patients with locally advanced CAP were
enrolled in the study. Patient characteristics are shown in
Table I. Figs. 1 and 2
show the AP-PA and LL fields defined in the individual patients.
Table II shows the results of the
analysis, in terms of field margins using the different beam
energies. Figs. 3 and 4 show the suggested fields obtained using
the ‘recommended’ margins for a cobalt unit.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Number | % |
|---|
| Age, years median
(range) | 66 (46–82) |
|
| Sex
(male/female) | 9/6 | 60/40 |
| BMI, median
(range) | 25 (20–30) |
|
 | Table II.Field definitions. |
Table II.
Field definitions.
| Field | Margin |
| Co60 | 6 MV | 15 MV |
|---|
|
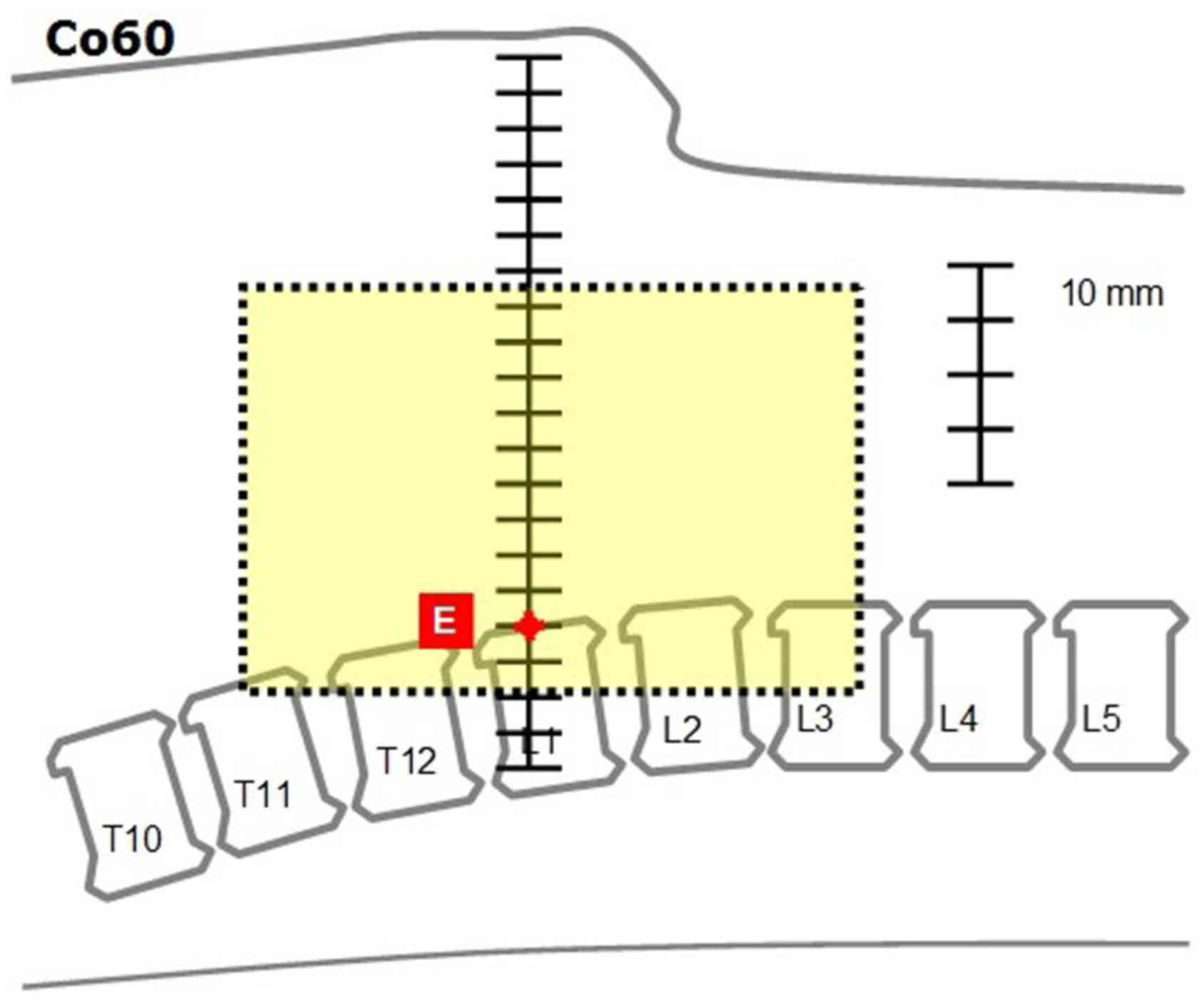
Anterior-posterior | Cranial | From point A (middle
of T11 vertebra): Caudally | 0 | 5 | 10 |
|
| Caudal | From point B (bottom
of the duodenal wall): Caudally | 15 | 10 | 5 |
|
| Right | From point C (most
external point of the duodenum): Laterally | 10 | 8 | 8 |
|
| Left | From point D (left
margin of L1 vertebra): Laterally | 15 | 13 | 13 |
| Lateral | Cranial | Same as
anterior-posterior | 0 | 5 | 10 |
|
| Caudal | Same as
anterior-posterior | 15 | 10 | 5 |
|
| Anterior | From point E
(anteriorsurface of L1 vertebra): Anteriorly | 95 | 93 | 93 |
|
| Posterior | From point E
(anterior surface of L1 vertebra): Posteriorly | 20 | 18 | 18 |
Discussion
A dosimetric evaluation was performed in 15 patients
with locally advanced CAP to define the standard fields for
palliative RT with 2D technique. Previous guidelines for 2D RT were
available (7). However, these
guidelines were provided for tumor and prophylactic nodal
irradiation (PNI). In our study, guidelines are provided for the
irradiation of the primary tumor only. In fact, our recommendations
are aimed at standardization of palliative RT, for which PNI is
unnecessary. In fact, PNI produces higher toxicity, with a negative
impact on quality of life (QoL) (9).
The analysis was conducted by defining the CTV as
the head and body of the pancreas since they account for most sites
of CAP. Therefore, the guidelines given in this study apply only to
tumors in these locations. In the CTV, the retropancreatic space
was also included. The region behind the pancreas, referred to as
the ‘mesopancreas’ (10), contains a
rich nerve plexus accompanying the lymphatic vessels (11). The infiltration of this nerve plexus
can cause pain, frequently present in these patients (1). Therefore, to broadly include this
potential pain site of origin, the anterior half of the great
vessels was included in the CTV.
For the definition of the set-up margin, 1 cm in all
directions was used. We must recognize that this margin may be
lower especially in cases of systematic use of portal imaging
(12). However, our study is
addressed to less technologically equipped centers, and therefore
probably without these practicality setting.
In this analysis, field margins were defined using
the minimum size to ensure a minimum dose to the target of 90% of
the prescribed dose. This constraint is less restrictive than the
limits recommended by ICRU 62 which provides a minimum dose to the
target of 95%. However, the constraint Dmin
>90% seemed more appropriate considering our
palliative treatment concept. More so, the use of this dose limit
can probably reduce the risk of wide irradiation of OARs,
particularly the intestine and stomach. We felt it was important to
reduce the toxicity profile with respect to OARs since our goal was
to improve QoL.
This study included 15 patients with different
anatomical features, as indicated from the large range of body mass
index (Table I). This sample size
seemed reasonable to define the standard dimensions of the
irradiation fields. However, we can not exclude that these fields
may be inadequate in some patients. Therefore, it is recommended
that a qualitative assessment of the tumor site be undertaken, such
as using a diagnostic CT scan. Particularly, this would provide an
assessment of the cranial field limit appropriate for the inclusion
of the tumor in the treated volume. A qualitative assessment of
this type may also enable reduction of the field size in some
patients.
Our study provides descriptive information only
about field sizes and location, and not on dose and fractionation.
Therefore, a subsequent dosimetric study has been planned in order
to identify the dose to be administered with this technique taking
into account current dose-volume constraints (13). This analysis will also determine the
actual feasibility of treatment based on cobalt equipment. Thus,
these results will be potentially useful in centers with this
modality only.
In conclusion, with this paper we are providing a
convenient tool for 2D target delineation of CAP in less equipped
centres.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All relevant data and its supporting information
files are within the paper.
Authors' contributions
Concept and design involved MB, SaC, FD, GB, SiC and
AGM. Treatment planning, analysis and interpretation of the data
was performed by AM, MB, CMD, AG and AGM. Drafting of the article
was performed by MB, FD, SaC, and AGM. Critical revision of the
article for important intellectual content involved TW, TS, AFMK,
MAS and AGM.
Ethics approval and consent to
participate
All patients enrolled in the study signed a consent
form for the use of their data and CT images for performing this
analysis. The study was approved by the institutional board of
Research and Care Foundation ‘Giovanni Paolo II’.
Consent to publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saif MW: Pancreatic neoplasm in 2011: An
update. JOP. 12:316–321. 2011.PubMed/NCBI
|
|
2
|
Morganti AG, Trodella L, Valentini V,
Barbi S, Macchia G, Mantini G, Turriziani A and Cellini N: Pain
relief with short term irradiation in locally advanced carcinoma of
the pancreas. J Pall Care. 19:258–262. 2003.
|
|
3
|
NCCN Clinical Practice Guidelines in
Oncology (NCCN Guidelines), . Pancreatic Adenocarcinoma. version 3.
2017.http://www.nccn.org/professionals/physicians/pdf/prostate.pdf27th–September.
2017
|
|
4
|
Adebamowo CA and Akarolo-Anthony S: Cancer
in Africa: Opportunities for collaborative research and training.
Afr J Med Sci. 38 Suppl 2:S5–S13. 2009.
|
|
5
|
Mugambe Kigula JB and Wegoye P: Pattern
and experience with cancers treated with the Chinese GWGP80 cobalt
unit at mulago hospital, kampala. East Afr Med J. 77:523–525.
2000.PubMed/NCBI
|
|
6
|
Sharma V, Gaye PM, Wahab SA, Ndlovu N,
Ngoma T, Vanderpuye V, Sowuhami A, Dawotola DA, Kigula-Mugambe J
and Jeremic B: Palliative radiation therapy practice for advanced
esophageal carcinoma in Africa. Dis Esophagus. 23:240–243. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Willett CG, Czito BJ and Bendell JC:
Cancer of the pancreasHalperin EC, Perez CA and Brady LW: Perez and
Brady's principles and practices of radiation oncology. 5th
edition. Lippincott, Williams & Wilkins; Philadelphia PA: pp.
1336–31348. 2008
|
|
8
|
Song YC, You JQ, Yuan ZY, Wang W, Li XY
and Wang P: A preliminary probe into the movement of pancreatic
lesions and factors that influence it. Br J Radiol. 83:505–508.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morganti AG, Trodella L, Valentini V,
Macchia G, Alfieri S, Smaniotto D, Luzi S, Costamagna G, Doglietto
GB and Cellini N: Concomitant gemcitabine (‘Gemzar’) and extended
nodal irradiation in the treatment of pancreatic and biliary
carcinoma: A phase I study. Onkologie. 26:325–329. 2003.PubMed/NCBI
|
|
10
|
Gockel I, Domeyer M, Wolloscheck T,
Konerding MA and Junginger T: Resection of the mesopancreas (RMP):
A new surgical classification of a known anatomical space. World J
Surg Oncol. 5:442007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noto M, Miwa K, Kitagawa H, Kayahara M,
Takamura H, Shimizu K and Ohta T: Pancreas head carcinoma:
Frequency of invasion to soft tissue adherent to the superior
mesenteric artery. Am J Surg Pathol. 29:1056–1061. 2005.PubMed/NCBI
|
|
12
|
Yovino S, Poppe M, Jabbour S, David V,
Garofalo M, Pandya N, Alexander R, Hanna N and Regine WF:
Intensity-modulated radiation therapy significantly improves acute
gastrointestinal toxicity in pancreatic and ampullary cancers. Int
J Radiat Oncol Biol Phys. 79:158–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bentzen SM, Constine LS, Deasy JO,
Eisbruch A, Jackson A, Marks LB, Ten Haken RK and Yorke ED:
Quantitative analyses of normal tissue effects in the clinic
(QUANTEC): An introduction to the scientific issues. Int J Radiat
Oncol Biol Phys. 76 3 Suppl:S3–S9. 2010. View Article : Google Scholar : PubMed/NCBI
|