Introduction
The mortality rates of patients with advanced
non-small cell lung cancer (NSCLC) remain high (1). To improve this poor prognosis, several
adjuvant chemotherapies have been administered in patients with
completely resected NSCLC, but the improvement of the survival rate
is not ideal, and patients sometimes struggle with adverse effects,
like nausea, neutropenia, and fatigue (2–10).
Ideally, we would be able to predict the effects of
chemotherapeutic agents and regimens for patients who received
chemotherapy, especially for postoperative adjuvant chemotherapy,
because whether or not adjuvant chemotherapy reduces the rate of
recurrence is unclear. Even with cytotoxic anticancer drugs, the
predictive factors of the therapeutic effect would ideally be
revealed in a manner similar to that observed for molecular
targeted therapy (11–13).
Recently, the expression of some proteins has been
reported as a predictor of the efficacy of cytotoxic
chemotherapeutic agents. Excision repair cross-complementation
group 1 (ERCC1) is a DNA repair gene in the nucleotide excision
repair pathway that is activated when platinum-based
chemotherapeutic agents form DNA adducts (14). High ERCC1 expression in several
cancers has been reported in association with resistance to
platinum-based treatment (15–17).
Class III β-tubulin (TUBB3) is a major component of the
microtubules that are targeted by taxane-based agents, which exert
their growth inhibitory effects through the inhibition of
microtubule dynamics, resulting in the growth arrest of tumor cells
at the G2-M phase (18). High
expression of TUBB3 has been reported in association with
resistance to taxane-based treatment in human cancers (19–21).
Thymidylate synthase (TS) is an enzyme that generates
deoxythymidine monophosphate, which is subsequently phosphorylated
to thymidine triphosphate for use in DNA synthesis and repair. High
expression of TS has been reported in association with fluorouracil
(5FU)-based chemotherapy (including S-1 agent) resistance in
various cancers (22–24). Dihydropyrimidine dehydrogenase (DPD)
is the initial and rate-limiting enzyme in degrading 5-FU to
2-fluoro-β-alanine (25), and high
expression of DPD has been reported in association with resistance
to 5-FU-based chemotherapies (26–28).
Orotate phosphoribosyltransferase (OPRT) is an enzyme involved in
pyrimidine biosynthesis and contributes to the conversion of 5-FU
into fdUMP, an active form of 5-FU. Low expression of OPRT has been
reported in association with resistance to 5-FU-based
chemotherapies (29,30).
In this study, we investigated the expression of
several proteins in completely resected NSCLC patients who received
carboplatin plus paclitaxel (CP) or S-1 regimen as adjuvant
chemotherapy.
Patients and methods
Patients
A multicenter randomized feasibility study of CP vs.
S-1 in patients with locally advanced completely resected NSCLC was
conducted. Forty patients underwent complete resection and were
diagnosed with pathological stage II or IIIA NSCLC (the 7th edition
of the Tumor-Node-Metastasis classification) (31) at Nagoya City University Hospital
(Nagoya, Japan) and its affiliated hospitals between January 2008
and December 2013.
Written informed consent was obtained from all
patients, and the study protocol was approved by the Institutional
Review Board of each participating institution (Nagoya City
University Hospital No. 45-13-0020). This study was registered on
the UMIN Clinical Trial database (ID:000001510). We have reported
on details of this study (32). In
this paper, we evaluated the relationships between the protein
expression and the prognosis of patients who received adjuvant
chemotherapy after complete surgical resection. The randomization
was performed centrally at the Department of Oncology, Immunology
and Surgery, Nagoya City University Graduate School of Medical
Sciences (Nagoya, Japan).
Design of the study and treatment
schedule
The patients were randomly assigned either to arm A
(21 cases) receiving CP bi-weekly or to arm B (19 cases) receiving
S-1. Among the 40 patients, two patients assigned to arm A could
not continue the adjuvant chemotherapy because of a Grade 4
allergic reaction (anaphylactic shock) during the first cycle of
paclitaxel infusion. We excluded these two patients from this
additional study and investigated the 38 patients who received
adjuvant chemotherapy over two courses.
The infusing dosage of paclitaxel was 120 mg
m−2 on days 1 and 15. Carboplatin at an area under the
curve (AUC 3) dose was also administered on days 1 and 15. The
patients received adjuvant chemotherapy with carboplatin plus
paclitaxel every four weeks for up to four cycles. Calvert's
formula was used to calculate the dose of the AUC for carboplatin
(33), while the creatinine
clearance was determined with the Jellifie formula (34). The dosage of S-1 was established as
follows: patients with a body surface area (BSA) <1.25
m2 received 40 mg twice a day (80 mg/day), those with
BSA of ≥1.25 m2 but <1.5 m2 received 50 mg
twice a day (100 mg/day), and those with a BSA ≥1.5 m2
received 60 mg twice a day (120 mg/day). S-1 was administered for
two weeks followed by a one-week rest period for up to one year.
Both arms A and B continued on the above prescription unless any
evidence for relapse, other malignancies, or severe adverse events
were identified.
Recurrence was diagnosed on the basis of imaging
study findings. Chest and abdominal computed tomography and
positron emission tomography plus head magnetic resonance imaging
were performed at 6- and 12-month intervals, respectively. In
addition, when patients complained of any symptoms or exhibited
elevated tumor markers on blood tests, imaging studies were
performed.
Protein expression by
immunohistochemistry
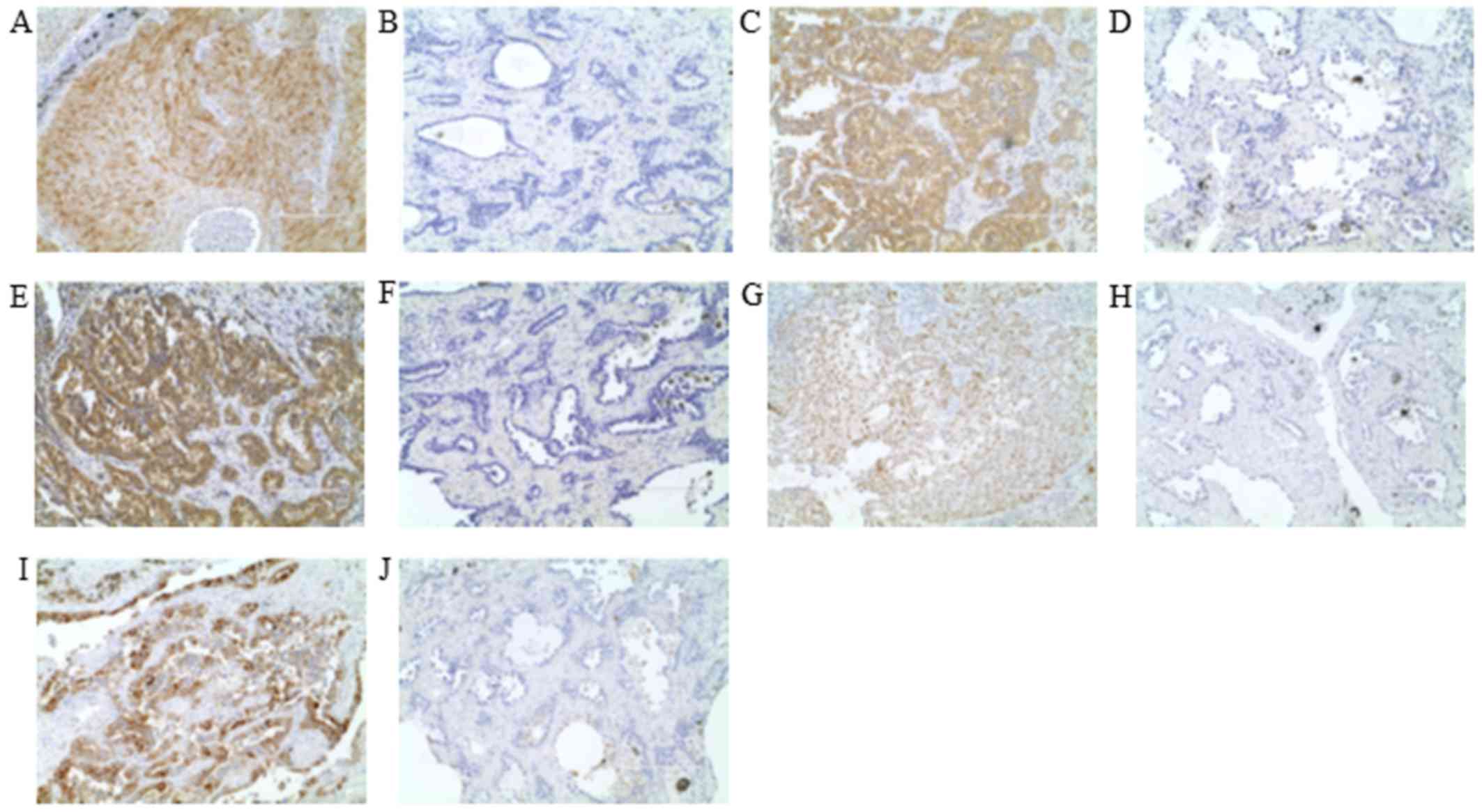
The ERCC1 protein expression was evaluated by
immunohistochemistry (IHC) using an anti-ERCC1 antibody (clone 8F1;
Abcam, Cambridge, UK). We used a standard protocol for the
immunostaining of the samples. The details of the method were
previously described (35). Tumor
nuclear staining intensity was graded on a scale of 0–3. The
percentage of positive tumor nuclei was graded on a scale of 0–3.
The percentage of positive tumor nuclei was evaluated, and a
proportion score was attributed (0 if 0%; 0.1 if 1–9%; 0.5 if
10–49%; 1.0 if ≥ 50%), as previously described (36,37).
The antibody against TUBB3 was an anti-class III
β-tubulin monoclonal antibody (clone TUJ1; Covance, Inc.,
Princeton, NJ, USA). Having over 50% of positive cells with a
staining intensity of 2 was considered TUBB3-positive (35).
TS protein was evaluated by IHC using recombinant
human TS-specific antibody (clone RTSSA; Taiho Pharmaceutical, Co.,
Ltd., Saitama, Japan). The slides were examined at low
magnification, and the intensity of cytoplasmic staining was scored
as follows: 0, no staining or faint staining; 1+, moderate
staining; 2+, strong staining. We classified scores of 0 as
negative and scores of 1+ and 2+ as positive for the TS antibody.
We also judged cases with <10% of tumor cells with moderate or
strong staining as being negative (38).
OPRT protein expression was evaluated by IHC using
an anti-OPRT polyclonal antibody (Taiho Pharmaceutical, Co., Ltd.)
The staining was the same as for TS (38). Scores of 0 and 1+ were classified as
negative and scores of 2+ as positive for the OPRT protein. We also
judged cases with <10% of tumor cells with moderate or strong
staining as being negative.
DPD protein expression was evaluated by IHC using
anti-DPD polyclonal antibody RDPDPA (dilution: 1:400; Taiho
Pharmaceutical, Co., Ltd.) The staining was the same as the
previously described method (28).
Scores of 0 and 1+ were classified as negative and scores of 2+ as
positive for the DPD protein. We also judged cases with <10% of
tumor cells with moderate or strong staining as being negative.
All immunostained sections were evaluated by
separate investigator without knowledge of the patients' clinical
data to evaluated H-scoring accurately. Representative positive and
negative cases of each IHC are shown in Fig. 1.
Statistical analysis
The sample size was determined based on a phase II
study reported by Kawamura et al (39) applying docetaxel plus gemcitabine as
an adjuvant chemotherapy in 35 patients. The number of patients in
each arm was calculated using the Fleming method and found to be 32
per arm (32). However, sufficient
data for patients in the study could not be gathered within the
study period.
The characteristics, disease-free survival (DFS),
and the overall survival (OS) of 38 patients who received over two
courses of adjuvant chemotherapy were analyzed. The five-year DFS
and OS were examined by the Kaplan-Meier method, and the difference
in the two arms was calculated by the log-rank test. The
differences in the rate of adverse events were evaluated by the
χ2 test. All of the data were analyzed with the EZR
software version 1.33 (www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html)
(40). P≤0.05 was considered to
indicate a statistically significant difference.
Results
Patients' characteristics
Forty patients with stage II or IIIA NSCLC who had
received surgically complete resection were enrolled. Among the 40
patients, 2 were excluded due to the cessation of adjuvant
chemotherapy because of a grade 4 allergic reaction (anaphylactic
shock) induced by paclitaxel. The patients' characteristics are
presented in Table I. Briefly, the
patients were 7 females and 31 males ranging in age from 39–75
years, with a mean age of 63.6 years. There were no significant
differences in the clinicopathological characteristics between arms
A and B.
 | Table I.Characteristics of the 38 patients
recruited to the present study. |
Table I.
Characteristics of the 38 patients
recruited to the present study.
|
Characteristics | All patients
(n=38) | CBDCA+PTX
(n=19) | S-1 (n=19) | P-value |
|---|
| Observation period,
months | 15–98/67 | 19–98/67 | 15–87/67 | 0.951 |
| Sex, n |
|
|
|
|
|
Male | 31 | 14 | 17 | 0.405 |
|
Female | 7 | 5 | 2 |
|
| Age, years | 39–75/63.6 | 47–73/64.4 | 39–75/62.9 | 0.529 |
| Histological type,
n |
|
|
|
|
|
Adenocarcinoma | 24 | 11 | 13 | 0.737 |
|
Squamous cell carcinoma | 13 | 7 | 6 |
|
|
Others | 1 | 1 | 0 |
|
| Pathological stage
(IIA/IIB/IIIA), n | 17/11/10 | 9/5/5 | 8/6/5 | 0.980 |
| ERCC1
(Positive/negative), n | 18/20 | 10/9 | 8/11 | 0.746 |
| TUBB3
(Positive/negative), n | 17/21 | 9/10 | 8/11 | 0.980 |
| TS
(Positive/negative), n | 21/17 | 11/8 | 10/9 | 0.980 |
| OPRT
(Positive/negative), n | 16/22 | 7/12 | 9/10 | 0.743 |
| DPD
(Positive/negative), n | 22/16 | 14/5 | 8/11 | 0.091 |
Protein expression on IHC
The ERCC1 IHC staining was positive in 18/38 cases
(47%) in all patients. The positive cases were 10/19 (53%) in arm A
and 8/19 (42%) in arm B, and there was no significant difference in
the ERCC1 protein expression among the various adjuvant
chemotherapy regimens. No association between the expression of
ERCC1 and clinicopathological factors was identified (data not
shown).
The TUBB3 IHC staining was positive in 17/38 cases
(45%) in all patients. The positive cases were 9/19 (47%) in arm A
and 8/19 (42%) in arm B, and there was no significant difference in
the TUBB3 protein expression among adjuvant chemotherapy regimens.
No association between the expression of TUBB3 and
clinicopathological factors was identified (data not shown).
The TS IHC staining was positive in 21/38 cases
(55%) in all patients. The positive cases were 11/19 (58%) in arm A
and 10/19 (53%) in arm B, and there was no significant difference
in the TS protein expression among adjuvant chemotherapy regimens.
No association between the expression of TS and clinicopathological
factors was identified (data not shown).
The OPRT IHC staining was positive in 16/38 cases
(42%) in all patients. The positive cases were 7/19 (37%) in arm A
and 9/19 (47%) in arm B, and there was no significant difference in
the OPRT protein expression among adjuvant chemotherapy regimens.
No association between the expression of OPRT and
clinicopathological factors was identified (data not shown).
The DPD IHC staining was positive in 22/38 cases
(58%) in all patients. The positive cases were 14/19 (74%) in arm A
and 8/19 (42%) in arm B, and there was no significant difference in
the DPD protein expression among adjuvant chemotherapy regimens. No
association between the expression of DPD and clinicopathological
factors was identified (data not shown).
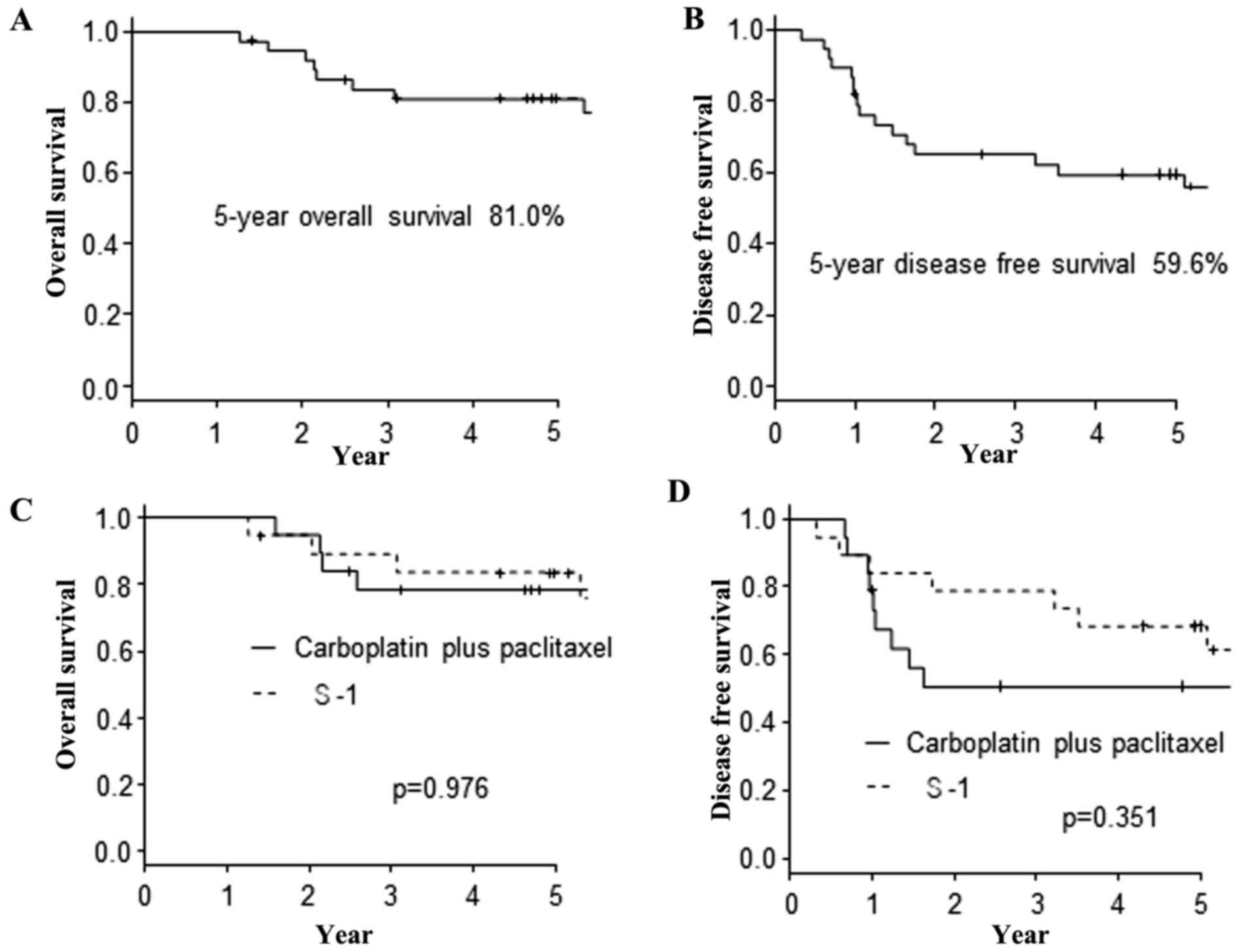
The survival
The correlations between the OS plus DFS and the
clinicopathological factors of the 38 patients are summarized in
Table II. No factors, including the
protein expression, were found to have significantly influenced the
OS or DFS. Furthermore, there were no significant differences in
the OS and DFS between the CP and S-1 adjuvant chemotherapy
regimens. The 5-year OS and DFS of 38 patients was 81.0 and 59.6%,
respectively (Fig. 2A and B). The
Kaplan-Meier curves based on the adjuvant chemotherapy regimens are
shown in Fig. 2C and D.
 | Table II.Correlation with overall survival
plus disease free survival and clinicopathological factors. |
Table II.
Correlation with overall survival
plus disease free survival and clinicopathological factors.
|
|
|
| Overall
survival | Disease free
survival |
|---|
|
|
|
|
|
|
|---|
| Factor | Subgroup | Total n (n=38) | 5-year survival
(%) | P-value | 5-year survival
(%) | P-value |
|---|
| Age, years | ≤65/>65 | 20/18 | 78.8/83.3 | 0.182 | 63.6/55.6 | 0.898 |
| Sex | Male/female | 31/7 | 76.5/100 | 0.070 | 56.8/71.4 | 0.398 |
| Tissue type |
Adenocarcinoma/others | 24/14 | 87.1/70.0 | 0.399 | 58.3/60.2 | 0.477 |
| Pathological
stage | IIA/IIB or
IIIA | 17/21 | 87.8/75.2 | 0.085 | 69.1/52.4 | 0.250 |
| Chemotherapy
regime | CP/S-1 | 19/19 | 78.6/83.6 | 0.976 | 50.8/68.4 | 0.351 |
| ERCC1 |
Positive/negative | 18/20 | 76.0/85.0 | 0.773 | 70.6/50.0 | 0.111 |
| TUBB3 |
Positive/negative | 17/21 | 87.5/75.6 | 0.696 | 64.7/54.8 | 0.869 |
| TS |
Positive/negative | 21/17 | 74.4/88.2 | 0.092 | 50.1/70.6 | 0.140 |
| OPRT |
Positive/negative | 16/22 | 86.2/77.3 | 0.783 | 66.1/54.5 | 0.502 |
| DPD |
Positive/negative | 22/16 | 86.1/73.7 | 0.824 | 66.5/50.0 | 0.331 |
The correlations between the OS plus DFS and the
clinicopathological factors of the 19 patients who received CP
adjuvant chemotherapy are summarized in Table III. There were no factors found to
have significantly influenced the OS or DFS in the patients who
received the CP regimen. The protein expressions of ERCC1 and TUBB3
did not affect the OS or DFS.
 | Table III.Correlation with overall survival
plus disease free survival and clinicopathological factors for
Carboplatin plus paclitaxel patients. |
Table III.
Correlation with overall survival
plus disease free survival and clinicopathological factors for
Carboplatin plus paclitaxel patients.
|
|
|
| Overall
survival | Disease free
survival |
|---|
|
|
|
|
|
|
|---|
| Factor | Subgroup | Total n (n=19) | 5-year survival
(%) | P-value | 5-year survival
(%) | P-value |
|---|
| Age, years | ≤65/>65 | 9/10 | 76.2/80.0 | 0.598 | 63.5/40.0 | 0.281 |
| Sex | Male/female | 14/5 | 70.7/100 | 0.134 | 47.6/60.0 | 0.601 |
| Tissue type |
Adenocarcinoma/others | 11/8 | 90.9/60.0 | 0.325 | 36.4/72.9 | 0.172 |
| Pathological
stage | IIA/IIB or
IIIA | 9/10 | 76.2/80.0 | 0.473 | 53.3/50.0 | 0.902 |
| ERCC1 |
Positive/negative | 10/9 | 58.3/100 | 0.773 | 67.5/33.3 | 0.129 |
| TUBB3 |
Positive/negative | 9/10 | 77.8/78.7 | 0.527 | 44.4/57.1 | 0.502 |
| TS |
Positive/negative | 11/8 | 71.6/87.5 | 0.310 | 53.0/50.0 | 0.700 |
| OPRT |
Positive/negative | 7/12 | 68.6/83.3 | 0.824 | 68.6/41.7 | 0.321 |
| DPD |
Positive/negative | 14/5 | 77.9/80.0 | 0.806 | 62.9/20.0 | 0.164 |
The correlations between the OS plus DFS and the
clinicopathological factors of the 19 patients who received S-1
adjuvant chemotherapy are summarized at Table IV. There were no factors found to
have significantly influenced the OS in the patients who received
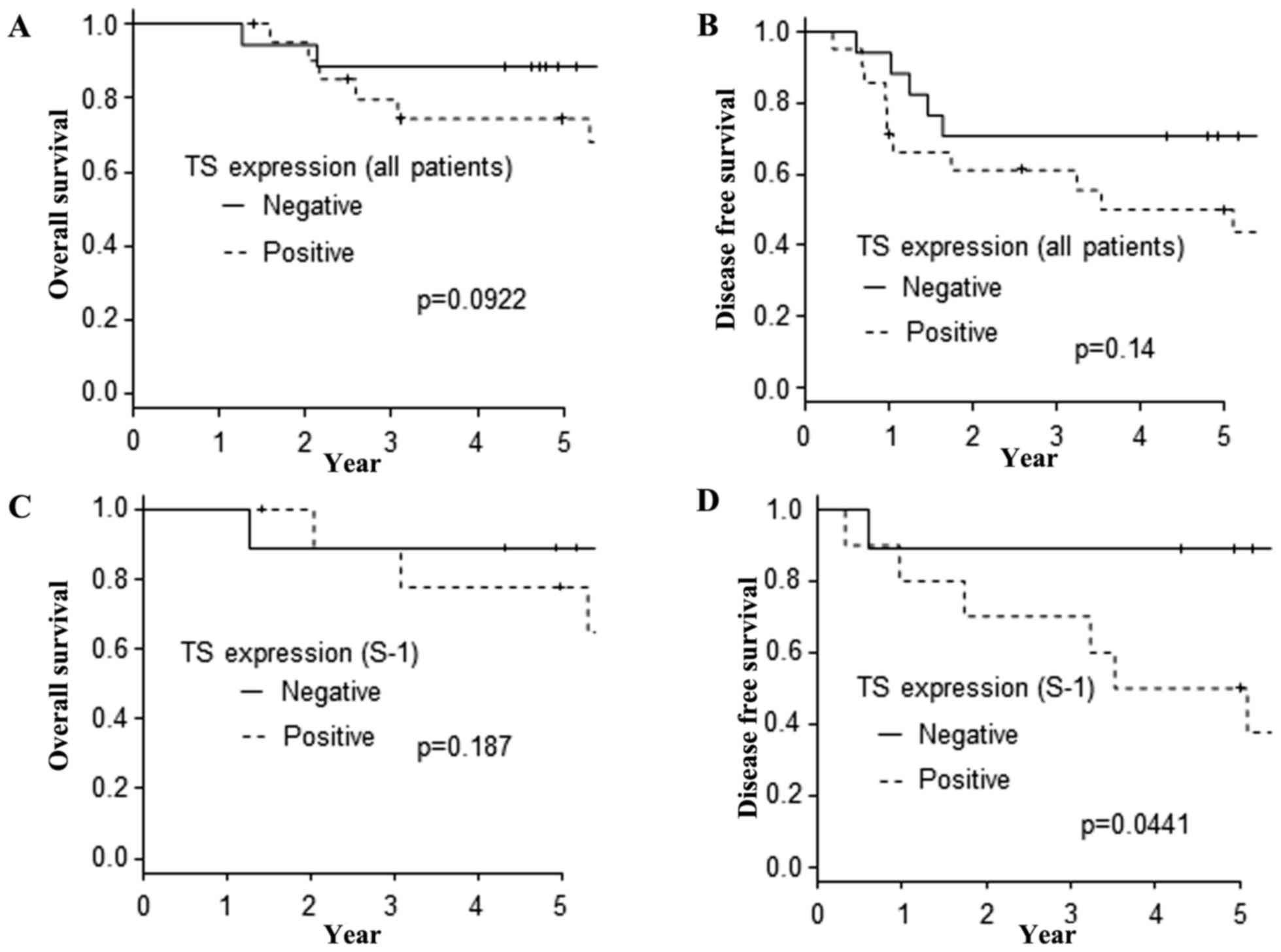
the S-1 regimens. In the analysis of the DFS, the protein
expression of TS was the only significant prognostic factor.
However, the protein expression of TS did not affect the OS
(Fig. 3A) or DFS (Fig. 3B) in the investigation of all 38
patients. Furthermore, the protein expression of TS did not affect
the OS (Fig. 3C) in the
investigation of the 19 patients who received S-1 adjuvant
chemotherapy. However, when we limited our investigation to the DFS
of the patients who received S-1 adjuvant chemotherapy, the
TS-negative cases showed a longer DFS than the TS-positive cases
(Fig. 3D).
 | Table IV.Correlation with overall survival
plus disease free survival and clinicopathological factors for S-1
patients. |
Table IV.
Correlation with overall survival
plus disease free survival and clinicopathological factors for S-1
patients.
|
|
|
| Overall
survival | Disease free
survival |
|---|
|
|
|
|
|
|
|---|
| Factor | Subgroup | Total n (n=19) | 5-year survival
(%) | P-value | 5-year survival
(%) | P-value |
|---|
| Age, years | ≤65/>65 | 11/8 | 80.8/87.5 | 0.202 | 63.6/75.0 | 0.389 |
| Sex | Male/female | 17/2 | 81.6/100 | 0.316 | 64.7/100 | 0.297 |
| Tissue type |
Adenocarcinoma/others | 13/6 | 83.9/83.3 | 0.839 | 76.9/50.0 | 0.570 |
| Pathological
stage | IIA/IIB or
IIIA | 8/11 | 100/70.7 | 0.065 | 87.5/54.5 | 0.072 |
| ERCC1 |
Positive/negative | 8/11 | 100/72.7 | 0.142 | 75.0/63.6 | 0.333 |
| TUBB3 |
Positive/negative | 8/11 | 100/72.7 | 0.241 | 87.5/54.5 | 0.346 |
| TS |
Positive/negative | 10/9 | 77.8/88.9 | 0.187 | 50.0/88.9 | 0.044 |
| OPRT |
Positive/negative | 9/10 | 100/70.0 | 0.496 | 66.7/70.0 | 0.783 |
| DPD |
Positive/negative | 8/11 | 100/70.7 | 0.587 | 75.0/63.6 | 0.721 |
Discussion
The survival of patients with advanced lung cancer
is still unfavorable compared with malignant tumors of other organs
(1). Recently, improved outcomes
have been achieved with molecular-targeted therapy for select
patients with epidermal growth factor receptor (EGFR)-activating
mutations or ALK translocation (11–13).
Understanding the genetic and molecular variations that affect the
efficacy of chemotherapeutic agents may improve patient care by
allowing physicians to optimize treatment for each patient. Even
with cytotoxic anticancer drugs, it would be useful to know the
factors predictive of a therapeutic effect before starting the
administration of chemotherapy.
In this study, we evaluated the expression of
several proteins in 38 patients with stage II and IIIA NSCLC who
had received CP or S-1 as adjuvant chemotherapy. The 5-year OS and
DFS of these 38 patients were 81.0 and 59.6%, respectively. These
findings are comparable to those that have been reported recently
(1,41). Concerning the OS analysis, the EGFR
mutation status has been shown to influence the prognosis after
relapse (11–13). Molecular-targeted therapeutic drugs
apparently extend the OS in cases with EGFR mutations. It is
therefore difficult to evaluate the effect of adjuvant chemotherapy
on the OS in our small-scale study, because we don't have the data
of gene mutations about all patients of this study. We should
evaluate the DFS to clarify the relationship between protein
expression and adjuvant chemotherapy efficacy. We should check the
gene mutations (EGFR and ALK) to evaluated the effect of adjuvant
chemotherapy on the OS in the future studies.
The CP regimen is considered as a standard
chemotherapy regimen for recurrent and advanced lung cancer
(42–46). We used the regimen of bi-weekly
paclitaxel plus carboplatin to be able to complete the adjuvant
chemotherapy without interruption due to side effects. As S-1 is
considered more effective than UFT, long-term S-1 administration
may be promising as an adjuvant chemotherapy regimen for advanced
lung cancer (47). Indeed, several
studies have shown that S-1 administration as adjuvant chemotherapy
is associated with significant survival benefits following
surgically complete resection for NSCLC (47,48). In
this study, the 5-year OS and DFS were almost the same between the
S-1 group and the CP group.
We investigated the protein expressions of ERCC1 and
TUBB3, which are believed to be associated with the effect of
platinum- and taxane-based chemotherapies, respectively.
Previously, ERCC1-positive cases were reported to show more
resistance to platinum-based chemotherapy than negative cases
(16), but no relationship was noted
between the ERCC1 expression and the prognosis, even in the
patients who received the CP regimen in this study. We obtained
similar findings concerning the TUBB3 expression. TUBB3-negative
cases have previously been reported to show a better prognosis than
positive ones. The prognostic effect of TUBB3 expression observed
in this study, even in the patients who received CP regimen, was
not consistent with prior published reports in the setting of
advanced NSCLC (35,49,50).
This discrepancy may be attributed to the small patient population
in this study.
We also evaluated the protein expressions of TS,
DPD, and OPRT, which are believed to be associated with the effect
5-FU-related agents, including S-1. Specifically, the
overexpression of TS and DPD have been reported to be associated
with resistance to S-1 (26–28). In contrast, the overexpression of
OPRT was reported to be associated with a better prognosis in
patients who received S-1 chemotherapy (30). In the present study, the expression
of DPD and OPRT showed no association with the OS or DFS, even in
the patients who received S-1 chemotherapy. The expression of TS
did not have an association with the OS or DFS in the total
population or with the OS in the 19 patients who received S-1.
However, in the analysis of the DFS of the 19 patients who received
S-1, the patients with TS overexpression showed a significantly
poorer prognosis than the TS-negative patients.
One limitation associated with this study was the
small patient population, as only 19 cases received S-1 and 19
cases received CP. Among the 40 patients, 2 were excluded due to
the cessation of adjuvant chemotherapy because of a grade 4
allergic reaction (anaphylactic shock) induced by paclitaxel. The
frequency of the anaphylactic shock (5%) was higher than previous
reports. We think that the small sample size of this study will
affect to the result. However, the adverse effects of S-1 were
tolerable, and S-1 chemotherapy may be considered a promising
adjuvant chemotherapy for patients with advanced disease who have
undergone complete surgical resection. Further large-scale analyses
of the relationship between TS expression and chemotherapeutic
effects are desired. Moreover, we should evaluate the relationship
among each protein expression in a large-scale clinical trial in
the future.
We herein showed that TS is a potentially useful
biomarker to help identify patients who will benefit from S-1
adjuvant chemotherapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KO and MY designed the present study. KO, KN, TY,
TK, TN, MS, SM, HH, OK and MY collected the patients' data. KO, TT,
TS, RO, TW and RN analyzed the patients' data. KO was a major
contributor in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients, and the study protocol was approved by the Institutional
Review Board of each participating institution (Nagoya City
University Hospital no. 45-13-0020). This study was carried out in
accordance with the Declaration of Helsinki.
Consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Detterbeck FC, Chansky K, Groome P,
Bolejack V, Crowley J, Shemnski L, Kennedy C, Krasnik M, Peake M,
Rami-Porta R, et al: The IASLC lung cancer staging project:
Methodology and validation used in the development of proposals for
revision of the stage classification of NSCLC in the forthcoming
(eighth) edition of the TNM classification of lung cancer. J Thorac
Oncol. 11:1433–1446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kis MG, Gaspar LE, Chaft JE, Kennedy EB,
Azzoli CG, Ellis PM, Lin SH, Pass HI, Seth R, Shepherd FA, et al:
Adjuvant systemic therapy and adjuvant radiation therapy for stage
I to IIIA completely resected non-small-cell lung cancers: American
society of clinical oncology/cancer care ontario clinical practice
guideline update. J Clin Oncol. 35:2960–2974. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pisters KM, Evans WK, Azzoli CG, Kris MG,
Smith CA, Desch CE, Somerfield MR, Brouwers MC, Darling G, Ellis
PM, et al: Cancer care onario and American society of clinical
oncology adjuvant chemotherapy and adjuvant radiation therapy for
stages I–IIIA resectable non-small cell lung cancer guideline. J
Clin Oncol. 25:5506–5518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crinò L, Weder W, van Meerbeeck J and
Felip E: ESMO Guidelines Working Group: Early stage and locally
acvanced (non-metastatic) non-small-cell lung cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21 Suppl 5:v103–v115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
NSCLC Meta-analyses Collaborative Group, .
Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le
Chevalier T, Le Pechoux C, Parmar MK, Pignon JP, et al: Adjuvant
chemotherapy, with or without postoperative radiotherapy, in
operable non-small-cell lung cancer: Two meta-analyses of
individual patient data. Lancet. 375:1267–1277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Früh M, Rolland E, Pignon JP, Seymour L,
Ding K, Tribodet H, Winton T, Le Chevalier T, Scagliotti GV,
Douillard JY, et al: Pooled analysis of the effect of age on
adjuvant cisplatin-based chemotherapy for completely resected
non-small-cell lung cancer. J Clin Oncol. 26:3573–3581. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hotta K, Matsuo K, Ueoka H, Kiura K,
Tabata M and Tanimoto M: Role of adjuvant chemotherapy in patients
with resected non-small-cell lung cancer: Reappraisal with
meta-analysis of randomized controlled trials. J Clin Oncol.
22:3860–3867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sedrakyan A, Van Der Meullen J, O'Byrne K,
Prendiville J, Hill J and Treasure T: Postoperative chemotherapy
for non-small cell lung cancer: A systematic review and
meta-analysis. J Thorac Cardiovasc Surg. 128:414–419. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berghmans T, Paesmans M, Meert AP, Mascaux
C, Lothaire P, Lafitte JJ and Sculier JP: Survival improvement in
resectable non-small cell lung cancer with (neo)adjuvant
chemotherapy: Results of a meta-analysis of the literature. Lung
Cancer. 49:13–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kelly K, Crowley J, Bunn PA Jr, Presant
CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR,
Moore DF, et al: Randomized phase III trial of paclitaxel plus
carboplatin versus vinorelbine plus cisplatin in the treatment of
patients with advanced non-small-cell lung cancer: A Southwest
Oncology Group trial. J Clin Oncol. 19:3210–3218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epedermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwak EL, Bang YJ, Camidge DR, Shaw AT,
Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, et al:
Anaplastic lymphoma kinase inhibition in non-small-cell lung
cancer. N Engl J Med. 363:1693–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang D and Lippard SJ: Cellular processing
of platinum anticancer drugs. Nature Rev Drug Disc. 4:307–320.
2005. View
Article : Google Scholar
|
|
15
|
Altaha R, Liang X, Yu JJ and Reed E:
Excision repair cross complementing-group 1: Gene expression and
platinum resistance. Int J Mol Med. 14:959–970. 2004.PubMed/NCBI
|
|
16
|
Lord RV, Brabender J, Gandara D, Alberola
V, Camps C, Domine M, Cardenal F, Sánchez JM, Gumerlock PH, Tarón
M, et al: Low ERCC1 expression correlates with prolonged survival
after cisplatin plus gemcitabine chemotherapy in non-small cell
lung cancer. Clin Cancer Res. 8:2286–2291. 2002.PubMed/NCBI
|
|
17
|
Metzger R, Leichman CG, Danenberg KD,
Danenberg PV, Lenz HJ, Hayashi K, Groshen S, Salonga D, Cohen H,
Laine L, et al: ERCC1 mRNA levels complement thymidylate synthase
mRNA levels in predicting response and survival for gastric cancer
patients receiving combination cisplatin and fluorouracil
chemotherapy. J Clin Onclo. 16:309–316. 1998. View Article : Google Scholar
|
|
18
|
Burkhart CA, Kavallaris M and Horwitz Band
S: The role of beta-tubulin isotypes in resistance to antimitotic
drugs. Biochem Biophys Acta. 1471:O1–9. 2001.PubMed/NCBI
|
|
19
|
Katsetos CD, Legido A, Perentes E and Mörk
SJ: Class III beta-tubulin isotype: a key cytoskeletal protein at
the crossroads of developmental neurobiology and tumor
neuropathology. J Child Neurol. 18:851–867. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu Q and Luduena RF: Removal of beta III
isotype enhances taxol induced microtubule assembly. Cell Struct
Funct. 18:173–182. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamath K, Wilson L, Cabral F and Jordan
MA: BetaIII-tubulin induces paclitaxel resistance in association
with reduced effects on microtubule dynamic instability. J Biol
Chem. 280:12902–12907. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lenz HJ, Leichman CG, Danenberg KD,
Danenberg PV, Groshen S, Cohen H, Laine L, Crookes P, Silberman H,
Baranda J, et al: Thymidylate synthase mRNA level in adenocarcinoma
of the stomach: A predictor for primary tumor response and overall
survival. J Clin Oncol. 14:176–182. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takeda M, Okamoto I, Hirabayashi N, Kitano
M and Nakagawa K: Thymidylate synthase and dihydropyrimidine
dehydrogenase expression levels are associated with response to S-1
plus carboplatin in advanced non-small cell lung cancer. Lung
Cancer. 73:103–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ogiuchi Y, Maruoka Y, Ando T, Kobayashi M
and Ogiuchi H: Thymidylate synthase, thymidine phosphorylase and
orotate phosphoribosyl transferase levels as predictive factors of
chemotherapy in oral squzmous cell carcinoma. Acta Histochem
Cytochem. 41:39–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Diasio RB and Harris BE: Clinical
pharmacology of 5-fluorouracil. Clin Parmacokinet. 16:215–237.
1989. View Article : Google Scholar
|
|
26
|
Oguri T, Achiwa H, Bessho Y, Muramatsu H,
Maeda H, Niimi T, Sato S and Ueda R: The role of thymidylate
synthase and dihydropyrimidine dehydrogenase in resistance to
5-fluorouracil in human lung cancer cells. Lung Cancer. 49:345–351.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Etienne MC, Chéradame S, Fischel JL,
Formento P, Dassonville O, Renée N, Schneider M, Thyss A, Demard F
and Milano G: Response to fluorouracil therapy in cancer patients:
The role of tumoral dihydorpyrimidine dehydrogenase activity. J
Clin Oncol. 13:1663–1670. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyoshi T, Kondo K, Toba H, Yoshida M,
Fujino H, Kenzaki K, Sakiyama S, Takehisa M and Tangoku A:
Predictive value of thymidylate synthase and dihydropyrimidine
dehydrogenase expression in tumor tissue, regarding the efficacy of
postoperatively administered UFT (Tegafur+Uracil) in patients with
non-small cell lung cancer. Anticancer Res. 27:2641–2648.
2007.PubMed/NCBI
|
|
29
|
Sakurai Y, Sakamoto K, Sugimoto Y, Yoshida
I, Masui T, Tonomura S, Inaba K, Shoji M, Nakamura Y, Uyama I, et
al: Orotate phosphoribosyltransferase levels measured by a newly
established enzyme-linked immunosorbent assay in gastric carcinoma.
Cancer Sci. 97:492–498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi IS, Lee HS, Lee KW, Kim H, Kim KH,
Kim YJ, Kim JH, Kim WH and Lee JS: Biomarker analysis in patients
with advanced gastric cancer treated with S-1 plus cisplatin
chemotherapy: Orotate phosphoribosyltransferase expression is
associated with treatment outcomes. Med Oncol. 28:991–998. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; Oxford: 2009
|
|
32
|
Okuda K, Yano M, Tatematsu T, Nakamae K,
Yamada T, Kasugai T, Nishida T, Sano M, Moriyama S, Haneda H, et
al: S-1 vs. paclitaxel plus carboplatin as adjuvant chemotherapy
for completely resected stage II/IIIA non-small-cell lung cancer.
Mol Clin Oncol. 8:73–79. 2018.PubMed/NCBI
|
|
33
|
Calvert AH, Newell DR, Gumbrell LA,
O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME and
Wiltshaw E: Carboplatin dosage: Prospective evaluation of a simple
formula based on renal function. J Clin Oncol. 7:1748–1756. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jellifie RW and Jlliffe SM: A computer
program for estimation of creatine clearance from unstable serum
creatine levels, age, sex, and weight. Math Biosci. 14:17–24. 1972.
View Article : Google Scholar
|
|
35
|
Okuda K, Sasaki H, Dumontet C, Kawano O,
Yukiue H, Yokoyama T, Yano M and Fujii Y: Expression of excision
repair cross-complementation group 1 and class III beta-tubulin
predict survival after chemotherapy for completely resected
non-small cell lung cancer. Lung Cancer. 62:105–112. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Al Haddad S, Zhang Z, Leygue E, Snell L,
Huang A, Niu Y, Hiller-Hitchcock T, Hole K, Murphy LC and Watson
PH: Psoriasin (S100A7) expression and invasive breast cancer. Am J
Pathol. 155:2057–2067. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Handara-Luca A, Bilal H, Bertrand LC and
Fouret P: Extra-cellular signal-regulated ERK-1/ERK-2 pathway
activation in human salivary gland mucoepidermoid carcinoma:
association to aggressive tumor behavior and tumor cell
proliferation. Am J Pathol. 163:957–967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yokota K, Sasaki H, Okuda K, Shitara M,
Hikosaka Y, Moriyama S, Yano M and Fujii Y: Expression of
thymidylate synthase and orotate phosphoribosyltransferase in
thymic carcinoma. Exp Ther Med. 4:589–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kawamura M, Eguchi K, Izumi Y, Yamato Y,
Koike T, Sakaguchi H, Hada E and Kobayashi K: Phase II trial of
gemcitabine and docetaxel in patients with completely resected
stage IIA-IIIA non-small-cell lung cancer. Cancer Chemother
Pharmacol. 60:495–501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kanda Y: Investigation of the freely
available easy-to-use sofrware ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Asamura H, Goya T, Koshiishi Y, Sohara Y,
Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, et
al: A Japanese lung cancer registry study: Prognosis of 13,010
resected lung cancers. J Thorac Oncol. 3:46–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ohe Y, Ohashi Y, Kubota K, Tamura T,
Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y and Fukuoka
M: Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm cooperative study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamashita Y, Kataoka K, Ishida T, Matsuura
M, Seno N, Mukaida H, Miyahara E, Miyata Y, Okita R, Shimizu K, et
al: A feasibility study of postoperative adjuvant therapy of
carboplatin and weekly paclitaxel for completely resected non-small
cell lung cancer. J Thorac Oncolo. 3:612–616. 2008. View Article : Google Scholar
|
|
45
|
Ichiki M, Kawasaki M, Takayama K, Ninomiya
K, Kuba M, Iwami F, Miyazaki N, Oishi K, Takeo S, Aizawa H and
Nakanishi Y: A multi-center phase II study of carboplatin and
paclitaxel with a biweekly schedule in patients with advanced
non-small-cell lung cancer. Kyushu thoracic oncology group trial.
Cancer Chemother Pharmacol. 58:368–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sugaya M, Uramoto H, Uchiyama A, Nagashima
A, Nakanishi R, Sakata H, Nakanishi K, Hanagiri T and Yasumoto K:
Phase II trial of adjuvant chemotherapy with bi-weekly carboplatin
plus paclitaxel in patients with completely resected non-small cell
lung cancer. Anticancer Res. 30:3039–3044. 2010.PubMed/NCBI
|
|
47
|
Iwamoto Y, Mitsudomi T, Sakai K, Yamanaka
T, Yoshioka H, Takahama M, Yoshimura M, Yoshino I, Takeda M,
Sugawara S, et al: Randomized phase II study of adjuvant
chemotherapy with long-term S-1 versus Cisplatin+S-1 in completely
resected stage II–IIIA non-small cell lung cancer. Clin Cancer Res.
21:5245–5252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Okumura S, Sasaki T, Satoh K, Kitada M,
Nagase A, Yatsuyanagi E and Ohsaki Y: Feasibility of adjuvant
chemotherapy with S-1 consisting of a 4-week administration and a
two-week rest period in patients with completely resected non-small
cell lung cancer. Mol Clin Oncol. 1:124–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mozzetti S, Ferlini C, Concolino P,
Filippetti F, Raspaglio G, Prislei S, Gallo D, Martinelli E,
Ranelletti FO, Ferrandina G and Scambia G: CClass III beta-tubulin
overexpression is a prominent mechanism of paclitaxel resistance in
ovarian cancer patients. Clin Cancer Res. 11:298–305.
2005.PubMed/NCBI
|
|
50
|
Paradiso A, Mangia A, Chiriatti A, Tommasi
S, Zito A, Latorre A, Schttulli F and Lorusso V: Biomarkers
predictive for clinical efficacy of taxol-based chemotherapy in
advanced breast cancer. Ann Oncol. 16 Suppl 4:iv14–iv19. 2005.
View Article : Google Scholar : PubMed/NCBI
|