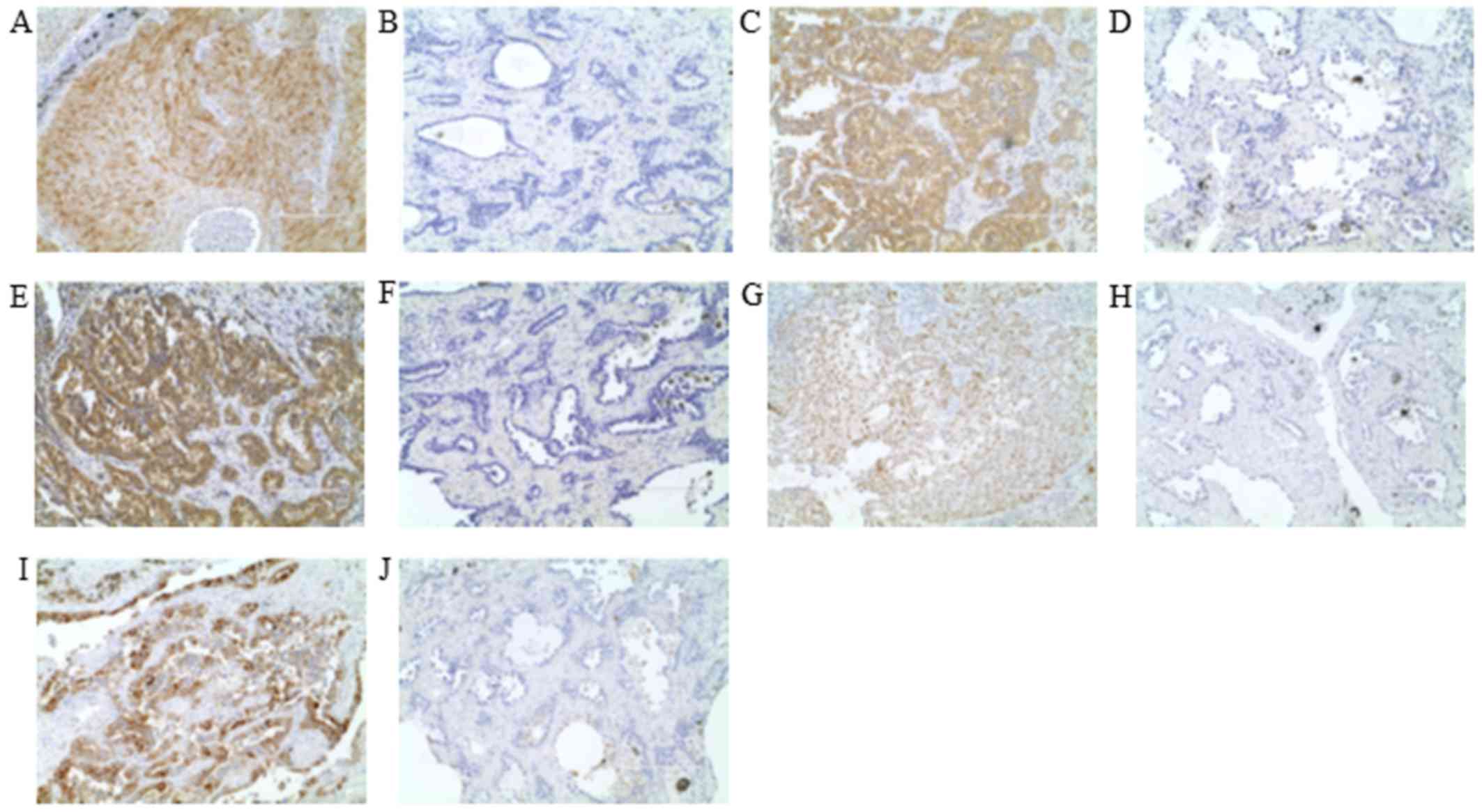
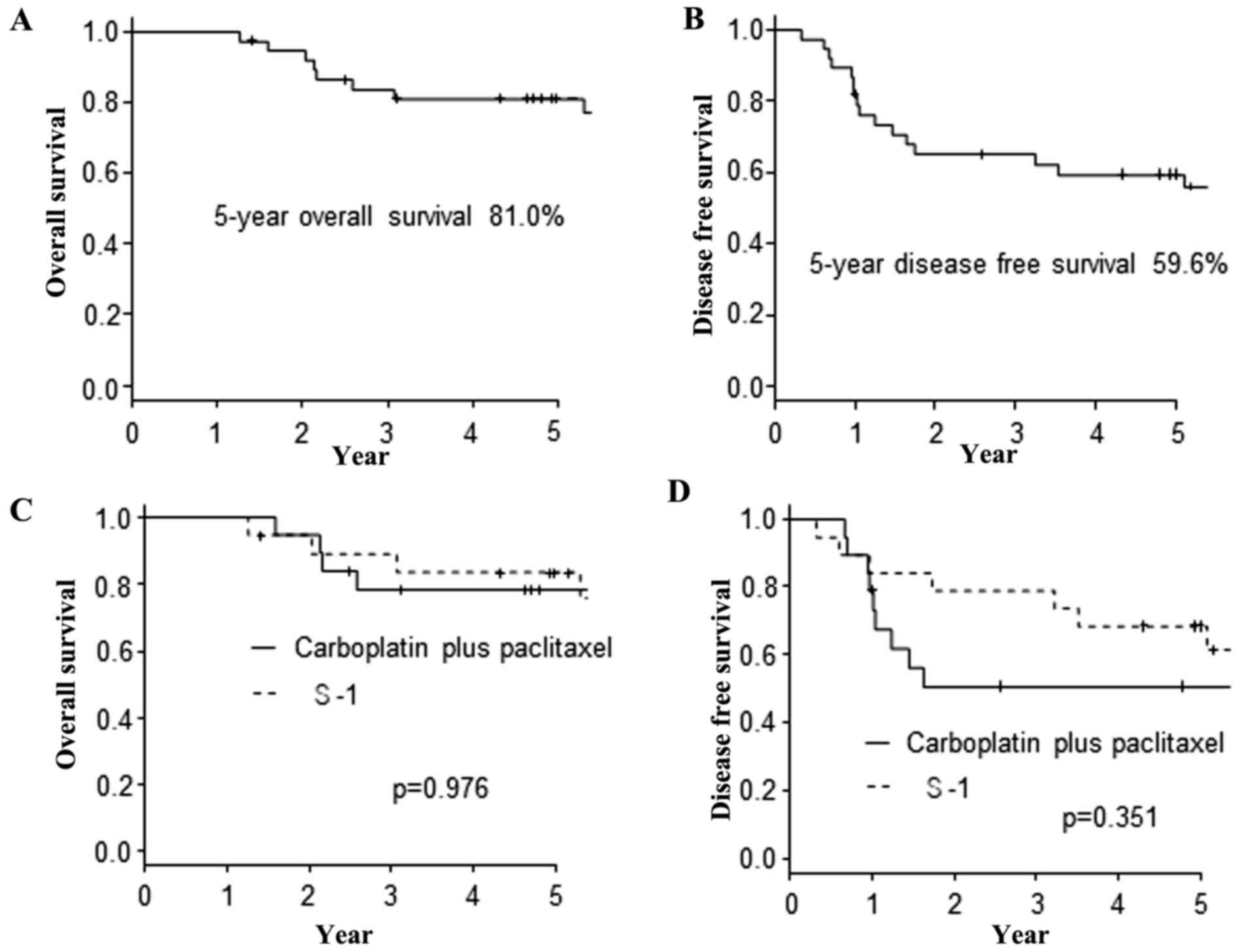
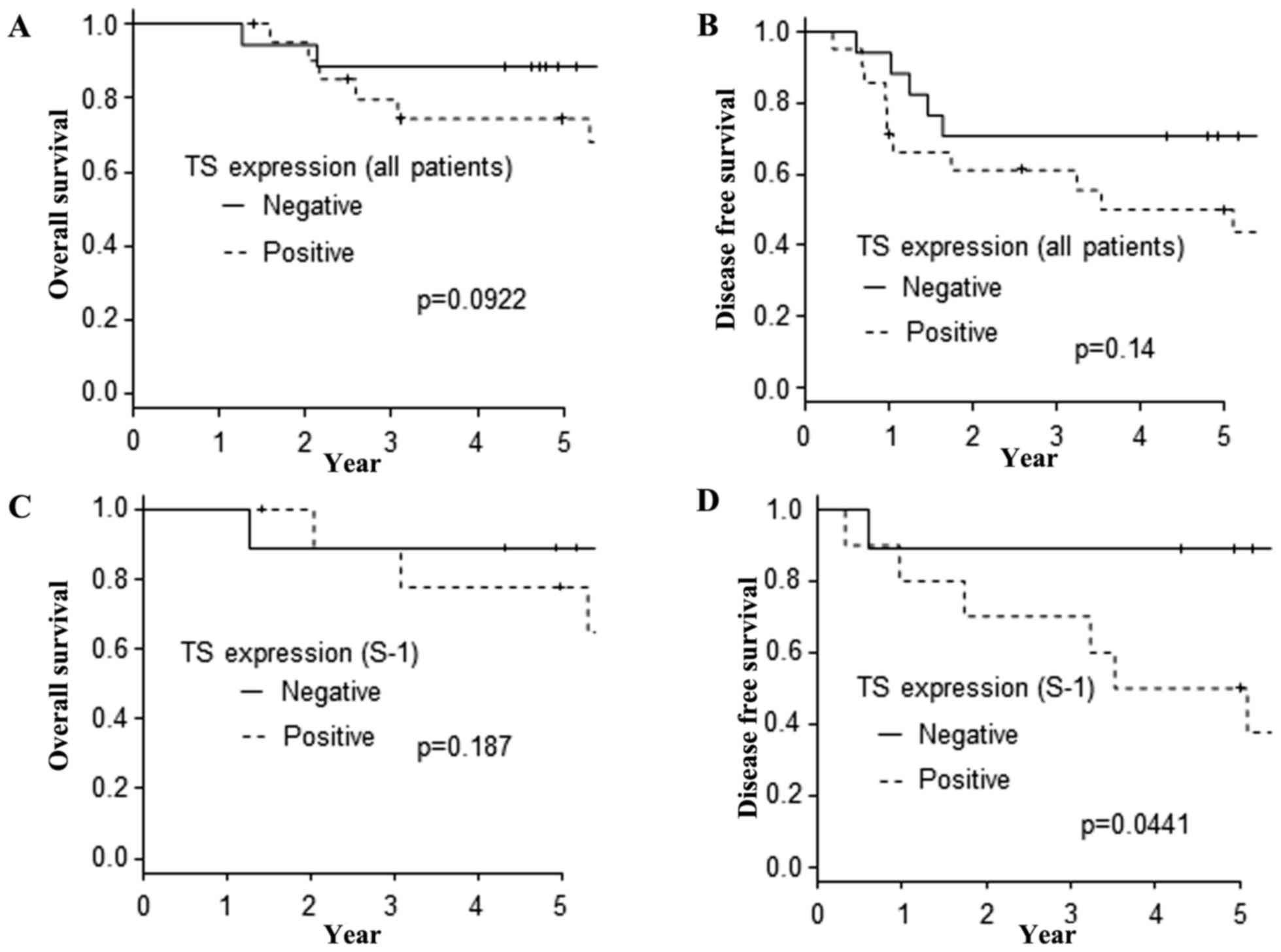
|
1
|
Detterbeck FC, Chansky K, Groome P,
Bolejack V, Crowley J, Shemnski L, Kennedy C, Krasnik M, Peake M,
Rami-Porta R, et al: The IASLC lung cancer staging project:
Methodology and validation used in the development of proposals for
revision of the stage classification of NSCLC in the forthcoming
(eighth) edition of the TNM classification of lung cancer. J Thorac
Oncol. 11:1433–1446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kis MG, Gaspar LE, Chaft JE, Kennedy EB,
Azzoli CG, Ellis PM, Lin SH, Pass HI, Seth R, Shepherd FA, et al:
Adjuvant systemic therapy and adjuvant radiation therapy for stage
I to IIIA completely resected non-small-cell lung cancers: American
society of clinical oncology/cancer care ontario clinical practice
guideline update. J Clin Oncol. 35:2960–2974. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pisters KM, Evans WK, Azzoli CG, Kris MG,
Smith CA, Desch CE, Somerfield MR, Brouwers MC, Darling G, Ellis
PM, et al: Cancer care onario and American society of clinical
oncology adjuvant chemotherapy and adjuvant radiation therapy for
stages I–IIIA resectable non-small cell lung cancer guideline. J
Clin Oncol. 25:5506–5518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crinò L, Weder W, van Meerbeeck J and
Felip E: ESMO Guidelines Working Group: Early stage and locally
acvanced (non-metastatic) non-small-cell lung cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21 Suppl 5:v103–v115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
NSCLC Meta-analyses Collaborative Group, .
Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le
Chevalier T, Le Pechoux C, Parmar MK, Pignon JP, et al: Adjuvant
chemotherapy, with or without postoperative radiotherapy, in
operable non-small-cell lung cancer: Two meta-analyses of
individual patient data. Lancet. 375:1267–1277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Früh M, Rolland E, Pignon JP, Seymour L,
Ding K, Tribodet H, Winton T, Le Chevalier T, Scagliotti GV,
Douillard JY, et al: Pooled analysis of the effect of age on
adjuvant cisplatin-based chemotherapy for completely resected
non-small-cell lung cancer. J Clin Oncol. 26:3573–3581. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hotta K, Matsuo K, Ueoka H, Kiura K,
Tabata M and Tanimoto M: Role of adjuvant chemotherapy in patients
with resected non-small-cell lung cancer: Reappraisal with
meta-analysis of randomized controlled trials. J Clin Oncol.
22:3860–3867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sedrakyan A, Van Der Meullen J, O'Byrne K,
Prendiville J, Hill J and Treasure T: Postoperative chemotherapy
for non-small cell lung cancer: A systematic review and
meta-analysis. J Thorac Cardiovasc Surg. 128:414–419. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berghmans T, Paesmans M, Meert AP, Mascaux
C, Lothaire P, Lafitte JJ and Sculier JP: Survival improvement in
resectable non-small cell lung cancer with (neo)adjuvant
chemotherapy: Results of a meta-analysis of the literature. Lung
Cancer. 49:13–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kelly K, Crowley J, Bunn PA Jr, Presant
CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR,
Moore DF, et al: Randomized phase III trial of paclitaxel plus
carboplatin versus vinorelbine plus cisplatin in the treatment of
patients with advanced non-small-cell lung cancer: A Southwest
Oncology Group trial. J Clin Oncol. 19:3210–3218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epedermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwak EL, Bang YJ, Camidge DR, Shaw AT,
Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, et al:
Anaplastic lymphoma kinase inhibition in non-small-cell lung
cancer. N Engl J Med. 363:1693–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang D and Lippard SJ: Cellular processing
of platinum anticancer drugs. Nature Rev Drug Disc. 4:307–320.
2005. View
Article : Google Scholar
|
|
15
|
Altaha R, Liang X, Yu JJ and Reed E:
Excision repair cross complementing-group 1: Gene expression and
platinum resistance. Int J Mol Med. 14:959–970. 2004.PubMed/NCBI
|
|
16
|
Lord RV, Brabender J, Gandara D, Alberola
V, Camps C, Domine M, Cardenal F, Sánchez JM, Gumerlock PH, Tarón
M, et al: Low ERCC1 expression correlates with prolonged survival
after cisplatin plus gemcitabine chemotherapy in non-small cell
lung cancer. Clin Cancer Res. 8:2286–2291. 2002.PubMed/NCBI
|
|
17
|
Metzger R, Leichman CG, Danenberg KD,
Danenberg PV, Lenz HJ, Hayashi K, Groshen S, Salonga D, Cohen H,
Laine L, et al: ERCC1 mRNA levels complement thymidylate synthase
mRNA levels in predicting response and survival for gastric cancer
patients receiving combination cisplatin and fluorouracil
chemotherapy. J Clin Onclo. 16:309–316. 1998. View Article : Google Scholar
|
|
18
|
Burkhart CA, Kavallaris M and Horwitz Band
S: The role of beta-tubulin isotypes in resistance to antimitotic
drugs. Biochem Biophys Acta. 1471:O1–9. 2001.PubMed/NCBI
|
|
19
|
Katsetos CD, Legido A, Perentes E and Mörk
SJ: Class III beta-tubulin isotype: a key cytoskeletal protein at
the crossroads of developmental neurobiology and tumor
neuropathology. J Child Neurol. 18:851–867. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu Q and Luduena RF: Removal of beta III
isotype enhances taxol induced microtubule assembly. Cell Struct
Funct. 18:173–182. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamath K, Wilson L, Cabral F and Jordan
MA: BetaIII-tubulin induces paclitaxel resistance in association
with reduced effects on microtubule dynamic instability. J Biol
Chem. 280:12902–12907. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lenz HJ, Leichman CG, Danenberg KD,
Danenberg PV, Groshen S, Cohen H, Laine L, Crookes P, Silberman H,
Baranda J, et al: Thymidylate synthase mRNA level in adenocarcinoma
of the stomach: A predictor for primary tumor response and overall
survival. J Clin Oncol. 14:176–182. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takeda M, Okamoto I, Hirabayashi N, Kitano
M and Nakagawa K: Thymidylate synthase and dihydropyrimidine
dehydrogenase expression levels are associated with response to S-1
plus carboplatin in advanced non-small cell lung cancer. Lung
Cancer. 73:103–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ogiuchi Y, Maruoka Y, Ando T, Kobayashi M
and Ogiuchi H: Thymidylate synthase, thymidine phosphorylase and
orotate phosphoribosyl transferase levels as predictive factors of
chemotherapy in oral squzmous cell carcinoma. Acta Histochem
Cytochem. 41:39–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Diasio RB and Harris BE: Clinical
pharmacology of 5-fluorouracil. Clin Parmacokinet. 16:215–237.
1989. View Article : Google Scholar
|
|
26
|
Oguri T, Achiwa H, Bessho Y, Muramatsu H,
Maeda H, Niimi T, Sato S and Ueda R: The role of thymidylate
synthase and dihydropyrimidine dehydrogenase in resistance to
5-fluorouracil in human lung cancer cells. Lung Cancer. 49:345–351.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Etienne MC, Chéradame S, Fischel JL,
Formento P, Dassonville O, Renée N, Schneider M, Thyss A, Demard F
and Milano G: Response to fluorouracil therapy in cancer patients:
The role of tumoral dihydorpyrimidine dehydrogenase activity. J
Clin Oncol. 13:1663–1670. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyoshi T, Kondo K, Toba H, Yoshida M,
Fujino H, Kenzaki K, Sakiyama S, Takehisa M and Tangoku A:
Predictive value of thymidylate synthase and dihydropyrimidine
dehydrogenase expression in tumor tissue, regarding the efficacy of
postoperatively administered UFT (Tegafur+Uracil) in patients with
non-small cell lung cancer. Anticancer Res. 27:2641–2648.
2007.PubMed/NCBI
|
|
29
|
Sakurai Y, Sakamoto K, Sugimoto Y, Yoshida
I, Masui T, Tonomura S, Inaba K, Shoji M, Nakamura Y, Uyama I, et
al: Orotate phosphoribosyltransferase levels measured by a newly
established enzyme-linked immunosorbent assay in gastric carcinoma.
Cancer Sci. 97:492–498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi IS, Lee HS, Lee KW, Kim H, Kim KH,
Kim YJ, Kim JH, Kim WH and Lee JS: Biomarker analysis in patients
with advanced gastric cancer treated with S-1 plus cisplatin
chemotherapy: Orotate phosphoribosyltransferase expression is
associated with treatment outcomes. Med Oncol. 28:991–998. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; Oxford: 2009
|
|
32
|
Okuda K, Yano M, Tatematsu T, Nakamae K,
Yamada T, Kasugai T, Nishida T, Sano M, Moriyama S, Haneda H, et
al: S-1 vs. paclitaxel plus carboplatin as adjuvant chemotherapy
for completely resected stage II/IIIA non-small-cell lung cancer.
Mol Clin Oncol. 8:73–79. 2018.PubMed/NCBI
|
|
33
|
Calvert AH, Newell DR, Gumbrell LA,
O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME and
Wiltshaw E: Carboplatin dosage: Prospective evaluation of a simple
formula based on renal function. J Clin Oncol. 7:1748–1756. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jellifie RW and Jlliffe SM: A computer
program for estimation of creatine clearance from unstable serum
creatine levels, age, sex, and weight. Math Biosci. 14:17–24. 1972.
View Article : Google Scholar
|
|
35
|
Okuda K, Sasaki H, Dumontet C, Kawano O,
Yukiue H, Yokoyama T, Yano M and Fujii Y: Expression of excision
repair cross-complementation group 1 and class III beta-tubulin
predict survival after chemotherapy for completely resected
non-small cell lung cancer. Lung Cancer. 62:105–112. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Al Haddad S, Zhang Z, Leygue E, Snell L,
Huang A, Niu Y, Hiller-Hitchcock T, Hole K, Murphy LC and Watson
PH: Psoriasin (S100A7) expression and invasive breast cancer. Am J
Pathol. 155:2057–2067. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Handara-Luca A, Bilal H, Bertrand LC and
Fouret P: Extra-cellular signal-regulated ERK-1/ERK-2 pathway
activation in human salivary gland mucoepidermoid carcinoma:
association to aggressive tumor behavior and tumor cell
proliferation. Am J Pathol. 163:957–967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yokota K, Sasaki H, Okuda K, Shitara M,
Hikosaka Y, Moriyama S, Yano M and Fujii Y: Expression of
thymidylate synthase and orotate phosphoribosyltransferase in
thymic carcinoma. Exp Ther Med. 4:589–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kawamura M, Eguchi K, Izumi Y, Yamato Y,
Koike T, Sakaguchi H, Hada E and Kobayashi K: Phase II trial of
gemcitabine and docetaxel in patients with completely resected
stage IIA-IIIA non-small-cell lung cancer. Cancer Chemother
Pharmacol. 60:495–501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kanda Y: Investigation of the freely
available easy-to-use sofrware ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Asamura H, Goya T, Koshiishi Y, Sohara Y,
Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, et
al: A Japanese lung cancer registry study: Prognosis of 13,010
resected lung cancers. J Thorac Oncol. 3:46–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ohe Y, Ohashi Y, Kubota K, Tamura T,
Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y and Fukuoka
M: Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm cooperative study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamashita Y, Kataoka K, Ishida T, Matsuura
M, Seno N, Mukaida H, Miyahara E, Miyata Y, Okita R, Shimizu K, et
al: A feasibility study of postoperative adjuvant therapy of
carboplatin and weekly paclitaxel for completely resected non-small
cell lung cancer. J Thorac Oncolo. 3:612–616. 2008. View Article : Google Scholar
|
|
45
|
Ichiki M, Kawasaki M, Takayama K, Ninomiya
K, Kuba M, Iwami F, Miyazaki N, Oishi K, Takeo S, Aizawa H and
Nakanishi Y: A multi-center phase II study of carboplatin and
paclitaxel with a biweekly schedule in patients with advanced
non-small-cell lung cancer. Kyushu thoracic oncology group trial.
Cancer Chemother Pharmacol. 58:368–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sugaya M, Uramoto H, Uchiyama A, Nagashima
A, Nakanishi R, Sakata H, Nakanishi K, Hanagiri T and Yasumoto K:
Phase II trial of adjuvant chemotherapy with bi-weekly carboplatin
plus paclitaxel in patients with completely resected non-small cell
lung cancer. Anticancer Res. 30:3039–3044. 2010.PubMed/NCBI
|
|
47
|
Iwamoto Y, Mitsudomi T, Sakai K, Yamanaka
T, Yoshioka H, Takahama M, Yoshimura M, Yoshino I, Takeda M,
Sugawara S, et al: Randomized phase II study of adjuvant
chemotherapy with long-term S-1 versus Cisplatin+S-1 in completely
resected stage II–IIIA non-small cell lung cancer. Clin Cancer Res.
21:5245–5252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Okumura S, Sasaki T, Satoh K, Kitada M,
Nagase A, Yatsuyanagi E and Ohsaki Y: Feasibility of adjuvant
chemotherapy with S-1 consisting of a 4-week administration and a
two-week rest period in patients with completely resected non-small
cell lung cancer. Mol Clin Oncol. 1:124–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mozzetti S, Ferlini C, Concolino P,
Filippetti F, Raspaglio G, Prislei S, Gallo D, Martinelli E,
Ranelletti FO, Ferrandina G and Scambia G: CClass III beta-tubulin
overexpression is a prominent mechanism of paclitaxel resistance in
ovarian cancer patients. Clin Cancer Res. 11:298–305.
2005.PubMed/NCBI
|
|
50
|
Paradiso A, Mangia A, Chiriatti A, Tommasi
S, Zito A, Latorre A, Schttulli F and Lorusso V: Biomarkers
predictive for clinical efficacy of taxol-based chemotherapy in
advanced breast cancer. Ann Oncol. 16 Suppl 4:iv14–iv19. 2005.
View Article : Google Scholar : PubMed/NCBI
|