Introduction
Cancer is a major public health problem worldwide,
and there are approximately 15.5 million patients with a history of
cancer in the United States (1);
thus, there is great public concern about cancer therapy. The
development of anticancer drugs, with the shift from traditional
chemotherapies to molecular-targeted therapies in the last decade,
have dramatically improved the clinical outcome of cancer patients,
and have resulted in prolonging the life span of these patients
(2). Traditional chemotherapies
target the cell cycle of malignant cells as well as that of normal
cells, resulting in adverse effects on cytotoxicity such as
alopecia, gastrointestinal toxicity, myelotoxicity and
cardiotoxicity. In contrast, molecular-targeted therapies with
monoclonal antibodies specifically target malignant cells. The
specificity of molecular targeted therapies for malignant cells is
the reduction of side effects as well as more effective treatment
of various kinds of cancer, when compared with traditional standard
chemotherapies. However, adverse effects on cardiac function (i.e.,
cardiotoxicity) for several molecular target agents have been
reported (3,4).
Trastuzumab, a monoclonal antibody administered for
breast cancer patients, interferes with human epidermal growth
factor receptor 2 (HER2), which is a kind of trans-membrane
tyrosine kinase that regulates cell growth, cell survival,
adhesion, migration, and differentiation (5,6). HER2 is
overexpressed in approximately 25% of breast cancer cases, and
HER2-positive breast cancer tends to have a high risk of
metastasis, resistance for anticancer drugs, and recurrence,
because overactivation of HER2 signaling both promotes cell
proliferation and inhibits cell death (7). Trastuzumab significantly improves
overall survival in patients with HER2-positive breast cancer by
blocking HER2 signaling (8).
Although HER2 signaling also plays an essential role in the
maintenance of cardiomyocyte function (9,10), there
is recent increasing concern about the adverse effects of
trastuzumab-induced cardiotoxicity (TIC), which is occasionally
life-threatening. Unfortunately, the risk factors and predictors of
TIC in breast cancer patients have yet to be fully elucidated
(3,11,12).
The aims of this study were to investigate the
predictors of TIC, and to consider appropriate management for such
patients.
Patients and methods
Study design and subjects
This was a retrospective study to investigate the
prevalence of TIC and its predictors. The clinical data of
consecutive 450 breast cancer patients, who had been referred to
our department to undergo echocardiography before chemotherapy
and/or operation between 2003 and 2015, were investigated,
including baseline characteristics, echocardiographic parameters,
and occurrence of TIC or all-cause death. Trastuzumab was
administered as a loading dose of 8 mg/kg, followed by 6 mg/kg
every three weeks (13). We
evaluated several co-morbidities and past histories in the medical
records that may be associated with occurrence of TIC and
mortality, such as the presence and/or past history of
hypertension, dyslipidemia, diabetes mellitus, chronic kidney
disease, ischemic heart disease, arrhythmia, valvular heart disease
(VHD) and heart failure, as well as past treatment of anthracycline
or radiation. Echocardiography was performed by experienced
echocardiographers using standard techniques. The definition of TIC
was: i) Manifestation of decompensated heart failure, based on the
Framingham criteria (14); or ii)
<55% of left ventricular ejection fraction (LVEF) and a
reduction of LVEF of >10% from baseline to after trastuzumab
treatment in asymptomatic patients (15). Out of 450 patients, 119 patients
received trastuzumab therapy. We divided the 119 patients into two
groups based on the presence or absence of TIC (TIC group, n=13 and
non-TIC group, n=106).
Statistical analysis
Normally distributed data are presented as mean ±
SD. The baseline characteristics between the two groups were
compared using the independent Student's t-test for parametric
variables, and the Chi-square test was used for categorical
variables. Comparisons of data at baseline and after trastuzumab
treatment were analyzed using the paired Student's t-test. We
performed logistic regression analysis allowing for interaction
between the onset of TIC and each possible confounding factor.
These analyses were performed using a statistical software package
(SPSS version 24.0; IBM, Armonk, NY, USA), and a P-value of
<0.05 was considered statistically significant. The study
protocol was approved by the ethics committee of Fukushima Medical
University.
Results
The baseline characteristics of the 119 patients are
presented in Table I. There were no
significant differences, except for the presence of VHD, in the
baseline characteristics and echocardiographic parameters between
the TIC and non-TIC groups before initiation of trastuzumab
(Table I). The details of VHD were
as follows: i) Moderate mitral regurgitation, n=4; ii) moderate
aortic regurgitation, n=3; and iii) moderate tricuspid
regurgitation, n=5. Slight-to-mild VHD was not considered as
significant VHD, and there were no patients with severe VHD in the
present study. Seventy-two patients (60.5%) received a combination
therapy with anthracycline, 38 (31.9%) received radiation therapy,
and 27 (22.6%) received both therapies. In the follow-up period
(mean, 1,410 days), symptomatic heart failure occurred in two
patients (1.6%), and 11 patients (9.2%) had asymptomatic impairment
of cardiac function, as determined by echocardiography. The
impairment of cardiac function was ameliorated by discontinuing
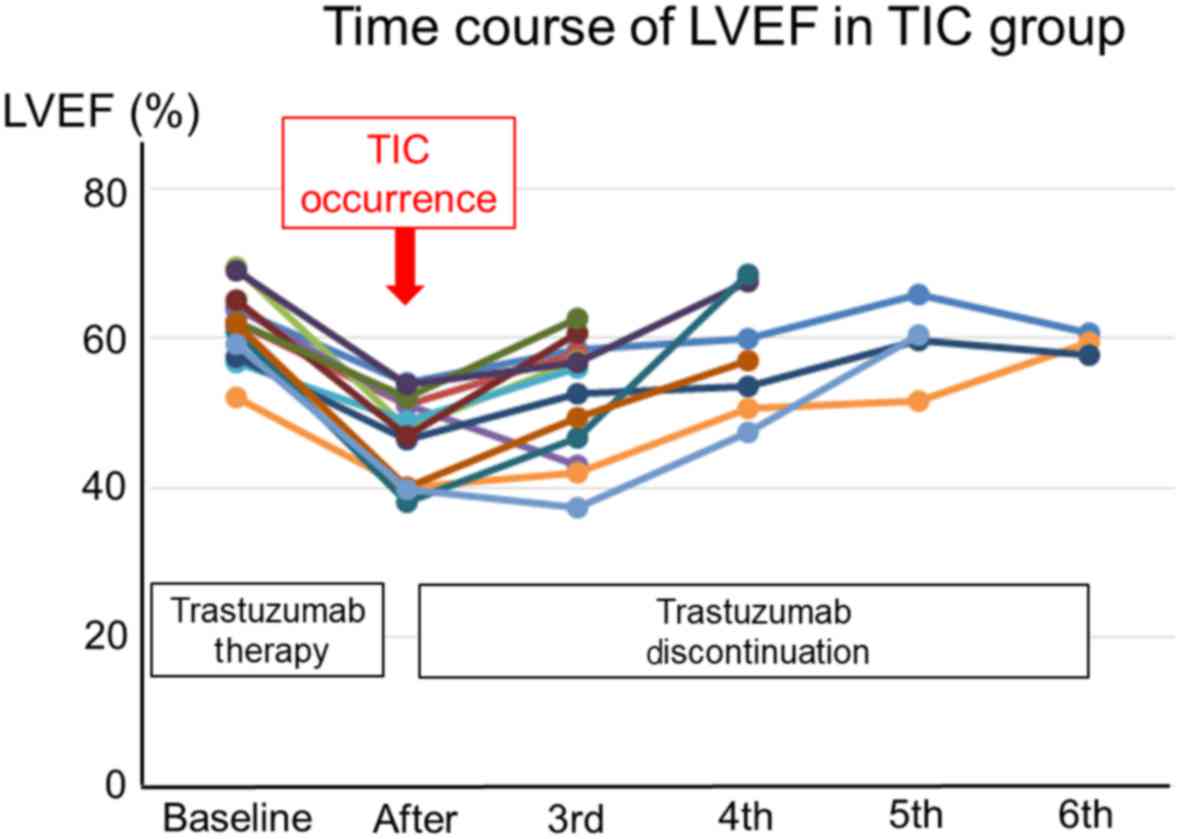
trastuzumab. The time courses of LVEF in each patient with TIC are
presented in Fig. 1. The LVEF in all
TIC patients was recovered after discontinuing trastuzumab. Twenty
patients (16.8%) died due to cancer-related causes, and no patients
died of cardiac death. Within 3 to 6 months of initiation of
treatment with trastuzumab, the TIC group showed increased volumes
of the left ventricle and atrium, and decreases of the fractional
shortening, LVEF and E′. In the present logistic regression
analysis, only the presence of VHD at baseline was a predictor of
TIC, and there was no other predictor for TIC, including baseline
characteristics, other therapies and baseline echocardiographic
parameters, in the present study (Table
II).
 | Table I.Patient demographics. |
Table I.
Patient demographics.
|
| TIC (−) (n=106) | TIC (+) (n=13) | P-value |
|---|
| Demographics |
| Age
(years) | 56.7±11.1 | 51.9±9.1 | 0.093 |
| Female
sex (n, %) | 106 (100) | 13 (100) | 0.806 |
| Body mass
index (kg/cm2) | 22.5±3.9 | 20.1±2.7 | 0.062 |
| Co-morbidity |
|
Hypertension (n, %) | 22
(20.8) | 0 (0) | 0.174 |
|
Dyslipidemia (n, %) | 22 (20.8) | 2 (15.4) | 0.841 |
| Diabetes
mellitus (n, %) | 10 (9.4) | 1 (7.7) | 0.919 |
| Chronic
kidney disease (n, %) | 7 (5.6) | 1 (7.7) | 0.879 |
| Ischemic
heart disease | 2 (1.9) | 0 | 0.617 |
|
Arrhythmia | 5 (4.7) | 1 (7.7) | 0.644 |
| Valvular
heart disease | 4 (3.8) | 3 (23.1) | 0.047 |
|
MR/AR/TR | 1 (0.9)/2 | 3 (23.1)/1 |
|
|
| (1.9)/3 (2.8) | (7.7)/2 (15.4) |
|
| Heart
failure | 1 (0.9) | 1 (7.7) | 0.074 |
| Laboratory data |
|
Hemoglobin (g/dl) | 11.7±1.5 | 11.4±1.4 | 0.514 |
|
Creatinine (mg/dl) | 0.59±0.12 | 0.65±0.14 | 0.250 |
|
Estimated GFR
(ml/min/m2) | 82.9±20.2 | 87.5±25.2 | 0.461 |
| Combination
therapy |
|
Anthracycline (n, %) | 62 (58.5) | 10 (76.9) | 0.163 |
|
Radiation therapy (n, %) | 36 (34.0) | 2 (15.4) | 0.221 |
|
Anthracycline and radiation
therapy (n, %) | 26 (24.5) | 1 (7.7) | 0.293 |
| Echocardiographic
parameters (Baseline and after trastuzumab) |
| IVS (mm):
Baseline | 9.1±1.3 | 9.8±0.9 | 0.098 |
|
After | 9.4±3.3 | 9.8±1.9 | 0.627 |
| PW (mm):
Baseline | 9.6±3.1 | 9.7±0.6 | 0.805 |
|
After | 9.3±1.5 | 9.9±1.4 | 0.270 |
| LVDd (mm):
Baseline | 42.2±4.9 | 43.5±3.6 | 0.360 |
|
After | 43.1±4.9 |
48.1±4.1a | 0.004 |
| LVDs (mm):
Baseline | 26.1±4.9 | 28.0±3.8 | 0.146 |
|
After | 26.7±4.4 |
35.5±5.6a | <0.001 |
| FS (%):
Baseline | 38.0±8.1 | 33.5±4.7 | 0.196 |
|
After | 37.5±7.3 |
23.6±8.7a | <0.001 |
| LA diameter (mm):
Baseline | 31.2±5.3 | 31.5±7.6 | 0.862 |
|
After | 31.2±6.5 | 34.2±5.2 | 0.164 |
| LA volume (ml):
Baseline | 35.7±16.1 | 37.3±19.9 | 0.783 |
|
After | 35.5±17.3 |
54.8±17.6a | 0.005 |
| LVEDV (ml):
Baseline | 63.4±19.6 | 68.2±11.0 | 0.385 |
|
After | 65.0±21.2 |
90.4±17.1b | 0.001 |
| LVESV (ml):
Baseline | 22.7±8.3 | 26.1±4.9 | 0.149 |
|
After | 24.0±10.1 |
48.6±15.5b | <0.001 |
| LVEF (%):
Baseline | 64.3±5.7 | 61.9±4.7 | 0.147 |
|
After | 64.2±5.5 |
46.9±8.0b | <0.001 |
| TRPG (mmHg):
Baseline | 17.3±6.6 | 18.3±6.9 | 0.663 |
|
After | 19.8±7.3 | 22.8±9.3 | 0.228 |
| E (m/sec):
Baseline | 0.68±0.16 | 1.1±1.6 | 0.321 |
|
After | 0.71±0.17 | 0.82±0.33 | 0.333 |
| A (m/sec):
Baseline | 0.73±0.18 | 0.65±0.16 | 0.086 |
|
After | 0.73±0.16 | 0.66±0.19 | 0.235 |
| E′ (cm/sec):
Baseline | 8.7±2.9 | 8.2±1.6 | 0.564 |
|
After | 9.3±4.3 |
5.6±3.9a | 0.030 |
| IVC (mm):
Baseline | 11.8±2.8 | 12.3±2.6 | 0.570 |
|
After | 12.3±3.3 |
14.8±4.0a | 0.047 |
 | Table II.Univariate logistic regression
analysis to determine factors related to TIC. |
Table II.
Univariate logistic regression
analysis to determine factors related to TIC.
| Factors | β coefficient | P-value | OR | 95% CI |
|---|
| Age | 0.037 | 0.135 | 0.962 | 0.913–1.012 |
| Body mass
index | −0.213 | 0.248 | 0.808 | 0.666–0.118 |
| Hypertension | −18.637 | 0.998 | – | – |
| Dyslipidemia | −0.398 | 0.592 | 0.672 | 0.156–2.883 |
| Diabetes
mellitus | −0.315 | 0.712 | 0.730 | 0.137–3.879 |
| Chronic kidney
disease | −0.064 | 0.884 | 0.938 | 0.398–2.212 |
| Ischemic heart
disease | −19.123 | 0.999 | – | – |
| Arrhythmia | 0.521 | 0.647 | 1.683 | 0.181–15.637 |
| Valvular heart
disease | 2.035 | 0.015 | 7.650 | 1.496–39.114 |
| Heart failure | 2.169 | 0.134 | 8.750 | 0.514–149.079 |
| Combination with
anthracycline therapy | 0.861 | 0.210 | 2.366 | 0.615–9.096 |
| Combination with
radiation therapy | −1.735 | 0.102 | 0.176 | 0.022–1.413 |
| Combination with
anthracycline and radiation therapy | −19.361 | 0.998 | – | – |
| LVDd (mm) | 0.058 | 0.357 | 1.060 | 0.936–1.200 |
| LVEF (%) | −0.078 | 0.149 | 0.925 | 0.832–1.028 |
| LA diameter
(mm) | 0.09 | 0.860 | 1.009 | 0.911–1.119 |
| E wave (m/sec) | 0.988 | 0.306 | 2.686 | 0.400–18.017 |
| A wave (m/sec) | −3.135 | 0.118 | 0.043 | 0.001–2.219 |
| E` wave
(cm/sec) | −0.076 | 0.560 | 0.927 | 0.718–1.196 |
Discussion
Although trastuzumab improves the prognosis of
patients with HER2-positive breast cancer, TIC is an important
issue. The exact mechanism of TIC is still remains unknown;
however, several mechanisms have been suspected (16,17).
HER2 conditional knockout mice generally develop severe dilated
cardiomyopathy (16). Thus, HER2,
the preferred dimerization partner for all other ErbB receptors,
particularly ErbB4 in cardiomyocytes, is thought to have a variety
of roles in the normal physiology of the heart, including
morphology, hypertrophic growth, excitation-contraction coupling,
and survival (16). Neuregulin
activates both ErbB2 and ErbB4 receptor tyrosine kinase activity
and promotes growth, myofilament organization, and survival of
isolated cardiac myocytes (17). By
circulating regulating reactive oxygen species-induced
cardiomyocyte apoptosis, inducing cell-cycle activity, neuregulin
normally protects cardiomyocytes against stress, promoting
regeneration and improving cardiac function and survival (16). Inhibition of HER2 signaling with
trastuzumab antagonizes the effects of neuregulin (16).
In the present retrospective study, asymptomatic TIC
occurred in 9.2% of the study population, and symptomatic HF
occurred in 1.6%. These results are concordant with those of a
recent large retrospective cohort study by Bowles et al, of
12,500 women who had been diagnosed with breast cancer between 1999
and 2007 in the United states. That study reported a TIC occurrence
of 9.8% of the study population, and symptomatic HF occurrence in
2.7% (18). In addition, LVEF
recovery was observed in all patients with TIC after the
discontinuation of trastuzumab in the current study, which is
consistent with the findings of Bowles's study. Compared with no
chemotherapy, the incidence of TIC in Bowles's study was higher in
patients treated with anthracycline alone [adjusted hazard ratio
(HR)= 1.40, 95% confidence interval (CI): 1.11–1.76]. Although the
increased risk was similar to those of other chemotherapy (adjusted
HR=1.49, 95% CI: 1.25–1.77), the risk was highly increased in
patients treated with trastuzumab alone (adjusted HR= 4.12, 95% CI:
2.30–7.42) or anthracycline plus trastuzumab (adjusted HR= 7.19,
95% CI: 5.00–10.35) (18). Data from
clinical trials have indicated that anthracycline is associated
with an approximate 2% increase (19) in heart failure and/or cardiomyopathy
incidence, and anthracycline followed by trastuzumab is associated
with an approximate 4% increase (20). Although several other clinical
studies have investigated the risk factors or predictors for TIC
(12,21), said predictors TIC have yet to be
fully elucidated, and regular cardiac function monitoring has been
recommended (22).
Recently, a position paper by the European Society
of Cardiology reported that: i) Age, high body mass index >30
kg/m2, hypertension, previous or concomitant
anthracycline treatment, previous radiation therapy, previous LV
dysfunction are suggested factors associated with TIC (3); and that ii) cumulative dose, female
sex, age, chronic kidney disease, hypertension, previous radiation
therapy, concomitant chemotherapy, pre-existing cardiac disease
associated with increased wall stress are suggested factors that
are all associated with risk of cardiotoxicity following
anthracycline treatment (3).
In contrast to previous data (3,11,12,18,21),
in the present study, only presence of VHD was a novel predictor of
TIC, but not age, body mass index, other cardiac diseases, combined
anthracycline therapy or LVEF. We cannot fully explain the reasons
for the differences between said reports and the current results;
however, the impact of VHD on TIC were considered as follows:
First, VHD has not yet been fully focused on in previous studies in
TIC (12,18,21). In
contrast, the use of trastuzumab may be avoided in patients with
past histories of other cardiac diseases, as stipulated in the
recent trastuzumab guidelines (23).
Second, presence of VHD itself may indicate latent cardiac damage
associated with past endocarditis, radiation, chemotherapy and/or
secondary to LV dysfunction (3).
Radiation causes fibrosis and calcification of the aortic root,
aortic valve cusps, mitral valve annulus and base and mid portions
of the mitral valve leaflets, sparing the mitral valve tips and
commissures (3,24). Third, VHD is associated with
increased wall stress, which is a factor of cardiotoxicity
(3,11,12).
Fourth, mitral regurgitation results in the overestimation of
baseline LVEF. Additionally, with respect to concomitant
anthracycline therapy, cardiotoxicity with anthracyclines is
generally known to be dose-dependent. A cardiotoxicity incidence
occurred in 3–5% of patients administered anthracyclines at a dose
of 400 mg/m2, 7–26% at a dose of 550 mg/m2,
and 18–48% at a dose of 700 mg/m2 (3). In our study subjects, 72 patients
(60.5%) received anthracycline therapy, with a mean dose of 270±90
mg/m2; hence, the impact of anthracycline on
cardiotoxicity seems to be less in the present study than in
previous studies (3,11,12,18,21).
Moreover, some studies have reported that cardiac
diastolic dysfunction, evaluated by echocardiography before
chemotherapy, might also be a predictor for chemotherapy-induced
cardiotoxicity (25), since
diastolic dysfunction precedes the appearance of systolic
dysfunction in patients treated with chemotherapy (26). It has also been reported that the
evaluation of cardiac function using modalities other than
echocardiography, such as cardiac magnetic resonance imaging,
radionuclide imaging, and positron emission tomography/magnetic
resonance, may be able to predict TIC (11). On the other hand, the usefulness of
cardiac biomarkers, such as troponin I, troponin T, natriuretic
peptide, and high-sensitive C-reactive protein, as predictors for
TIC has not been proven yet (27),
although the elevation of troponin I in patients with TIC has been
reported to predict the irreversibility of LVEF decline and a
higher incidence of cardiac events (28). Furthermore, baseline clinical
characteristics, cardiac functional parameters and biomarkers have
not been well evaluated as predictors for TIC.
There are several limitations in the present study.
First, this was a retrospective study of a single institution, and
the number of study subjects was relatively small. The study
population might be insufficient, and thus it is possible that
there were additional factors associated with TIC that were not
detected. In addition, the small number of subjects may not be
enough to fully explain the relationship between VHD with TIC.
Although we considered the use of anthracycline, radiation and
combination therapies as risk factors of TIC, logistic regression
analysis demonstrated that these factors were not associated with
TIC in the present study. However, it should be noted that we could
not fully deny the respective impacts of these markers on TIC.
Another limitation is that we only examined echocardiography in the
present study, and cardiac biomarkers and other cardiac imaging
were not fully examined. Furthermore, unfortunately, because it is
a retrospective study, we could not fully check the amount of
trastuzumab dose or doses in each patient. Therefore, our results
are preliminary, and further studies with larger populations and
multifaceted evaluations using other cardiac imaging and/or
biomarkers are needed.
In the current study, we found that TIC occurred in
approximately 10% of all breast cancer patients who received
treatment with trastuzumab. The impaired cardiac function was
ameliorated by discontinuing trastuzumab. In addition, the presence
of valvular heart disease might be a possible predictor of TIC
occurrence in breast cancer patients. Our present data suggests the
importance of regular monitoring of cardiac function.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AS and AY drafted the article and designed this
study. MMT and MO performed statistical analysis. AK, TI and TO
obtained general data. YT designed this study, obtained general
data and revised the article critically for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol conforms to the ethical
guidelines of the 1975 Declaration of Hlsinki as reflected in a
prior approval by the institution's human research committee
(Fukushima Medical University). Patient consent was obtained at the
time of data collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Santhosh S, Kumar P, Ramprasad V and
Chaudhuri A: Evolution of targeted therapies in cancer:
Opportunities and challenges in the clinic. Future Oncol.
11:279–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zamorano JL, Lancellotti P, Rodriguez
Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ,
Lip GYH, Lyon AR, et al: Group ESCSD. 2016 esc position paper on
cancer treatments and cardiovascular toxicity developed under the
auspices of the esc committee for practice guidelines: The task
force for cancer treatments and cardiovascular toxicity of the
european society of cardiology (esc). Eur Heart J. 37:2768–2801.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lal H, Kolaja KL and Force T: Cancer
genetics and the cardiotoxicity of the therapeutics. J Am Coll
Cardiol. 61:267–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nikolai BC, Lanz RB, York B, Dasgupta S,
Mitsiades N, Creighton CJ, Tsimelzon A, Hilsenbeck SG, Lonard DM,
Smith CL and O'Malley BW: HER2 signaling drives DNA anabolism and
proliferation through SRC-3 phosphorylation and E2F1-regulated
genes. Cancer Res. 76:1463–1475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hudis CA: Trastuzumab-mechanism of action
and use in clinical practice. N Engl J Med. 357:39–51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pritchard KI, Shepherd LE, O'Malley FP,
Andrulis IL, Tu D, Bramwell VH and Levine MN: National Cancer
Institute of Canada Clinical Trials Group: HER2 and responsiveness
of breast cancer to adjuvant chemotherapy. N Engl J Med.
354:2103–2111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perez EA, Romond EH, Suman VJ, Jeong JH,
Sledge G, Geyer CE Jr, Martino S, Rastogi P, Gralow J, Swain SM, et
al: Trastuzumab plus adjuvant chemotherapy for human epidermal
growth factor receptor 2-positive breast cancer: Planned joint
analysis of overall survival from NSABP B-31 and NCCTG N9831. J
Clin Oncol. 32:3744–3752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Criscitiello C and Curigliano G: HER2
signaling pathway and trastuzumab cardiotoxicity. Future Oncol.
9:179–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Negro A, Brar BK and Lee KF: Essential
roles of Her2/erbB2 in cardiac development and function. Recent
Prog Horm Res. 59:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bloom MW, Hamo CE, Cardinale D, Ky B,
Nohria A, Baer L, Skopicki H, Lenihan DJ, Gheorghiade M, Lyon AR
and Butler J: Cancer therapy-related cardiac dysfunction and heart
failure: Part 1: Definitions, pathophysiology, risk factors and
imaging. Circ Heart Fail. 9:e0026612016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ezaz G, Long JB, Gross CP and Chen J: Risk
prediction model for heart failure and cardiomyopathy after
adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc.
3:e0004722014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldhirsch A, Gelber RD, Piccart-Gebhart
MJ, de Azambuja E, Procter M, Suter TM, Jackisch C, Cameron D,
Weber HA, Heinzmann D, et al: 2 years versus 1 year of adjuvant
trastuzumab for HER2-positive breast cancer (HERA): An open-label,
randomised controlled trial. Lancet. 382:1021–1028. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McKee PA, Castelli WP, McNamara PM and
Kannel WB: The natural history of congestive heart failure: The
framingham study. N Engl J Med. 285:1441–1446. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seidman A, Hudis C, Pierri MK, Shak S,
Paton V, Ashby M, Murphy M, Stewart SJ and Keefe D: Cardiac
dysfunction in the trastuzumab clinical trials experience. J Clin
Oncol. 20:1215–1221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown SA, Sandhu N and Herrmann J: Systems
biology approaches to adverse drug effects: The example of
cardio-oncology. Nat Rev Clin Oncol. 12:718–731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guglin M, Cutro R and Mishkin JD:
Trastuzumab-induced cardiomyopathy. J Card Fail. 14:437–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bowles EJ, Wellman R, Feigelson HS,
Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard
KA, Davis RL, et al: Risk of heart failure in breast cancer
patients after anthracycline and trastuzumab treatment: A
retrospective cohort study. J Natl Cancer Inst. 104:1293–1305.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith LA, Cornelius VR, Plummer CJ, Levitt
G, Verrill M, Canney P and Jones A: Cardiotoxicity of anthracycline
agents for the treatment of cancer: Systematic review and
meta-analysis of randomised controlled trials. BMC Cancer.
10:3372010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Russell SD, Blackwell KL, Lawrence J,
Pippen JE Jr, Roe MT, Wood F, Paton V, Holmgren E and Mahaffey KW:
Independent adjudication of symptomatic heart failure with the use
of doxorubicin and cyclophosphamide followed by trastuzumab
adjuvant therapy: A combined review of cardiac data from the
National Surgical Adjuvant breast and Bowel Project B-31 and the
North Central Cancer Treatment Group N9831 clinical trials. J Clin
Oncol. 28:3416–3421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu AF, Yadav NU, Eaton AA, Lung BY, Thaler
HT, Liu JE, Hudis CA, Dang CT and Steingart RM: Continuous
trastuzumab therapy in breast cancer patients with asymptomatic
left ventricular dysfunction. Oncologist. 20:1105–1110. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martin M, Esteva FJ, Alba E, Khandheria B,
Pérez-Isla L, García-Sáenz JA, Márquez A, Sengupta P and Zamorano
J: Minimizing cardiotoxicity while optimizing treatment efficacy
with trastuzumab: Review and expert recommendations. Oncologist.
14:1–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hensley ML, Schuchter LM, Lindley C,
Meropol NJ, Cohen GI, Broder G, Gradishar WJ, Green DM, Langdon RJ
Jr, Mitchell RB, et al: American society of clinical oncology
clinical practice guidelines for the use of chemotherapy and
radiotherapy protectants. J Clin Oncol. 17:3333–3355. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hering D, Faber L and Horstkotte D:
Echocardiographic features of radiation-associated valvular
disease. Am J Cardiol. 92:226–230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tassan-Mangina S, Codorean D, Metivier M,
Costa B, Himberlin C, Jouannaud C, Blaise AM, Elaerts J and
Nazeyrollas P: Tissue doppler imaging and conventional
echocardiography after anthracycline treatment in adults: Early and
late alterations of left ventricular function during a prospective
study. Eur J Echocardiogr. 7:141–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oreto L, Todaro MC, Umland MM, Kramer C,
Qamar R, Carerj S, Khandheria BK and Paterick TE: Use of
echocardiography to evaluate the cardiac effects of therapies used
in cancer treatment: What do we know? J Am Soc Echocardiogr.
25:1141–1152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferté C, Massard C, Cohen A, Soria JC and
Ederhy S: Trastuzumab-induced cardiotoxicity: Is it time for
troponin for all patients? Am J Clin Oncol. 35:183–184. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cardinale D, Colombo A, Torrisi R, Sandri
MT, Civelli M, Salvatici M, Lamantia G, Colombo N, Cortinovis S,
Dessanai MA, et al: Trastuzumab-induced cardiotoxicity: Clinical
and prognostic implications of troponin i evaluation. J Clin Oncol.
28:3910–3916. 2010. View Article : Google Scholar : PubMed/NCBI
|