Introduction
Lung cancer (LC) is the primary cause of
cancer-related deaths in the western world, LC being responsible
for more than one-quarter (27%) of all cancer deaths (1). Non-small-cell LC (NSCLC) is the main
histological subtype of LC and the leading cause of cancer death
(2). The last few decades have seen
no significant progress in extending the survival of LC patients
despite the multiple clinical trials using cytotoxic
chemotherapeutic agents, anti-growth factor-signaling agents and/or
radiotherapy (3). Because cytostatic
drugs are not specific against cancer cells, they show a low safety
profile and cause severe side-effects. Cancer research must now
focus on strategies showing similar or higher antitumor effects,
but with far fewer side-effects. Thus, safer and more
specific/effective drugs against LC must be investigated along with
the seeking and identification of novel molecular targets that
destroy cancer cells.
One of these novel molecular targets could be the
substance P (SP)/neurokinin-1 receptor (NK-1R) system (4–6). This
system is known to play an important role in cancer (e.g.,
neuroblastoma, retinoblastoma, melanoma, hepatoblastoma, LC, etc.)
(4–6). It has been reported that SP and NK-1R
antagonists [aprepitant (Emend®), L-733,060, L-732,138]
promote the proliferation and inhibition of human NSCLC and
small-cell LC (SCLC) cells (5,6),
respectively. Both human LC cells express isoforms of the NK-1R and
mRNA for the NK-1R; they overexpress the tachykinin 1 gene; NK-1R
is also known to be involved in their viability (5,6). In a
concentration-dependent manner, NK-1R antagonists inhibited the
growth of NSCLC and SCLC cell lines, the action being mediated by
the NK-1R (inducing LC cells to die by apoptosis) (5,6). All
together, the data suggest that the NK-1R is a promising target in
the treatment of cancer (e.g., LC) and that NK-1R antagonists
behave as potential antitumor drugs. The NK-1R antagonist
aprepitant was administered here because it is a drug currently
administered in clinical practice for the treatment of nausea and
vomiting induced by chemotherapy and because it is safe and
well-tolerated (7–9). Thus, aprepitant is a good candidate to
be tested in patients in which neither a surgical treatment
(pneumonectomy) nor chemotherapy is possible. This is the case of
the patient studied here.
Case report
Patient and treatment
Male (76 years; height: 187 cm; body weight: 82 kg)
diagnosed with squamous cell carcinoma in the right main bronchus
up to 2-cm from the carina. About 8×7 cm in diameter and, according
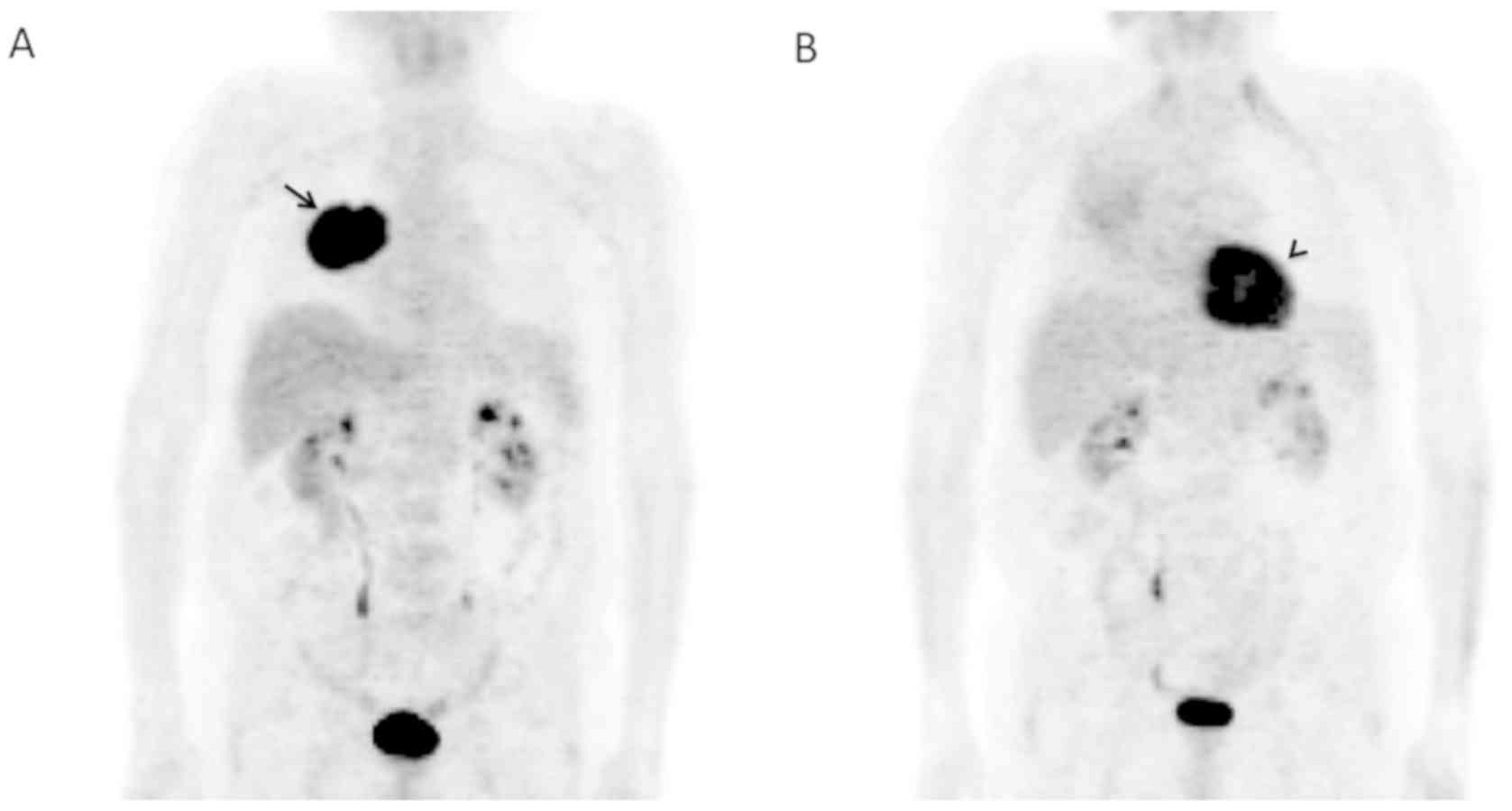
to the PET, forming a single mass without metastasis (Fig. 1A). No mediastinal lymphadenopathy.
Performance status (PS): Moderate. Respiratory function tests
showed that the patient was, at the limit, for pneumonectomy.
Histopathology: Moderate differentiated squamous cell carcinoma.
CEA (carcinoembryonic antigen): Normal. Diagnostic: NSCLC. Staging
of the patient was T4N3M0. Surgical treatment (pneumonectomy) was
not possible due to the proximity of the tumor mass to the right
main bronchus and because the patient had chronic obstructive
pulmonary disease (COPD) with limited respiratory functions (the
patient needed non-invasive mechanical home ventilation). Thus,
chemotherapy was not possible either. Treatment: Combination
therapy with radiotherapy and the NK-1R antagonist aprepitant
(compassionate use). The treatment was authorized by the Spanish
Medication Agency and the Spanish Ministry of Health. Regime of the
treatment was (45 days): 1) Radiotherapy on the right lung and
mediastinum (reaching up to 50.4 Gy (25 sessions) and then
overprinted to 65 Gy (8 additional sessions) and 2) administration
of aprepitant (1,140 mg/day). Thus, fifteen days after diagnosis,
radiotherapy and aprepitant were administered simultaneously.
According to the results obtained during in vitro
pre-clinical studies, the amount of the NK-1R antagonist used in
these studies (20 µM approximately) (10,11), was
extrapolated to the patient studied here.
Clinical course
Three weeks after treatment was started (Table I), no side-effects from aprepitant
were observed. The evolution was, from a clinical point of view,
good. Karnosfky: 90. Good appetite. The patient increased in body
weight from 82 to 87 kg. Physical examination: Good PS, coloration
and hydration of skin/mucous membranes. Dry radiodermatitis: Field
grade I. In both lungs: No dyspnea or vesicular murmur. No
lymphadenopathies. In abdomen, visceromegaly was not observed.
Chest CT showed a decrease of the lung mass (5.9×3.8 cm; initially
7×8 cm in diameter). The nodule of the posterior segment of the
right upper lobe was 4 mm, initially 7 mm. No other injuries were
observed. The hemogram revealed a discrete anemia (Hb: 10.6 g/dl).
Leucopenia grade I (3,400 leukocytes). Platelet level: Normal. The
erythrocyte sedimentation rate (ESR) was reduced from 95 to 27 mm.
Biochemical tests: No alteration.
 | Table I.Patient evolution time course:
Radiotherapy (50.4 Gy and then overprint to 65 Gy) and aprepitant
(45 days: 1,140 mg/day). |
Table I.
Patient evolution time course:
Radiotherapy (50.4 Gy and then overprint to 65 Gy) and aprepitant
(45 days: 1,140 mg/day).
| Clinical
evolution | Three weeks | Five weeks | Six months | One year |
|---|
| Performance
status | Good | Very good | Very good | Good |
| Appetite | Good | Good | Good | Not good, but the
patient maintained the weight |
| Side-effects | No | No | No | No |
| Body weight (kg) | From 82 to 87 | 90 | 90.4 | 90 |
| Dyspnea | No | No | No | No |
| Lymphoadenopathy | No | No | No | No |
| Visceromegaly | No | No | No | No |
| Hb (g/dl) | 10.6 | 10.6 | 13.5 | 10 |
| Leukocytes | 3,400 (leukopenia:
Grade I) | 5,200 | 5,200 | NA |
| ESR (mm) | From 95 to 27 | 25 | 25 | 46 |
| Dry
radiodermatitis | Grade I | Disappearance |
|
|
| Hepatorenal
biochemistry | Normal values | Normal values | Normal values | Normal values |
| Antigen squamous
cells | Normal value
(1.7) | NA | Normal value
(0.8) | Normal value
(1.2) |
| Right lung | Normal pulmonary
auscultation | Normal pulmonary
auscultation | Wheezing or sibilants
in the entire lung | The patient did not
ventilate the right lung because an atelectasis occurred due to
radiotherapy |
| Left lung | Normal pulmonary
auscultation | Normal pulmonary
auscultation | Normal pulmonary
auscultation | Normal auscultation
pulmonary |
| Chest CT Scan | Reduction of the lung
mass to 5.9×3.8 cm. (initially 7×8 cm) | Right hemitorax:
Pulmonary effusion increase. Density increase in the right
hemitorax at the level of the lower lobe with undefined borders and
with a tendency to coalesce. The right supraparahilar mass did not
show variations and loss of volume associated with the right lobe
was not observed | Right upper lobe
collapse. Pleural effusion of medium-low quantity. No tumor mass
observed. No hilar or mediastinal adenopathies | Right lung decreased
in size due to radiotherapy. No sign of recurrence in lung or
mediastinum |
Five weeks after the treatment started (Table I), the physical examination showed a
very good PS. Body weight: 90 kg. No dyspnea or expectoration. Good
appetite. Exploration: Sibilants in the entire right lung. No
peripheral adenopathy. Abdomen did not show visceromegaly. ESR: 25
mm. Hb: 10.6 g/dl. Leukocytes: 5,200. Glycaemia, transaminases, GGT
and alkaline phosphatase: Normal levels. Chest CT scan: Right
hemitorax, the pulmonary effusion increased as well as density at
the level of the lower lobe, showing undefined borders and a
tendency to coalesce. The right supraparahilar mass did not show
variations and loss of volume associated with the right lobe was
not found.
Six months after the treatment started (Table I; Fig.
1B), the patient showed a very good PS. Weight: 90.4 kg. No
dyspnea or spitting. Good appetite. Exploration: Wheezing or
sibilants in the entire right lung. Left lung: Normal pulmonary
auscultation. No peripheral lymphadenopathy or visceromegalies.
ESR: 25 mm. Hb: 13.5 g/dl. Leukocytes: 5,200. Chest CT scan: Right
upper lobe collapse. Pleural effusion of medium-low quantity. No
tumor mass was observed. Hilum and mediastinal adenopathies were
not observed. In the right lung, PET showed a great decrease of the
uptake which was seen to be diffused (Fig. 1B) compared to that observed when the
patient was first diagnosed with LC (Fig. 1A). In summary, excellent response to
treatment. No current neoplastic activity was observed.
One year later (Table
I). PS: Good (e.g., the patient walks frequently). No dyspnea.
Dry cough without changes. Appetite: Not good, but the patient has
maintained the weight gained. Physical examination showed no
peripheral adenopathy. Left lung: Normal pulmonary auscultation.
The patient did not ventilate the right lung, because an
atelectasis appeared due to radiotherapy. Abdomen did not show
visceromegaly. Hb: 10 g/dl. ESR: 46 mm. Normal hepatorenal
biochemistry. Antigen squamous cells: 1.20. Chest CT scan showed
that the right lung decreased in size, due to radiotherapy, and no
sign of recurrence in the lung/mediastinum was observed.
Two years later. Chest CT scan showed that the right
lung decreased in size due to radiotherapy. Compensatory pleural
effusion, without masses or ganglia, was observed. Lung or
mediastinum: No sign of recurrence was observed. Abdomen CT scan:
Normal. In summary, no sign of LC.
Three years later. Similar results to those observed
at two years. Due to causes not related to NSCLC, the patient
died.
Discussion
The current treatment for NSCLC is surgical
intervention, chemotherapy and radiotherapy. However, the patient
studied here could not be treated surgically because of having
chronic obstructive pulmonary disease (COPD) and, due to the poor
prognosis of the disease, chemotherapy was not administered. Thus,
the impossibility of carrying out either surgical treatment or the
administration of chemotherapy, darkened the prognosis.
Attempting to improve his life expectancy and
according to the results obtained in pre-clinical studies (10,11), the
NK-1R antagonist aprepitant was administered (compassionate use).
The amount of the NK-1R antagonist used in these pre-clinical
studies (20 µM approximately) was extrapolated (1,140 mg/day) to
the patient studied here. Moreover, in other pre-clinical study, it
was fully demonstrated that aprepitant, in a
concentration-dependent manner, promoted the apoptosis of NSCLC
cells (tumor cells die by apoptosis); that these cells overexpress
the NK-1R and that the NK-1R is involved in its viability (5). In a clinical trial to treat moderate to
severe depression, it was found that a dose of 300 mg/day of
aprepitant was safe, well-tolerated and showed side-effects similar
to the placebo (7). It is also known
that administration of aprepitant (375 mg/day) to HIV patients for
2 weeks was safe, well-tolerated and decreased the number of
CD4+ PD-1-positive cells along with the plasma level of
SP (8). It is known that LC cells
express PD-L1 (12). Additionally,
it has been reported that a patient, unmanageable to all standard
antiemetic therapies, suffering from breast cancer brain
metastasis, received aprepitant (80 mg/day; initially for seven
months) followed by an increase to 120 mg of the drug every third
day. In this patient, clinical conditions were improved, the level
of the CA153 tumor marker decreased (from 187 to 122), no
side-effects and good control of nausea/vomiting were observed
(13).
It is known that the PET technique, using labeled
fluoro deoxy glucose (FDG), is based on the Warburg effect. This
effect occurs in most cancer cells which pre-dominantly produce
energy by means of a high rate of glycolysis followed by lactic
acid fermentation (14). Growing
cancer cells show glycolytic rates up to 200 times higher than
those of their normal tissues of origin and this occurs even if
oxygen is plentiful (14). SP, in a
concentration-dependent manner, promotes glycogen breakdown and
favors the Warburg effect and then the glucose obtained would be
used by cancer cells to increase their metabolism (4). NK-1R antagonists inhibit this effect
which means that the action is mediated by the NK-1R and that these
antagonists could produce death of cancer cells by starvation
(4,15). NSCLC express SP and NK-1Rs and hence
aprepitant could block the glycogen breakdown in these cells,
counteracting the Warburg effect (4). Currently, the Warburg effect is
exclusively applied in clinical practice for diagnosis (PET);
however, it is also possible to take advantage of this mechanism
(as an antitumor strategy) by using NK-1R antagonists (e.g.,
aprepitant) (4).
It is also known that radiotherapy elicits
neurogenic inflammation (vasodilatation, increase the permeability
of blood vessels, extravasation of plasma proteins) and that this
inflammation is mediated by the NK-1R (4). Thus, it seems that aprepitant could
exert a dual effect: Antitumor activity (against the NSCLC cells
overexpressing the NK-1R) (4–6) and to
decrease the side-effects promoted by radiotherapy (16). In the patient studied here,
aprepitant could have exerted this dual effect. This idea is in
agreement with the results found here, since the patient did not
have nausea/vomiting and showed good general health condition (it
has also been reported that aprepitant exerts an antidepressant
action) (7). Moreover, despite
radiotherapy, the anemia did not get worse and only presented
leucopenia grade I (3,400 leukocytes). Two weeks after the
treatment was started, the number of leukocytes returned to normal
values. In biochemical tests, no alteration was observed due to the
treatment with aprepitant. This means that aprepitant (1,140 mg/day
for 45 days) was safe and well-tolerated and that the combination
therapy of radiotherapy and aprepitant (this NK-1R antagonist
promotes the apoptosis in cancer cells overexpressing the NK-1R)
(4–6,9–11) could be a good strategy against LC.
Finally, the USA National Comprehensive Cancer
Network (NCCN) recommends that the best management for cancer
patients (having a median overall survival of approximately 1 year)
and in which current therapeutic strategies failed, is to be
included in clinical trials testing new antitumor strategies
(17). In this case, the NCCN
encourages the participation of these patients in clinical trials.
Thus, the case studied here opens the door to develop in patients
with LC a clinical trial to test the use of aprepitant alone or in
combination therapy with radiotherapy.
Acknowledgements
The authors would like to thank Ms Diane Haun
(University of Utah, USA) for reviewing the English of the
manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MM conceived the study. MM and JCC performed the
research. JCC and JPC were responsible for clinical diagnosis. MM
and RC designed and supervised the study, collected, analyzed and
interpreted the data, and drafted the manuscript. All the authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The compassionate use of aprepitant was authorized
by the Spanish Medication Agency and the Spanish Ministry of
Health. All procedures performed in this study were followed in
accordance with the Helsinki Declaration. Written informed consent
was obtained from the patient.
Patient consent for publication
Written informed consent was obtained from the
patient.
Competing interests
USPTO Application no. 20090012086 ‘Use of
non-peptidic NK-1 receptor antagonists for the production of
apoptosis in tumor cells’ (Miguel Muñoz).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 68:7–30. 2016. View Article : Google Scholar
|
|
2
|
Subramanian J, Regenbogen T, Nagaraj G,
Lane A, Devarakonda S, Zhou G and Govindan R: Review of ongoing
clinical trials in non-small-cell lung cancer: A status report for
2012 from the clinicaltrials.gov web site. J Thorac Oncol.
8:860–865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hodkinson PH, MacKinnon A and Sethi T:
Targeting growth factors in lung cancer. Chest. 133:1209–1216.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muñoz M and Coveñas R: Involvement of
substance P and the NK-1 receptor in cancer progression. Peptides.
48:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muñoz M, González-Ortega A, Rosso M,
Robles-Frías MJ, Carranza A, Salinas-Martín MV and Coveñas R: The
substance P/neurokinin-1 receptor system in lung cancer: Focus on
the antitumor action of neurokinin-1 receptor antagonists.
Peptides. 38:318–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Muñoz M, Rosso M and Coveñas R:
Neurokinin-1 receptor antagonists in lung cancer therapy. Lett Drug
Des Discov. 14:1465–1476. 2017. View Article : Google Scholar
|
|
7
|
Kramer MS, Cutler N, Feighner J,
Shrivastava R, Carman J, Sramek JJ, Reines SA, Liu G, Snavely D,
Wyatt-Knowles E, et al: Distinct mechanism for antidepressant
activity by blockade of central substance P receptors. Science.
281:1640–1645. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tebas P, Tuluc F, Barrett JS, Wagner W,
Kim D, Zhao H, Gonin R, Korelitz J and Douglas SD: A randomized,
placebo controlled, double masked phase IB study evaluating the
safety and antiviral activity of aprepitant, a neurokinin-1
receptor antagonist in HIV-1 infected adults. PLoS One.
6:e241802011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muñoz M and Coveñas R: Safety of
neurokinin-1 receptor antagonists. Expert Opin Drugs Safety.
12:673–685. 2013. View Article : Google Scholar
|
|
10
|
Muñoz M, Pérez A, Coveñas R, Rosso M and
Castro E: Antitumoural action of L-733,060 on neuroblastoma and
glioma cell lines. Arch Ital Biol. 142:105–112. 2004.PubMed/NCBI
|
|
11
|
Muñoz M, Rosso M, Pérez A, Coveñas R,
Rosso R, Zamarriego C and Piruat JI: The NK1 receptor is involved
in the antitumoural action of L-733,060 and in the mitogenic action
of substance P on neuroblastoma and glioma cell lines.
Neuropeptides. 39:427–432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu H, Boyle TA, Zhou C, Rimm DL and Hirsch
FR: PD-L1 Expression in lung cancer. J Thorac Oncol. 11:964–975.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee M, McCloskey M and Staples S:
Prolonged use of aprepitant in metastatic breast cancer and a
reduction in CA153 tumour marker levels. Int J Cancer Clin Res.
3:0712016. View Article : Google Scholar
|
|
14
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Medrano S, Gruenstein E and Dimlich RV:
Substance P receptors on human astro-cytoma cells are linked to
glycogen breakdown. Neurosci Lett. 167:14–18. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alfieri AB and Cubeddu LX: Efectos de los
antagonistas de los receptores NK1 y de la dexametasona sobre la
inflamación neurogénica inducida por ciclofosfamida y por radiación
X, en la rata. Archivos Venezolanos de Farmacologia y Terapeutica.
23:61–66. 2004.
|
|
17
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|