Introduction
Pancreatic ductal adenocarcinoma (PDAC) represents
one of the most lethal and aggressive forms of cancer. The 5 year
survival rate of patients after surgical resection for PDAC remains
poor. In Japan, the 5 year survival rate is 18.8% (1). In the United States and Europe, it is
20.0% (2). However, the survival
rate of patients with PDAC has increased due to advancements in
multimodal therapies (3,4).
Serum albumin is the most abundant blood plasma
protein in humans and is produced by the liver (5). Low serum albumin levels may be caused
by liver disease (5), systematic
inflammation (6), the disease state
(7), malnutrition (8), and sarcopenia (9). Several studies have identified
clinicopathological prognostic factors associated with serum
albumin levels in patients with PDAC, including the C-reactive
protein (CRP)-to-albumin ratio (10), prognostic nutritional index (PNI)
(11), and modified Glasgow
Prognostic Score (mGPS) (12).
However, no studies have reported that postoperative serum albumin
levels predict survival in patients with PDAC.
Recently, important technological advances have
facilitated the identification of biomarkers for PDAC. For example,
DNA from tumor tissue and serum from PDAC patients have potential
clinical utility as biomarkers for monitoring treatment response
and predicting survival (13). We
previously reported the pre- and postoperative clinicopathological
characteristics of patients with PDAC who survived for >5 years
after curative resection (1995–2011) (14). We also reported that preoperative
serum albumin levels may be a predictive biomarker for achieving 5
year survival in patients with curatively resected PDAC. Thus, in
the present study, we investigated prognostic factors associated
with serum albumin, hypothesizing that the postoperative level
and/or recovery rate of serum albumin is associated with survival
after pancreatectomy.
Materials and methods
Patients
Data of patients who underwent intended curative
pancreatectomy for PDAC at our institution between January 1995 and
March 2016 were retrospectively reviewed. All patients had
histologically confirmed PDAC. Patients were excluded if they had
liver cirrhosis, R2 resection, or died within 12 months of
pancreatectomy. We conducted a retrospective observational study
using the ‘opt-out’ method of our hospital. The study was approved
by the Ethics Committee of Keio University School of Medicine.
Preoperative assessment
Demographic and clinical variables included age,
sex, surgical procedure, neoadjuvant chemoradiotherapy, operative
time, blood loss, and postoperative complications (e.g., pancreatic
fistula, intraabdominal bleeding, delayed gastric emptying, and
fluid collection), which were evaluated using the Clavien-Dindo
classification. Preoperative laboratory data, including the
CRP-to-albumin ratio, PNI, and mGPS, were also collected.
Since 2003, at our hospital, neoadjuvant
chemoradiotherapy has been administered to patients who were
diagnosed with T3/T4 disease according to the Union for
International Cancer Control Tumor-Node-Metastasis Classification
of Malignant Tumors (7th edition) (15).
Surgery and pathology
Surgical procedures included
pancreaticoduodenectomy, distal pancreatectomy, and total
pancreatectomy. D2 lymph node dissection was performed in all
patients. Pathological staging was determined according to the
Union for International Cancer Control Tumor-Node-Metastasis
Classification of Malignant Tumors (7th edition) (15). R0 resections were defined as cases
without gross or microscopic evidence of residual disease. R1
resections had microscopically positive margins, and R2 resections
still contained some gross tumor matter. Pathological features
associated with prognosis included histologically assessed tumor
size; distal bile duct, duodenal, serosal, retropancreatic tissue,
portal venous or arterial system, or extrapancreatic nerve plexus
invasion; other organ invasion; lymph node metastasis (LNM); and
lymphatic, venous, or intrapancreatic neural infiltration (16).
Perioperative portal vein infusion
chemotherapy
Since 1986, perioperative portal vein infusion
chemotherapy has been performed as a standard treatment at our
hospital to prevent liver recurrence and improve survival in
patients with PDAC who have undergone potentially curative
resection (17,18).
Pre- and postoperative serum
albumin
Preoperative serum albumin levels were measured
before pancreatectomy. Postoperative serum albumin levels were
evaluated at postoperative months (POMs) 3, 6, and 12. The
postoperative serum albumin recovery rate was defined as the serum
albumin level at POMs 3, 6, or 12 (g/dl) divided by the
preoperative serum albumin level (g/dl).
Follow-up
Patients were followed-up at POMs 1, 3, 6, and 12
Patients were also subject to semiannual reviews. Clinical
examinations, laboratory investigations, and abdominal computed
tomography scans (to detect tumor recurrence) were performed.
Patients were follow-up until death or March 2017.
Statistical analyses
Survival curves were plotted using the Kaplan-Meier
method and compared using the log-rank test. Disease-free survival
(DFS) was defined as the time interval between the date of surgery
and the date of recurrence or last follow-up. Overall survival (OS)
was defined as the time interval between the date of surgery and
the date of death or last follow-up. Categorical variables were
compared using the chi-square or Fisher's exact test. Cox
proportional hazards regression models were used to determine
independent prognostic factors between January 1995 and March 2016.
The optimum cutoff value for postoperative serum albumin levels at
POMs 3, 6, and 12 was 3.9 g/dl, according to our previous report
(14). In the present study, the
optimum cutoff values for albumin on POM 3, POM 6 and POM 12 were
also calculated beforehand and the results were 3.6, 3.7, 3.7 g/dl
respectively (Table SI). The
results were quite similar between using unified cutoff value (3.9
g/dl) and optimum cutoff values, therefore the unified cutoff value
of 3.9 g/dl was used. The association between early recurrence
(within 1 year) and the postoperative administration of adjuvant
chemotherapy was analyzed using Spearman's rank correlation
coefficient. All statistical analyses were conducted using
Statistical Package for the Social Sciences for Macintosh (software
v.23.0; IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics and surgical
procedures
In total, 247 patients underwent curative
pancreatectomy at our hospital between January 1995 and March 2016.
Fifty-one patients died within 12 months of surgery. Therefore, 196
patients were enrolled. The patient characteristics are summarized
in Table I. Among the 51 patients
who died within 12 months of surgery, 39 (76.5%) of deaths were due
to recurrence of PDAC, 8 (15.7%) were due to other diseases, and 4
(7.8%) were from unknown causes. Recurrent lesions in 39 patients
were associated with peritoneal dissemination, liver metastasis,
and local recurrence. There were no specific factors associated
with poor survival, but in patients that died within 12 months of
surgery because of PDAC, peritoneal dissemination and liver
metastasis were the main lesions of recurrence.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Patients
(n=196) |
|---|
| Age (years), median
(range) | 68 (38–87) |
| Sex, n
(%) |
|
| M | 130 (66.3) |
| F | 66 (33.7) |
| Surgical procedure,
n (%) |
|
| PD | 106 (54.1) |
| DP | 80 (40.8) |
| TP | 10 (5.1) |
| NACRT, n
(%) | 37 (18.9) |
| Pathological stage,
n (%)a |
|
| IA | 11 (5.6) |
| IB | 9 (4.6) |
|
IIA | 51 (26.0) |
|
IIB | 124 (63.3) |
|
III | 1 (0.5) |
| IV | 0 (0.0) |
| Perioperative PVI
chemotherapy, n (%) | 121 (62.7) |
| Adjuvant
chemotherapy, n (%)b | 132 (67.3) |
| Resection status,
n (%) |
|
| R0 | 150 (76.5) |
| R1 | 46 (23.5) |
Postoperative albumin levels, albumin
recovery rate, and survival
Kaplan-Meier survival curves of all patients are
shown in Fig. 1A and B. Patients
with postoperative serum albumin levels of ≥3.9 g/dl at POMs 3 and
6 did not exhibit a significantly longer DFS or OS. However,
patients with postoperative serum albumin levels of ≥3.9 g/dl at
POM 12 did exhibit a significantly longer DFS and OS (both
P<0.001; Fig. 2A and B).
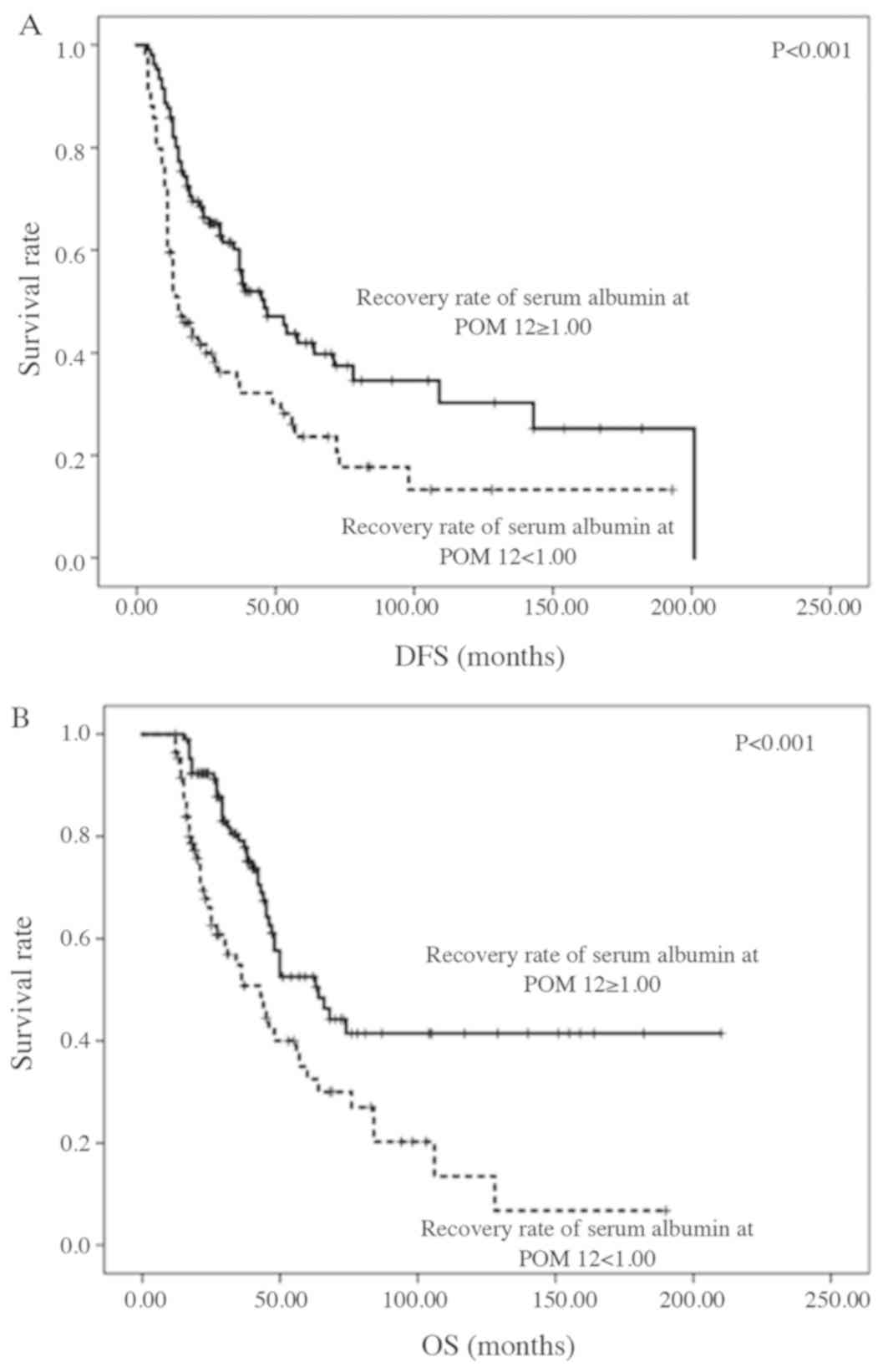
Patients with a postoperative serum albumin recovery rate of ≥1.00
at POM 3 had a significantly longer DFS (P=0.047). Patients
with a postoperative serum albumin recovery rate of ≥1.00 at POM 12
had a significantly longer DFS and OS (P<0.001 and
P=0.001, respectively; Fig. 3A
and B).
The results of the univariate and multivariate
analyses of DFS and OS are shown in Table II. In the univariate analysis, the
CRP-to-albumin ratio, PNI, and mGPS were not significant prognostic
factors for DFS or OS. The postoperative albumin level and albumin
recovery rate at POMs 3 and 6 were also not significant prognostic
factors for DFS or OS. However, the postoperative albumin level and
albumin recovery rate at POM 12 were significant prognostic factors
for DFS. Lymph node involvement, venous involvement, and LNM were
also significant prognostic factors for DFS. Neural involvement and
LNM were significant prognostic factors for OS. Multivariate
analysis confirmed venous involvement (HR: 0.75; P=0.030),
LNM (HR: 1.65; P=0.022), serum albumin level at POM 12 (≥3.9
g/dl) (HR: 0.60; P=0.017), and serum albumin recovery rate
at POM 12 (≥1.00) (HR: 0.60; P=0.017) to be independent
prognostic factors for DFS. LNM (HR: 1.79; P=0.013) and
serum albumin level at POM 12 (≥3.9 g/dl) (HR: 0.60;
P=0.033) were independent prognostic factors for OS
(Table II).
 | Table II.Univariate and multivariate analyses
of clinicopathological factors for DFS and OS. |
Table II.
Univariate and multivariate analyses
of clinicopathological factors for DFS and OS.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
|
| DFS | OS | DFS | OS |
|---|
|
|
|
|
|
|
|
|---|
|
|
| HR (95.0% CI) | P-value | HR (95.0% CI) | P-value | HR (95.0% CI) | P-value | HR (95.0% CI) | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
|
|
|
<68 | 99 | 1.13
(0.79–1.62) | 0.514 | 1.34
(0.89–2.03) | 0.158 | – | – | – | – |
|
≥68 | 97 | – |
| – |
| – |
| – |
|
| Sex |
|
|
|
|
|
|
|
|
|
| F | 66 | 0.87
(0.60–1.26) | 0.457 | 1.09
(0.70–1.67) | 0.711 | – | – | – | – |
| M | 130 | – |
| – |
| – |
| – |
|
| NACRT |
|
|
|
|
|
|
|
|
|
| Y | 3 | 0.77
(0.47–1.26) | 0.292 | 0.66
(0.37–1.16) | 0.147 | – | – | – | – |
| N | 15 | – |
| – |
| – |
| – |
|
| Surgical
procedure |
|
|
|
|
|
|
|
|
|
| PD |
| 1.01
(0.71–1.44) | 0.964 | 1.04
(0.69–1.57) | 0.840 | – | – | – | – |
|
DP/TP |
| – |
| – |
| – |
| – |
|
| Operative time
(min) |
|
|
|
|
|
|
|
|
|
|
<495 |
| 1.01
(0.70–1.46) | 0.948 | 1.07
(0.71–1.62) | 0.754 | – | – | – | – |
|
≥495 | 84 | – |
| – |
| – |
| – |
|
| Blood loss (g) |
|
|
|
|
|
|
|
|
|
|
<500 |
| 0.91
(0.63–1.31) | 0.607 | 1.01
(0.67–1.53) | 0.971 | – | – | – | – |
|
≥500 | 85 | – |
| – |
| – |
| – |
|
| Postoperative
complicationsa |
|
|
|
|
|
|
|
|
|
| Y |
| 1.03
(0.70–1.51) | 0.878 | 1.45
(0.95–2.19) | 0.083 | – | – | – | – |
| N |
| – |
| – |
| – |
| – |
|
| Perioperative PVI
chemotherapy |
|
|
|
|
|
|
|
|
|
| Y |
| 0.95
(0.66–1.37) | 0.790 | 0.86
(0.57–1.31) | 0.485 | – | – | – | – |
| N |
| – |
| – |
| – |
| – |
|
| Lymph node
involvement |
|
|
|
|
|
|
|
|
|
|
0–1 |
| 0.59
(0.42–0.85) | 0.005a | 0.83
(0.55–1.25) | 0.371 | 0.73
(0.49–1.10) | 0.130 | – | – |
|
2–3 |
| – |
| – |
| Ref. |
| – |
|
| Venous
involvement |
|
|
|
|
|
|
|
|
|
|
0–1 |
| 0.53
(0.42–0.77) | 0.001a | 0.73
(0.48–1.10) | 0.135 | 0.75
(0.41–0.96) | 0.030a | – | – |
|
2–3 | 4 | – |
| – |
| Ref. |
| – |
|
| Age (years) neural
involvement |
|
|
|
|
|
|
|
|
|
|
0–1 |
| 0.79
(0.55–1.14) | 0.204 | 0.65
(0.43–0.99) | 0.046a | – | – | 0.75
(0.48–1.16) | 0.197 |
|
2–3 | 4 | – |
| – |
| – |
| Ref. |
|
| LNM |
|
|
|
|
|
|
|
|
|
|
Neg. |
| 1.90
(1.31–2.76) | 0.001a | 1.62
(1.06–2.48) | 0.026a | Ref. |
| Ref. |
|
|
Pos. |
| – | – | 1.65
(1.07–2.52) | 0.022a |
| 1.79
(1.13–2.85) |
| 0.013a |
| CRP/Alb |
|
|
|
|
|
|
|
|
|
|
<0.003 |
| 1.03
(0.72–1.47) | 0.880 | 1.40
(0.93–2.10) | 0.108 | – | – | – | – |
|
≥0.003 |
| – | – | – | – |
|
|
|
|
| PNI |
|
|
|
|
|
|
|
|
|
|
<49.5 |
| 1.06
(0.69–1.63) | 0.795 | 1.17
(0.73–1.86) | 0.520 | – | – | – | – |
|
≥49.5 |
| – | – | – |
| – |
|
|
|
| mGPS |
|
|
|
|
|
|
|
|
|
| 0 | 1 | 1.14
(0.76–1.73) | 0.527 | 1.37
(0.86–2.16) | 0.183 | – | – | – | – |
|
1–2 |
| – | – | – | – |
|
|
|
|
| Preoperative Alb
(g/dl) |
|
|
|
|
|
|
|
|
|
|
<3.9 |
| 0.74
(0.52–1.07) | 0.108 | 0.74
(0.49–1.12) | 0.154 | – | – | – | – |
|
≥3.9 | 4 | – | – | – |
| – |
|
|
|
| Alb at POM 3
(g/dl) |
|
|
|
|
|
|
|
|
|
|
<3.9 | 9 | 0.94
(0.65–1.34) | 0.723 | 0.91
(0.60–1.37) | 0.640 | – | – | – | – |
|
≥3.9 |
| – |
| – | – | – |
|
|
|
| Alb at POM 6
(g/dl) |
|
|
|
|
|
|
|
|
|
|
<3.9 |
| 0.75
(0.53–1.08) | 0.119 | 0.73
(0.48–1.09) | 0.122 | – | – | – | – |
|
≥3.9 | 1 | – | – | – | – |
|
|
|
|
| Alb at POM 12
(g/dl) |
|
|
|
|
|
|
|
|
|
|
<3.9 |
| 0.50
(0.35–0.71) |
<0.001a | 0.51
(0.34–0.76) | 0.001a | Ref. |
| Ref. |
|
|
≥3.9 |
| – | – | 0.60
(0.40–0.91) | 0.017a | 0.60
(0.37–0.97) |
|
| 0.033a |
| Alb recovery rate
at POM 3b |
|
|
|
|
|
|
|
|
|
|
<1.00 |
| 0.68
(0.46–1.00) | 0.052 | 0.89
(0.58–1.37) | 0.600 | – | – | – | – |
|
≥1.00 |
| – | – | – | – |
|
|
|
|
| Alb recovery rate
at POM 6c |
|
|
|
|
|
|
|
|
|
|
<1.00 |
| 0.91
(0.63–1.31) | 0.601 | 0.79
(0.52–1.21) | 0.271 | – | – | – | – |
|
≥1.00 |
| – | – |
| – | – |
|
|
|
| Alb recovery rate
at POM 12d |
|
|
|
|
|
|
|
|
|
|
<1.00 |
| 0.51
(0.35–0.73) |
<0.001a | 0.47
(0.31–0.71) |
<0.001a | Ref. |
| Ref. |
|
|
≥1.00 | 1 | – | – | 0.60
(0.39–0.91) | 0.017a | 0.62
(0.38–1.00) | 0.051 |
| aP<0.05 |
Adjuvant chemotherapy and early
recurrence
Patients with a serum albumin level of <3.9 g/dl
at POMs 6 and 12 did not receive postoperative adjuvant
chemotherapy compared to those with a serum albumin level of ≥3.9
g/dl at POMs 6 and 12 (P=0.038 and P=0.009,
respectively). These patients also exhibited significantly early
recurrence (within 1 year) (P=0.029 and P=0.001,
respectively).
Discussion
We investigated the prognostic significance of pre-
and postoperative serum albumin levels and the serum albumin
recovery rate for predicting survival after pancreatectomy in
patients with PDAC. Our results showed that serum albumin levels at
POM 12 were an independent prognostic factor for both DFS and OS,
and that the serum albumin recovery rate at POM 12 was an
independent prognostic factor for DFS. Compared with other
prognostic factors or scores (e.g., the CRP-to-albumin ratio, PNI,
and mGPS), serum albumin levels may be straightforward because they
do not require any parameters. Furthermore, patients with a
postoperative serum albumin level of ≥3.9 g/dl at POM 12 or a
postoperative serum albumin recovery rate of ≥1.00 at POM 12
exhibited a significantly longer survival.
Albumin is a constitutive hepatic protein that
accounts for 50.0% of serum proteins produced by the liver and is
regulated by various factors (5).
Low serum albumin levels are associated with advanced PDAC
(7), malnutrition (8), and sarcopenia (9). Obstructive cholangitis or pancreatitis
is usually improved after surgery. Thus, in contrast to
preoperative serum albumin levels, low postoperative serum albumin
levels may be associated with cancer recurrence or inflammation,
especially postoperative serum albumin levels at POM 12. Serum
albumin levels have been associated with survival in oncologic
patients in locally or metastatic types of cancer, and a low serum
albumin may be due to a sustained systemic inflammatory response
from an aggressive metabolically active tumor (5). Preoperative and postoperative ongoing
inflammation leads to the production of acute phase proteins, and
if this process is prolonged or aggressive, such as in advanced
PDAC, it can lead to significant depletion of protein reserves and
a decrease in the body strength (6).
A postoperative lower serum albumin or insufficient albumin
recovery may represent the metabolic changes in pancreatic cancer
after surgery, and just against pancreatic cancer which is one of
the most lethal and aggressive forms of cancer, serum albumin may
be a potential biomarker for predicting earlier recurrence as well
as tumor markers (19,20). Matsuda et al (21), reported that insufficient albumin
recovery at POM 3 correlated with a poor prognosis in patients with
esophageal cancer who had undergone transthoracic esophagectomy. In
our study of patients with PDAC, the serum albumin recovery rate at
POM 3 was predictive of DFS. Moreover, patients with a
postoperative serum albumin recovery rate of ≥1.00 at POM 3 had a
longer DFS. Insufficient albumin recovery correlates with a poor
prognosis in patients with various types of cancer.
A number of overlapping factors may result in
postoperative hypoalbuminemia. It is unclear whether improving
preoperative serum albumin levels leads to a favorable prognosis.
However, considering these factors, we should endeavor to intervene
after surgery. Early nutritional support contributes to patient
survival (22–24). In the review article reported by
Gilliland et al (25), they
recommended that pancreatic cancer patients with moderately
decreased albumin levels (<3.0 mg/dl) or weight loss >5%
should still receive some form of nutritional supplementation prior
to surgery, or feeding jejunostomy tubes intraoperatively to avoid
undesirable patient outcomes associated with an insufficient
nutritional intervention. Shu et al (26), suggested in their meta-analysis that
postoperative early enteral nutrition (EEN) for patients with
digestive tract surgery improves the nutritional status, promotes
the functional recovery of the digestive system and reduces the
risk of postoperative complications; they also reported a
significant higher level of postoperative serum albumin in patients
with ENN than those without EEN. It may be more effective to insert
an enteral tube after surgery as early nutritional support for
patients with poor nutritional status or with lower albumin level
before surgery. However, it is unclear whether postoperative EEN
influences long-term prognosis of PDAC, such as DFS or OS; thus,
further studies are needed.
Okada et al (27), reported that a good nutritional
status during chemotherapy is closely associated with the
occurrence of adverse events and chemotherapy response to
FOLFOX/FIRI therapy in patients with colorectal cancer. It
prolonged DFS and OS in response to FOLFOX/FIRI therapy. In this
study, patients with a serum albumin level of <3.9 g/dl at POMs
6 and 12 did not receive postoperative adjuvant chemotherapy
compared to those with a serum albumin level of ≥3.9 g/dl at POMs 6
and 12. These patients also exhibited significantly early
recurrence (within 1 year). Maintaining a good nutritional status
during adjuvant chemotherapy may contribute to a higher response to
cancer. Nutritional support is an important intervention for
patients who are poorly nourished (e.g, with highly advanced
cancer, such as PDAC).
According to JASPAC-01 (3), which is one of the randomized
controlled trials of adjuvant chemoradiotherapy, we have typically
administrated S-1 as adjuvant chemotherapy, which is repeated every
6 weeks for up to four cycles since 2014, and before 2014, we have
mainly administrated gemcitabine as an adjuvant chemotherapy. We
have guessed that the patients with lower serum albumin levels or
insufficient albumin recovery at POM 12 tend to have lower serum
albumin levels or insufficient albumin recovery at POM 6.
Therefore, at POM 6, when adjuvant chemotherapy is finished, if the
patients have a lower serum albumin level, insufficient albumin
recovery, or high level of tumor markers associated with PDAC, such
as CA19-9, we should consider a continuation of S-1 as adjuvant
chemotherapy or a different administration, such as mFOLFILINOX,
which has recently been reported by Conroy et al (28), in a recent randomized control trial
of the adjuvant chemotherapy, ‘PRODIGE24/CCTGPA’.
Reduced serum albumin levels after surgical trauma
are also associated with systemic inflammation (29). Reduced postoperative serum albumin
levels are a marker of the stress response (30). Therefore, reducing postoperative
surgical stress may result in the optimal early recovery of
postoperative serum albumin levels. Anti-inflammatory drugs may be
effective for inhibiting inflammatory mediators. Methylprednisolone
treatment is associated with reduced interleukin-6 and
interleukin-8 inflammatory cytokine levels during esophagectomy for
esophageal carcinoma (31).
Moreover, Takata et al (32)
reported that postoperative ghrelin administration was effective
for improving the postoperative clinical course of patients with
esophageal cancer. However, there have been no reports of the
benefits of anti-inflammatory drug use during pancreatectomy.
Therefore, further research is needed.
This study has several limitations. First, this was
a retrospective study conducted at a single institution with a
relatively small number of patients. The prognostic significance of
postoperative serum albumin levels has not been verified in a
validation cohort. Second, the timing of serum albumin measurements
had not been established because this study was retrospective. In
our institution, patients were generally followed-up 1, 3, 6, and
12 months after surgery. Therefore, the timing of postoperative
serum albumin measurements was almost the same, although there were
patients who did not have blood tests at POMs 3, 6, and 12.
Although some hematological data were missing, the numbers were
small and assumed to be random. Therefore, even if the patients
with missing data were excluded, the outcomes of this study would
not change. However, further prospective studies are needed to
confirm these preliminary findings.
In conclusion, the postoperative level and recovery
rate of serum albumin are potential biomarkers for predicting the
prognosis of patients with PDAC who have undergone curative
resection. Further studies are needed to investigate the survival
benefit of increasing postoperative serum albumin levels in these
patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during this study are
available from the corresponding author on reasonable request.
Authors' contributions
YN, MK, and YK conceived and designed the study. YN
drafted the manuscript. MS, HY, YA, KT, GO, AT and YE analyzed the
data and critically revised the manuscript. All authors were
involved in data interpretation and drafting the manuscript and
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
Declaration of Helsinki and local ethical legislation. The present
study was approved by the Ethics Committee of Keio University
School of Medicine. Due to the retrospective nature of the study,
instead of obtaining informed consent from each patient,
participants were given the opportunity to opt-out.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Egawa S, Toma H, Ohigashi H, Okusaka T,
Nakano A, Hatori T, Maguchi H, Yanagisawa A and Tanaka M: Japan
pancreatic cancer registry; 30th year anniversary: Japan pancreas
society. Pancreas. 41:985–992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verma V, Li J and Lin C: Neoadjuvant
therapy for pancreatic cancer: Systematic review of postoperative
morbidity, mortality, and complications. Am J Clin Oncol.
39:302–313. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uesaka K, Boku N, Fukutomi A, Okamura Y,
Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto
H, et al: Adjuvant chemotherapy of S-1 versus gemcitabine for
resected pancreatic cancer: A phase 3, open-label, randomised,
non-inferiority trial (JASPAC 01). Lancet. 388:248–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farrugia A: Albumin usage in clinical
medicine: Tradition or therapeutic? Transfus Med Rev. 24:53–63.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gabay C and Kushner I: Acute-phase
proteins and other systemic responses to inflammation. N Eng J Med.
340:448–454. 1999. View Article : Google Scholar
|
|
7
|
Namendys-Silva SA, Gonzalez-Herrera MO,
Texcocano-Becerra J and Herrera-Gomez A: Hypoalbuminemia in
critically ill patients with cancer: Incidence and mortality. Am J
Hosp Palliat Care. 28:253–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fuhrman MP: The albumin-nutrition
connection: Separating myth from fact. Nutrition. 18:199–200. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Visser M, Kritchevsky SB, Newman AB,
Goodpaster BH, Tylavsky FA, Nevitt MC and Harris TB: Lower serum
albumin concentration and change in muscle mass: The health, aging
and body composition study. Am J Clin Nutr. 82:531–537. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haruki K, Shiba H, Shirai Y, Horiuchi T,
Iwase R, Fujiwara Y, Furukawa K, Misawa T and Yanaga K: The
C-reactive protein to albumin ratio predicts long-term outcomes in
patients with pancreatic cancer after pancreatic resection. World J
Surg. 40:2254–2260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanda M, Fujii T, Kodera Y, Nagai S,
Takeda S and Nakao A: Nutritional predictors of postoperative
outcome in pancreatic cancer. Br J Surg. 98:268–274. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imaoka H, Mizuno N, Hara K, Hijioka M,
Tajika M, Tanaka T, Ishihara M, Yogi T, Tsutsumi H, Fujiyoshi T, et
al: Evaluation of modified glasgow prognostic score for pancreatic
cancer: A retrospective cohort study. Pancreas. 45:211–217. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakano Y, Kitago M, Matsuda S, Nakamura Y,
Fujita Y, Imai S, Shinoda M, Yagi H, Abe Y, Hibi T, et al: KRAS
mutations in cell-free DNA from preoperative and postoperative sera
as a pancreatic cancer marker: A retrospective study. Br J Cancer.
118:662–669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakano Y, Kitago M, Shinoda M, Abe Y, Yagi
H, Hibi T, Takeuchi A, Aiura K, Itano O and Kitagawa Y: Clinical
predictive factors of long-term survival after curative resection
of pancreatic cancer: A retrospective study. Br J Cancer.
6:2278–2281. 2018.
|
|
15
|
Fujii-Nishimura Y, Nishiyama R, Kitago M,
Masugi Y, Ueno A, Aiura K, Kawachi S, Kawaida M, Abe Y, Shinoda M,
et al: Two cases of pathological complete response to neoadjuvant
chemoradiation therapy in pancreatic cancer. Keio J Med. 64:26–31.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Japan Pancreas Society: Classification of
pancreatic carcinoma. 2nd English ed. Tokyo. Kanehara2009.
|
|
17
|
Takahashi S, Aiura K, Saitoh J, Hayatsu S,
Kitajima M and Ogata Y: Treatment strategy for pancreatic head
cancer: Pylorus-preserving pancreatoduodenectomy, intraoperative
radiotherapy and portal catheterization. Digestion. 60
(Suppl):S130–S134. 1999. View Article : Google Scholar
|
|
18
|
Aiura K, Takahashi S, Matsui J, Ueda M and
Kitagawa Y: Beneficial effects of 5-Fluorouracil and heparin-based
portal infusion chemotherapy combined with mitomycin C and
cisplatin after curative resection of pancreatic cancer.
Pancreatology. 10:250–258. 2017. View Article : Google Scholar
|
|
19
|
McMillan DC, Watson WS, O'Gorman P, Tom
Preston, Scott HR and McArdle CS: Albumin concentrations are
primarily determined by the body cell mass and the systemic
inflammatory response in cancer patients with weight loss. Nutr
Cancer. 39:210–213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmid I, Schmitt M, Streiter M, Meilbeck
R, Haas RJ and Stachel DK: Effects of soluble TNF receptor II
(sTNF-RII), IL-1 receptor antagonist (IL-1ra), tumor load and
hypermetabolism on malnutrition in children with acute leukemia.
Eur J Med Res. 10:457–461. 2005.PubMed/NCBI
|
|
21
|
Matsuda S, Niihara M, Tsubosa Y, Sato H,
Takebayashi K, Kawamorita K, Mori K, Tsushima T, Yasui H, Takeuchi
H and Kitagawa Y: Clinical significance of postoperative recovery
of serum albumin levels in patients with esophageal cancer who
underwent transthoracic esophagectomy. Surg Today. 46:1138–1145.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ravasco P, Monteiro-Grillo I, Vidal PM and
Camilo ME: Dietary counseling improves patient outcomes: A
prospective, randomized, controlled trial in colorectal cancer
patients undergoing radiotherapy. J Clin Oncol. 23:1431–1438. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roxburgh CSD, Crozier JEM, Maxwell F,
Foulis AK, Brown J, McKee RF, Anderson JH, Horgan PG and McMillan
DC: Comparison of tumour based (Petersen Index) and
inflammation-based (Glasgow Prognostic Score) scoring systems in
patients undergoing curative resection for colon cancer. Br J
Cancer. 100:701–706. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McMillan DC, Crozier JE, Canna K, Angerson
WJ and McArdle CS: Evaluation of an inflammation based prognostic
score (GPS) in patients undergoing resection for colon and rectal
cancer. Int J Colorectal Dis. 22:881–886. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gilliland TM, Villafane-Ferriol N, Shah
KP, Shah RM, Tran Cao HS, Massarweh NN, Silberfein EJ, Choi EA, Hsu
C and McElhany CA: Nutritional and metabolic derangements in
pancreatic cancer and pancreatic resection. Nutrients. 9:E2432017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shu XL, Kang K, Gu LJ and Zhang YS: Effect
of early enteral nutrition on patients with digestive tract
surgery: A meta-analysis of randomized controlled trials. Exp Ther
Med. 12:2136–2144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okada S, Yamazaki S, Kaiga T, Funada T,
Kochi M and Takayama T: Impact of nutritional status in the era of
FOLFOX/FIRI-based chemotherapy. World J Surg Oncol. 15:162–169.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conroy T, Hammel P, Hebbar M, Ben
Abdelghani M, Wei AC, Raoul JL, Chone L, Francois E, Artru A, Biagi
JJ, et al: Folfirinox or gemcitabine as adjuvant therapy for
pancreatic cancer. N Engl J Med. 379:2395–2406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fleck A, Raines G, Hawker F, Trotter J,
Wallace PI, Ledingham IM and Calman KC: Increased vascular
permeability: A major cause of hypoalbuminaemia in disease and
injury. Lancet. 1:781–784. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hubner M, Mantziari S, Demartines N,
Pralong F, Coti-Bertrand P and Schafer M: Postoperative albumin
drop is a marker for surgical stress and a predictor for clinical
outcome: A pilot study. Gastroenterol Res Pract.
87431872016.PubMed/NCBI
|
|
31
|
Gao Q, Mok HP, Wang WP, Xiao F and Chen
LQ: Effect of perioperative glucocorticoid administration on
postoperative complications following esophagectomy: A
meta-analysis. Oncol Lett. 7:349–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takata A, Takiguchi S, Miyazaki Y, Miyata
H, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Mori M,
Kanagawa K and Doki Y: Randomized phase II study of the
anti-inflammatory effect of ghrelin during the postoperative period
of esophagectomy. Ann Surg. 262:230–236. 2015. View Article : Google Scholar : PubMed/NCBI
|