Introduction
Small bowel (SB) cancers are rare entities,
accounting for only about 2% of gastrointestinal (GI) malignancies
in the United States (U.S.) (1). Its
incidence in the U.S. has almost doubled between 1973 and 2004
(2). Early diagnosis poses a
challenge, as presenting symptoms, usually insidious and
nonspecific abdominal discomfort, are often-times vague and
difficult to recognize, which leads to an average delay in
diagnosis of 6–12 months (3,4). Less than 25% of tumors are discovered
at stage 1 or 2 (3). Diagnosis is
usually made when the patient has an acute GI emergency such as SB
obstruction (40%) or GI bleed (24%) (4). SB cancer is difficult to diagnose,
frequently presents at advanced stages, and has poor prognosis
(3,4). While surgical resection is the
treatment of choice for localized tumors, no standard treatment
protocol has been established for unresectable or metastatic
disease (4). We present the case of
a young woman with a case of infiltrating jejunal adenocarcinoma
with neuroendocrine differentiation (NED), whose diagnosis was made
when she came in with SB perforation.
Case report
A 45-year-old African American woman with a past
medical history of deep venous thrombosis, morbid obesity (body
mass index of 44), anemia, cholelithiasis, chronic back pain,
gastritis, metromenorrhagia and depression presented with severe
abdominal pain, nausea, vomiting, diarrhea, occasional flushing of
the skin, malaise, fever, shortness of breath, a recent 20-pound
weight loss and no urine output for two days. Vital signs were:
Blood pressure 74/49 mmHg, heart rate 140 bp, respiratory rate 22
bpm, temperature 100.9 F. Laboratory workup included white blood
count 29.9 k/µl, red blood count 3.37 m/µl, hemoglobin 7.5 g/dl,
platelet count 925 k/µl, blood urea nitrogen 36 mg/dl and
creatinine 4.8 mg/dl. Physical examination demonstrated generalized
abdominal tenderness and guarding, left flank pain and a 10 by 10
cm epigastric mass. Computed tomography (CT) of abdomen was
significant for a necrotic mass in the jejunum and high-grade SB
perforation. Urgent surgery revealed a necrotic, perforated jejunal
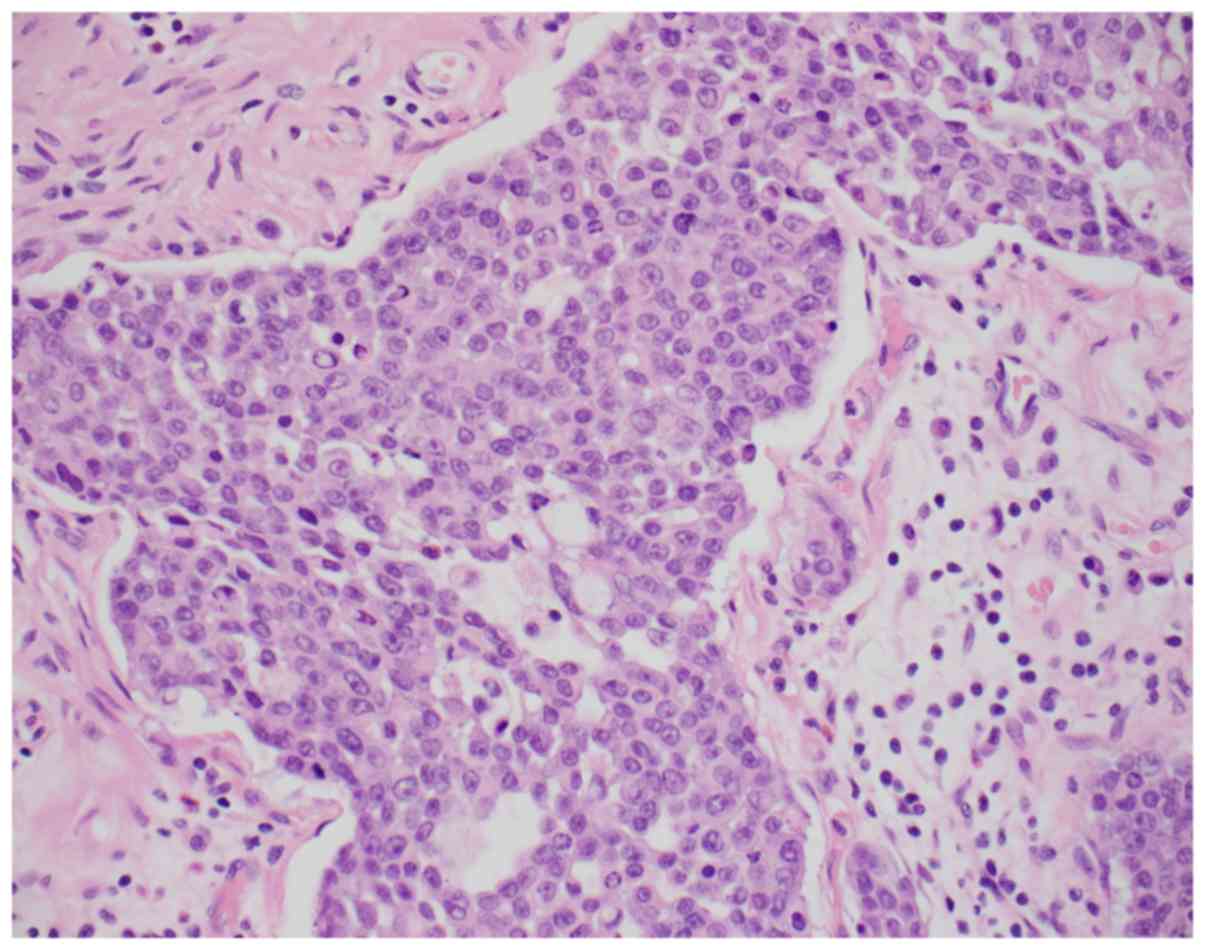
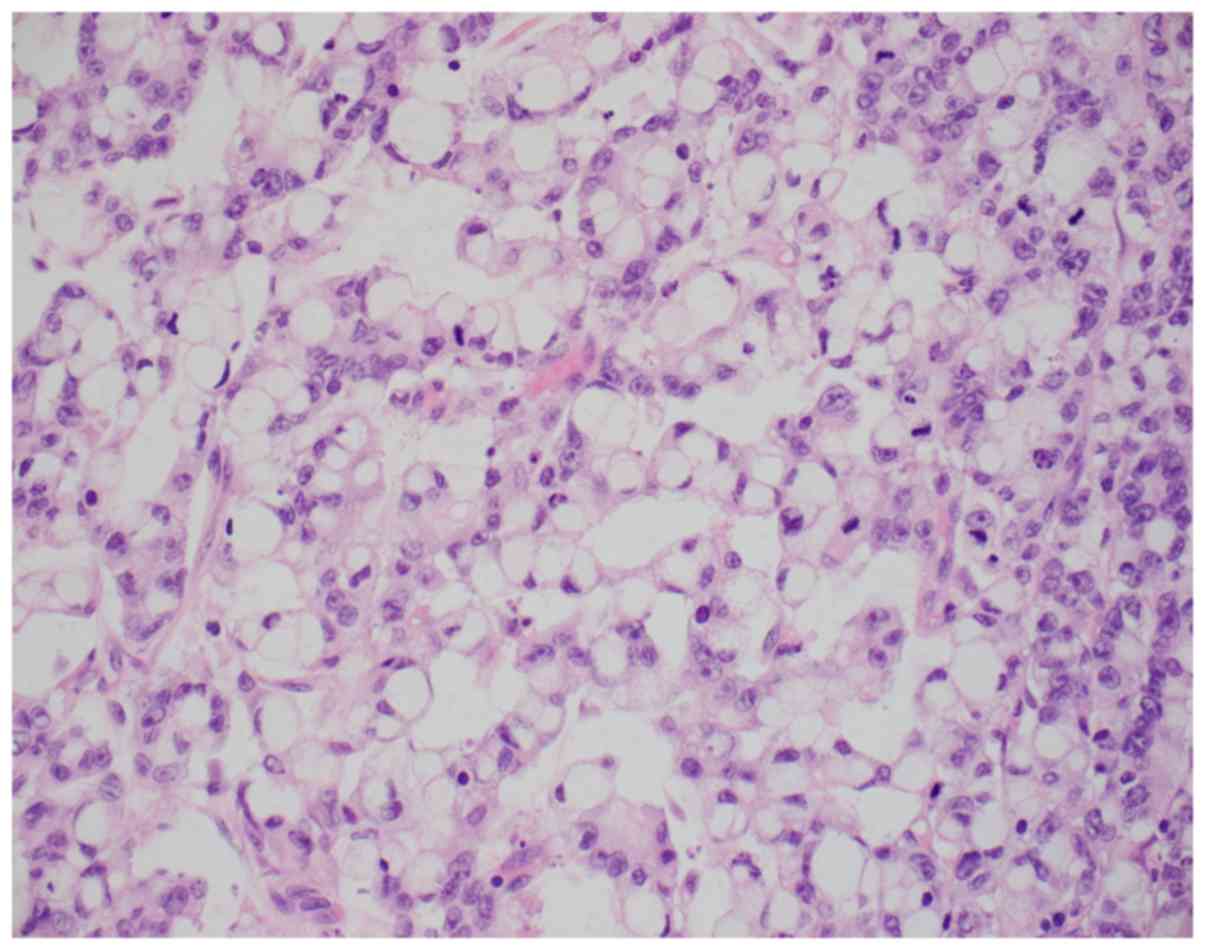
tumor invading the transverse colon. Histologically, the malignancy
was classified as infiltrating adenocarcinoma with areas
overexpressing synaptophysin, consistent with neuroendocrine
differentiation. In addition, solid areas with mucin production and
Signet-ring cells were also identified (Figs. 1 and 2). Immunohistochemically, the tumor cells
were positive for CDX2, CK20, and patchy positive for
synaptophysin. CK7, PAX-8, CD56 and chromogranin were negative.
Further investigation showed extensive abdominal carcinomatosis
with cerebral metastasis. The patient succumbed after 1 cycle of
chemotherapy with single-agent 5-fluorouracil (5-FU), less than 3
months after tumor debulking. 1 year and 9 months prior, the
patient was diagnosed with acute deep venous thrombosis in the
right popliteal and peroneal veins which was believed to be the
result of a very sedentary lifestyle. Patient was started on
warfarin. There was no family history of clotting abnormalities and
outpatient hereditary and acquired hypercoagulable workup testing
was negative. Half a year later, she started having diffuse
abdominal pain that was more pronounced in the days preceding the
onset of menstruation and tended to improve once bleeding stopped.
Upper and lower endoscopies were performed, which showed gastritis
and mild diffuse diverticulosis in the sigmoid and ascending colon,
respectively. Another 6 months later, the patient came in with
vomiting and severe abdominal pain, more prominent in the right
upper quadrant. On abdominal exam, Murphy's sign was positive.
After being taken off anticoagulation, she underwent elective
laparoscopic cholecystectomy, with only a few weeks of symptomatic
improvement. Shortly after, rapid deterioration led to the
previously detailed admission.
Discussion
The incidence of SB cancer in the U.S. has almost
doubled between 1973 and 2004 (2).
The number of African Americans affected is about twice that of
Caucasians, with most patients being diagnosed in the 5 to 7th
decade of life (5). Early diagnosis
poses a challenge, as presenting symptoms, are often-times vague
and difficult to recognize, which leads to an average delay in
diagnosis of 6–12 months (3,4). The typical presentation includes, as
seen in our patient, insidious and nonspecific abdominal
discomfort, vomiting, nausea, weight loss and anemia (6). Tumors are generally diagnosed in
advanced stages, when patients come in, as our patient did, with an
acute GI emergency (usually SB obstruction (40%) or GI bleed (24%)
(3,4).
The four major types of SB malignancies are
neuroendocrine tumors (37.4%), adenocarcinomas (36.9%), lymphomas
(17.3%) and stromal tumors (8.4%) (2). Their incidence has increased in recent
decades, a trend that shadows that of large bowel tumors (5). In a retrospective study on 2123
patients with small-bowel adenocarcinoma, the 5-year survival was
34.9%, lower than that of colon cancer (51.5%) (7). Large and SB cancers are geographically
correlated and patients likely share common environmental risk
factors, such as alcohol and smoking (1,5).
Increased consumption of refined carbohydrates, red meat and smoked
foods have also been linked to the development of SB malignancies
(8). The segment most commonly
involved in SB adenocarcinomas is the duodenum, followed by the
jejunum (9). However, specific
location within the small intestine does not appear to play a role
as far as prognosis for SB adenocarcinoma is concerned (6). SB neuroendocrine tumors, on the other
hand, are uncommonly found in the jejunum (<8%) (10). While proximal tumors are usually
nonfunctional, distal malignancies can be serotonin-producing
tumors that present with diarrhea, flushing, carcinoid heart
disease, bronchial constriction and abdominal pain, as seen in our
patient (10).
GI tract adenocarcinomas with NED are very rare. To
our knowledge, they were previously reported in the esophagus,
duodenum, stomach, ileum, colon and rectum but not in the jejunum
(11–15). Adenocarcinomas with NED are not to be
confused with mixed adenoneuroendocrine carcinomas (MANEC). MANEC
are also very rare malignancies with both gland-forming epithelial
and neuroendocrine components (16).
For a tumor to be classified as MANEC, however, both the
adenocarcinoma and the neuroendocrine component must be identified
in a proportion of at least 30% (16). A case of adenocarcinoma with patchy
neuroendocrine cells accounting for less than 30%, such as in this
case, will not be classified as MANEC. To our knowledge, no reports
of MANEC tumors in the jejunum have been published to date
either.
Older imaging studies have proven limited in their
evaluation of the SB: Barium small bowel follow-through has a
modest sensitivity (60%) in tumor detection and so do CT and
magnetic resonance imaging (MRI) (47–80%), whereas push enteroscopy
can usually assess only up to 40% of the length of the SB (17). With the addition of CT
enterography/enteroclysis, designed specifically to evaluate the
small intestine, accuracy for SB neoplasm identification has
increased to 84.7–92.7% (18,19).
Magnetic resonance enterography has similar diagnostic performance
but is generally more expensive and less available (20).
Capsule endoscopy, although time consuming, is a
good imaging option when standard bowel evaluation does not reveal
obvious pathology, yet clinical suspicion remains high. In a
meta-analysis on 24 studies (530 patients, each of whom had
previously undergone a mean of 6.77 diagnostic studies, with
negative results), capsule endoscopy identified 87% of tumors while
other techniques (push enteroscopy, small bowel series, or
colonoscopy with ileoscopy) identified 13% (21). However, if a SB tumor is present,
retention of the capsule can occur in up to 10% of patients, which
subjects them to more invasive methods (enteroscopy or surgery) for
capsule retrieval (22,23).
Overtube-assisted enteroscopy is specifically useful
in the diagnosis of SB tumors because it can visualize the entire
small intestine, has a low rate of complications and allows for
specimen biopsy (23).
Overtube-assisted enteroscopy includes double-balloon,
single-balloon and spiral enteroscopy (23). While the concordance rate between
overtube-assisted enteroscopy and capsule endoscopy appears higher
than 90%, instances were reported in which one study was superior
to the other in identifying lesions, and vice versa (23). Thus, when clinical suspicion for a SB
malignancy remains high, both techniques can be utilized, for
improved diagnostic accuracy (23).
A major limiting factor, however, is that capsule endoscopy and
overtube-assisted enteroscopy are not yet widely available.
As of yet, there is no standard treatment for SB
cancer, including none for adenocarcinoma with NED. Recommendations
state that mixed tumors with well-differentiated endocrine cells
should be treated as adenocarcinomas, whereas, if the endocrine
cells are poorly differentiated, the mass ought to be treated as a
poorly differentiated endocrine carcinoma (24). While surgical resection is the
treatment of choice for localized SB tumors, no specific
recommendations have been formulated for unresectable or
disseminated disease (4). In
metastatic disease, surgery at the primary tumor site is often
times needed because of obstruction or bleeding (4). Despite increased use of surgery and
adjuvant chemotherapy (8.1% in 1985 vs. 23.8% in 2005), survival
rates in SB cancer have not improved (2). The chemotherapy of choice for adjuvant
treatment in metastatic small bowel adenocarcinoma has been
single-agent 5-fluorouracil (5-FU) (17). Adding a platinum agent to 5-FU might
be beneficial (17). CAPOX
(combination of capecitabine and oxaliplatin), FOLFOX (folinic
acid/5-FU/oxaliplatin) and FOLFIRI (folinic acid/5-FU/irinotecan)
are promising therapeutic options (17). More clinical trials are needed to
assess treatment methods for this rare malignancy.
To our knowledge, a case of adenocarcinomas with NED
has never been reported in the jejunum. Although SB cancer makes up
a relatively small percentage of all cancer types, its incidence
has dramatically risen in the last 40 years. However, a more active
surgical and chemotherapeutic approach, has not resulted in
improved survival rates. When patients present with insidious and
nonspecific abdominal discomfort, a possible SB malignancy can be
considered. If common diagnostic techniques are negative but
clinical suspicion remains high, investigative studies can be
expanded to include newer imaging. This could potentially identify
these tumors earlier, prevent the development of emergent GI
complications and offer a chance for cure. More clinical trials are
needed in order to find new and improved methods for diagnosis and
treatment.
Acknowledgements
The authors would like to thank Dr Dennis Bloomfield
(Director of Clinical Research at Richmond University Medical
Center, New York, ΝΥ, USA) for his editorial assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
EC, WZ and SS followed-up the patient. EC, WZ, IC
and AP conceived and designed the case report. EC, IC and AP
performed the literature review. EC, WZ, IC, AP and SS wrote the
manuscript. All authors revised the manuscript critically. All
authors have read and approved the final draft of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of this case report and any associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schottenfeld D, Beebe-Dimmer JL and
Vigneau FD: The epidemiology and pathogenesis of neoplasia in the
small intestine. Ann Epidemiol. 19:58–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY,
Bennett CL and Talamonti MS: Small bowel cancer in the United
States: Changes in epidemiology, treatment, and survival over the
last 20 years. Ann Surg. 249:63–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dabaja BS, Suki D, Pro B, Bonnen M and
Ajani J: Adenocarcinoma of the small bowel: Presentation,
prognostic factors, and outcome of 217 patients. Cancer.
101:518–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Wang Z, Liu N, Hao J and Xu X: Small
bowel adenocarcinoma of the jejunum: A case report and literature
review. World J Surg Oncol. 14:1772016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haselkorn T, Whittemore AS and Lilienfeld
DE: Incidence of small bowel cancer in the United States and
worldwide: Geographic, temporal, and racial differences. Cancer
Causes Control. 16:781–787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chaiyasate K, Jain AK, Cheung LY, Jacobs
MJ and Mittal VK: Prognostic factors in primary adenocarcinoma of
the small intestine: 13-year single institution experience. World J
Surg Oncol. 6:122008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Young JI, Mongoue-Tchokote S, Wieghard N,
Mori M, Vaccaro GM, Sheppard BC and Tsikitis VL: Treatment and
survival of small-bowel adenocarcinoma in the United States: A
comparison with colon cancer. Dis Colon Rectum. 59:306–315. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aparicio T, Zaanan A, Svrcek M,
Laurent-Puig P, Carrere N, Manfredi S, Locher C and Afchain P:
Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis
and treatment. Dig Liver Dis. 46:97–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun KK, Wu X, Liu G, Qian H and Shen X:
Primary adenocarcinoma of the small intestine presenting as
superior mesenteric artery syndrome: A case report. Oncol Lett.
11:1903–1906. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scherübl H, Jensen RT, Cadiot G, Stölzel U
and Klöppel G: Neuroendocrine tumors of the small bowels are on the
rise: Early aspects and management. World J Gastrointest Endosc.
2:325–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang KL, Yang Q, Cleary KR, Swisher SG,
Correa AM, Komaki R, Ajani JA, Rashid A, Hamilton SR and Wu TT: The
significance of neuroendocrine differentiation in adenocarcinoma of
the esophagus and esophagogastric junction after preoperative
chemoradiation. Cancer. 107:1467–1474. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fresno MF, Floriano P, Díaz Iglesias JM,
Ablanedo P, Pérez del Río MJ, Barneo L and Herrero A:
Neuroendocrine carcinoma of the ampullar region with oat-cell
histological features and adenocarcinoma. Rev Esp Enferm Dig.
89:60–64. 1997.(In Spanish). PubMed/NCBI
|
|
13
|
Kim JJ, Kim JY, Hur H, Cho YK and Han SU:
Clinicopathologic significance of gastric adenocarcinoma with
neuroendocrine features. J Gastric Cancer. 11:195–199. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matulewicz RS, Fryer JP, Yang XJ, Goyal R
and Hairston JC: Renal transplantation in the setting of prior
urinary diversion: A case of poorly differentiated adenocarcinoma
in an ileal conduit. Urol Case Rep. 3:53–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng YJ, Lai W, Liu L, Wu H, Luo XX, Wang
J and Chu ZH: Prognostic significance of neuroendocrine
differentiation in colorectal adenocarcinoma after radical
operation: A meta-analysis. J Gastrointest Surg. 18:968–976. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rindi G, Arnold R, Bosman FT, Capella C,
Klimstra DS, Klöppel G, Komminoth P and Solcia E: Nomenclature and
classification of neuroendocrine neoplasms of the digestive
systemWHO classification of tumours of the digestive system. 4th.
Bosman FT, Carneiro F, Hruban RH and Theise ND: International
agency for research on cancer (IARC); Lyon: pp. 13–14. 2010
|
|
17
|
Overman MJ: Recent advances in the
management of adenocarcinoma of the small intestine. Gastrointest
Cancer Res. 3:90–96. 2009.PubMed/NCBI
|
|
18
|
Hakim FA, Alexander JA, Huprich JE, Grover
M and Enders FT: CT-enterography may identify small bowel tumors
not detected by capsule endoscopy: Eight years experience at Mayo
Clinic Rochester. Dig Dis Sci. 56:2914–2919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pilleul F, Penigaud M, Milot L, Saurin JC,
Chayvialle JA and Valette PJ: Possible small-bowel neoplasms:
Contrast-enhanced and water-enhanced multidetector CT enteroclysis.
Radiology. 241:796–801. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ilangovan R, Burling D, George A, Gupta A,
Marshall M and Taylor SA: CT enterography: Review of technique and
practical tips. Br J Radiol. 85:876–886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lewis BS, Eisen GM and Friedman S: A
pooled analysis to evaluate results of capsule endoscopy trials.
Endoscopy. 37:960–965. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rondonotti E, Pennazio M, Toth E, Menchen
P, Riccioni ME, De Palma GD, Scotto F, De Looze D, Pachofsky T,
Tacheci I, et al: Small-bowel neoplasms in patients undergoing
video capsule endoscopy: A multicenter European study. Endoscopy.
40:488–495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sulbaran M, de Moura E, Bernardo W, Morais
C, Oliveira J, Bustamante-Lopez L, Sakai P, Mönkemüller K and
Safatle-Ribeiro A: Overtube-assisted enteroscopy and capsule
endoscopy for the diagnosis of small-bowel polyps and tumors: A
systematic review and meta-analysis. Endosc Int Open. 4:E151–E163.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hervieu V and Scoazec JY: Mixed endocrine
tumors. Ann Pathol. 25:511–528. 2005.(In French). View Article : Google Scholar : PubMed/NCBI
|