Introduction
Both management and treatment options for pleural
metastasis (PM) and malignant pleural effusion (MPE) are
continuously evolving, as seen by the introduction of tunneled
pleural catheters, chemical pleurodesis and combined procedures
(1-3). PM
is a common manifestation of several tumor entities exhibiting a
poor prognosis (4-6).
The treatment of PM is often interdisciplinary and includes
oncologist, pulmonologists, surgeons, anesthesiologists and other
specialists. Recently, pressurized intrathoracic aerosol
chemotherapy (PITAC) has been introduced to the clinical setting as
a modified version of the micropump© (MIP™; Reger
Medizintechnik)-based pressurized intraperitoneal aerosol
chemotherapy (PIPAC) (7). PITAC is
currently used in PM patients who especially suffer from recurrent
pleural effusions.
Two of the investigators of the present study have
worked as physicians at the Department of General Surgery, Marien
Hospital Herne (Herne, Germany), where PIPAC was introduced and
where numerous patients have received PITAC treatment. Following
PITAC treatment, patients exhibited good results with a significant
decrease or even remission of MPE production in the pleural cavity.
Currently, only limited clinical data is available on the efficacy
of intrathoracic aerosol chemotherapy by means of PITAC (7). In its present form, PITAC is defined as
a surgical procedure requiring an operation room, a defined amount
of surgical personnel, such as a general or thoracic surgeon and
surgical assistants, and materials as well as the MIP™, a
single-use, high-pressure injector pump.
However, these preconditions in conducting PITAC may
limit the opportunity to extend its application on a larger scale
in non-surgical disciplines. Therefore, it is important to
investigate whether, from a technical point of view, it is possible
to facilitate PITAC procedures and make them available for bedside
applications. Technical studies on PIPAC procedures have already
suggested that alternatives to the currently used MIP™ are possible
(8,9)
and its mode of operation could be improved (10,11).
In addition, studies have investigated the use of
different substances (12) and new
drugs (13) for PIPAC applications.
However, since intraperitoneal aerosol chemotherapy is
predominantly used by surgeons and gynecologists in cases of
peritoneal metastasis, its potential for the treatment of PM and
MPE has been of less research interest. Despite limited data on the
incidences of PM and peritoneal metastasis, the occurrence of MPE's
is assumed to be more widespread than PM, thus emphasizing the
relevance of aerosol chemotherapy in patients with MPE. The present
study aimed to demonstrate an easily applicable intrathoracic
aerosol chemotherapy bedside approach for potential clinical use.
The present reported version of intrathoracic aerosol chemotherapy
has the potential to expand to disciplines other than thoracic
surgery.
Materials and methods
Experimental set-up
Intrathoracic chemotherapy (ITC) was performed on
three swine at 10 min post-mortem. All experiments were performed
at the veterinary animal laboratories of Wroclaw University
(Wroclaw, Poland). Swine were premedicated with an intramuscular
injection of midazolam (0.1 mg/kg, Midanium 5 mg/ml; WZF Polfa
S.A.), medetomidine (0.02 mg/kg, Cepetor 1 mg/ml; Cp - Pharma
Handelsgesellschaft Mbh) and ketamine (8 mg/kg, Ketamina 100 mg/ml;
Biowet Puławy Sp. z o.o.) mixture. Euthanization was performed via
intravenous injection using sodium pentobarbital with pentobarbital
(50 mg/kg with 12 mg/kg, morbital 133.3 mg/ml + 26,7 mg/ml; Biowet
Puławy Sp. z o.o.) according to recommended protocols of the
Handbook of Veterinary Anesthesia (14,15).
Experiments were conducted after a previous
cardiovascular study on the swine. Fresh post-mortem swine cadavers
were placed in a supine position and fixed at all four extremities.
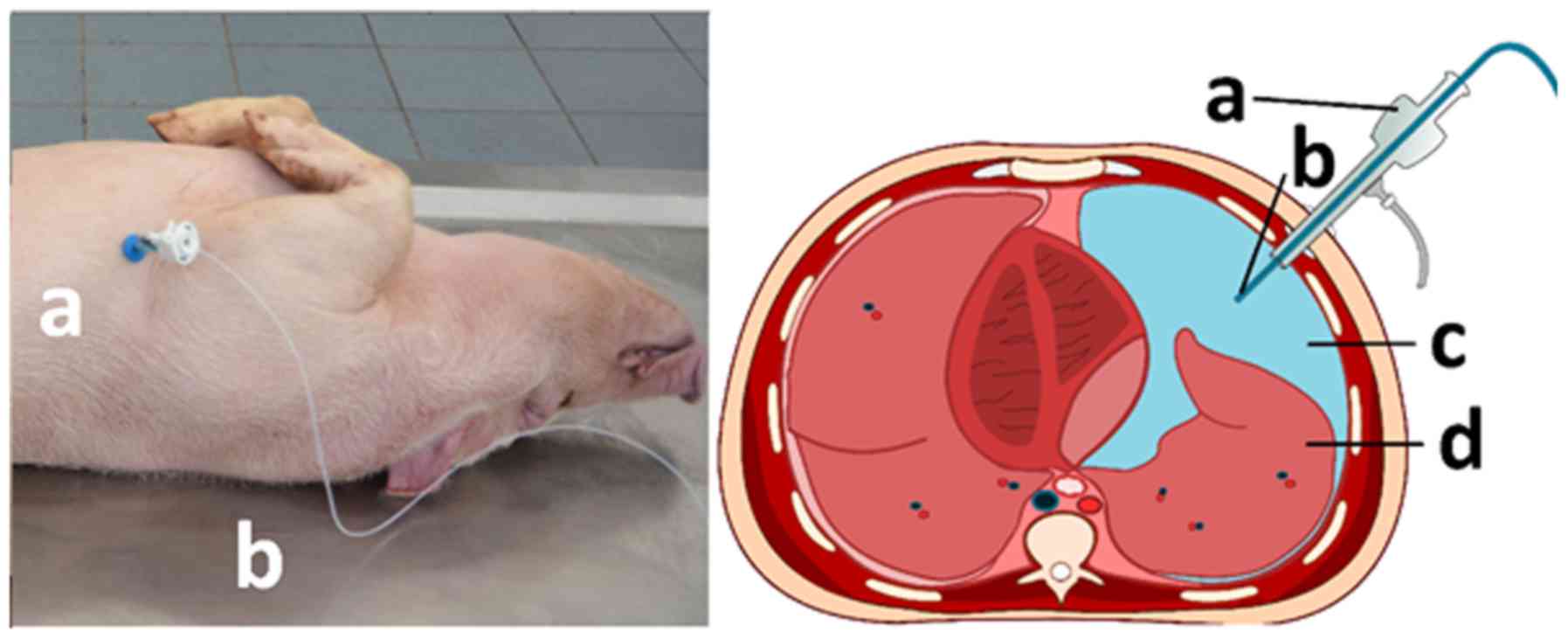
Using a scalpel, a small thoracic incision was made at the middle
mediothoracic line. The parietal pleura was manually perforated,
and a pneumothorax was established by placing a 5 mm trocar
(Kii®Balloon Blunt Tip system; Applied Medical).
Additionally, a 3 mm plastic tube was inserted through the trocar
to maintain the pneumothorax and a normal pressure environment
inside the chest and the surrounding environments. The surgical
thoracotomy entrance site was sutured to prevent air leakage
parallel to the placed trocar. To ensure that no leakage occurred
during the experiment, a total of 200-300 ml air was pumped into
the thorax at a pressure of 12 mmHg, resulting in a stable
insufflation of the wall for a few min. Subsequently, a 3 mm
plastic tube was inserted through the trocar to release any
remaining pressure in the thorax. After this, the tube was removed,
and the spray-catheter was placed into the trocar.
A doxorubicin solution (3 mg/50 ml NaCl 0.9%) was
aerosolized and delivered into the thorax with a 10 ml syringe and
a constant flow at 23˚C (Fig.
1).Five min after injecting the total doxorubicin solution, a
chest tube was inserted through the trocar. The trocar was then
removed from the chest and the chest tube was connected to a
suction system with an intersecting drain bottle. Subsequently, 30
min after the described procedure, the thorax tube was removed, and
the thoracic entrance site was completely sutured. The thorax was
surgically opened via thoracotomy and a total of eight tissue
samples were retrieved from each swine, including four tissue
samples from the visceral pleura and four tissue samples from the
parietal pleura.
Approval for the study was provided by The Ethical
and Veterinarian boards at Wroclaw University of Life and
Environmental Sciences (Wroclaw, Poland; approval no.
11/2018/P1).
Spray-catheter
The spray-catheter (PW-6C-1; Olympus Surgical
Technologies Europe) consists of a connecting device and a
high-pressure line connecting the shaft to the nozzle. The nozzle
head has a small central opening and the spray-catheter generates a
polydisperse aerosol. The spray-effect is achieved by manual
pressure on the connecting syringe.
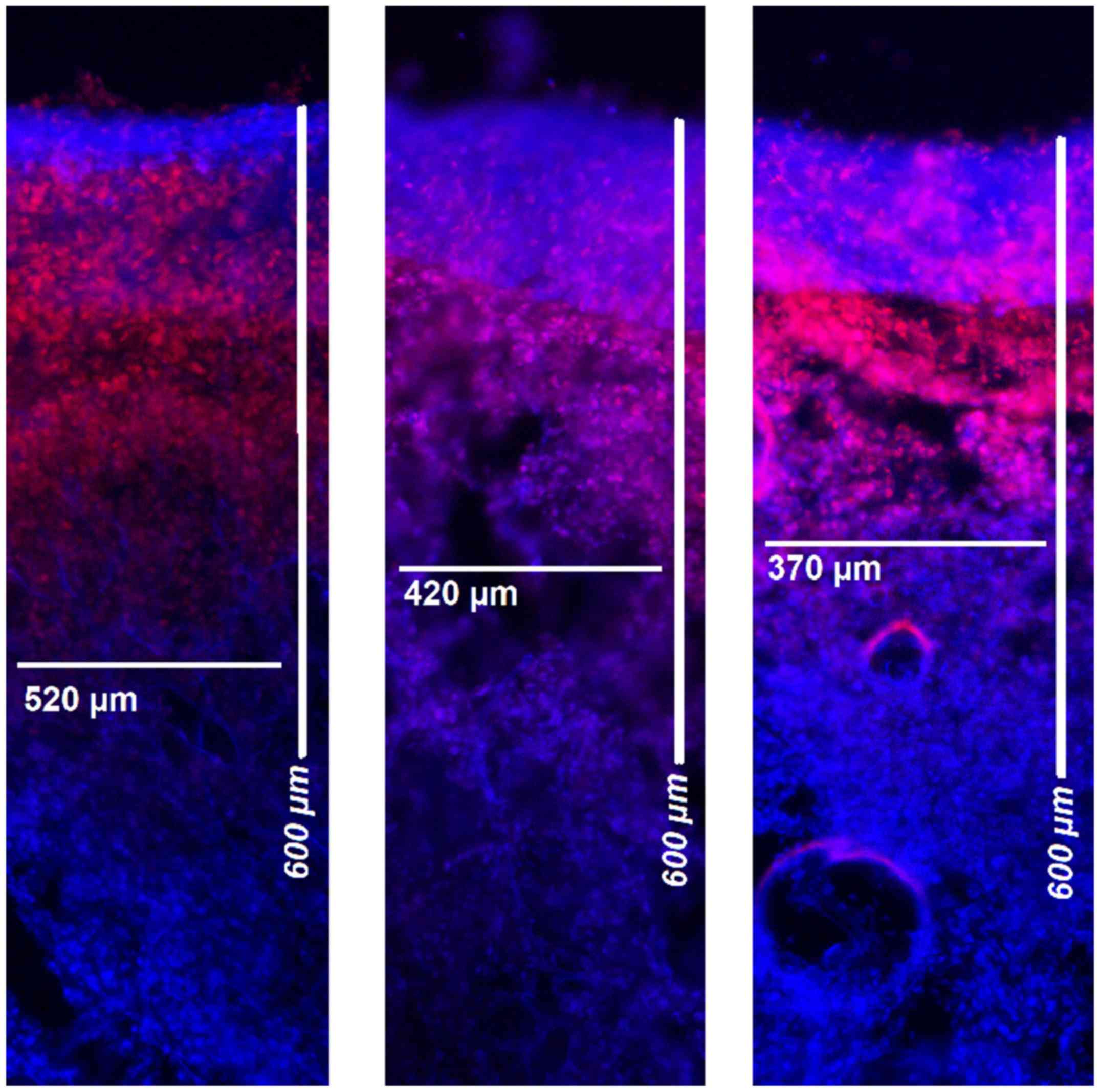
Microscopic analysis
Following treatments, tissue samples were rinsed
with sterile 0.9% NaCl solution to eliminate superficial
cytostatics and then immediately frozen in liquid nitrogen.
Cryosections were prepared from the visceral and parietal pleura.
Sections were mounted with VectaShield containing 1.5 µg/ml
4',6-diamidino-2-phenylindole (Thermo Fisher Scientific, Inc.) to
stain nuclei. The penetration depth of doxorubicin was determined
using a Nikon Eclipse 80i fluorescence microscope (magnification,
x10; Nikon Instruments Europe BV). The distance between the luminal
surface and the inner most positive staining for doxorubicin
accumulation was measured and reported in µm (Figs. 2 and 3).
Statistical analysis
Experiments were independently performed three times
and four tissue samples were retrieved from both the visceral and
the parietal pleura, respectively. In total, three cryosections per
tissue sample were subject to doxorubicin penetration measurements.
Data is presented as the mean ± SD. Statistical analyses were
performed using Sigma Plot (version 12; Systat Software, Inc.).
Student's t-test by ranks was used to analyze groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Technical feasibility
No complications were observed during the
procedures. ITC was applied in all three swine using a normal
pressure environment. Each swine procedure was performed by one
physician and one assistant in <1 h. The creation of an adequate
pneumothorax via small thoracotomy was possible and conducted
without using a double-lumen tube or intubation. The creation of a
pressurized cavity was not required to establish an adequate
working space for chemotherapeutic aerosol generation.
Intrathoracic chemotherapy
After placement of the chest tube and application of
negative pressure, part of the applied chemotherapeutic solution
was removed by the drainage system. Further thoracotomy revealed
detection of some residual chemotherapeutic solution in the
thoracic cavity. Therefore, removal of chemotherapeutic solution
was incomplete. Tissue samples were retrieved from different sites
of the pleural and visceral peritoneum. Following fluorescent
microscopy, tissue probes revealed doxorubicin contact within all
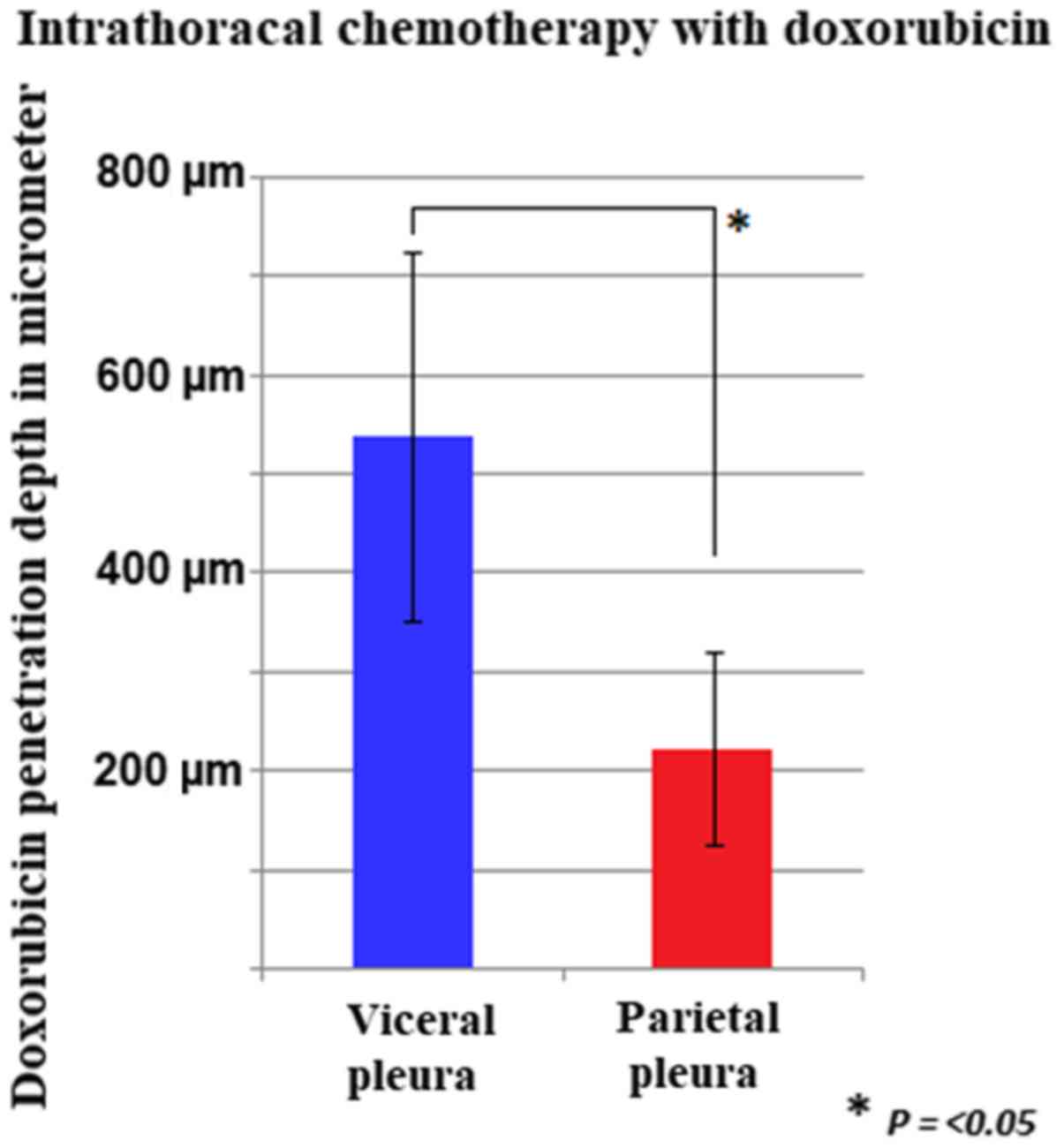
measured pleural locations. The mean tissue penetration rate was
540±186 µm for the visceral peritoneum and 224±97 µm for the
pleural peritoneum.
Discussion
Currently, research on pressurized aerosol
chemotherapy is of particular interest. Beside studies on technical
features (16,17), biological efficacy (18) and clinical relevance (19), the search for other applicational
modalities has received particular attention (20-23).
PITAC is an example of an applicational modality that stretches
beyond the current PIPAC. From the clinical experience of certain
investigators, multiple patients receiving PITAC have demonstrated
benefits from this therapy, which emphasizes its clinical efficacy,
particularly in the treatment of MPE. However, the main clinical
interest has been on PIPAC. This is rather attributable to factors
surrounding PITAC than to its outcome or efficacy. In peritoneal
metastasis, as opposed to PM, only few treatment options are
available in palliative cases.
In both PM and MPE treatment, a variety of different
catheters are used depending on the procedure performed, for
example, liquid chemotherapy installations or surgical procedures,
such as pleurectomy and talc poudrage (4). PITAC is currently performed in the
operating room and requires the use of a MIP™, a high-pressure
injector and specifically trained surgical personnel. However, many
patients with PM or MPE may not have access to a surgical
department or surgical time may not be allotted toward PITAC
procedures. Smaller and less complicated PIPAC approaches have
already been demonstrated (8). The
question remains as to whether it is technically feasible to reduce
the components of the MIP™ and the high-pressure injection device,
as well as change the pressurized environment for the intrathoracic
procedure.
From a technical point of view, a bedside approach
is feasible without needing the MIP (19). Rather, the currently less expensive
spray-catheter may be used instead. If these procedures are proven
safe from an occupational perspective, this finding may alleviate
the need for an operation room. The application of such procedures
may be open to other non-surgical doctors as they could use ITC for
the treatment of PM and MPE. Aerosol chemotherapy could be
administered bedside by any doctor who could be assisted by a
nurse, nurse assistant or medical student. While PIPAC technologies
require a pressurized abdominal cavity, there is no such
prerequisite for intrathoracic applications. Current data on the
effect of pressure on chemoaerosol penetration rates is
conflicting. While some studies report increased cytotoxicity with
increased pressure applied (24),
other studies do not demonstrate any such effect on drug
penetration rates (25).
However, these effects must be studied in a clinical
setting. The application of ITC via spray-catheter could be a novel
and technically feasible option for the treatment of PM and MPE.
More studies must be performed to thoroughly study both patient and
personnel safety in possible bedside applications. However, one
must be aware that bedside or outpatient conditions are different
from experimental conditions. Thus, the mere technical feasibility
of an approach may not equate to its applicability in the clinical
setting. Possible complications of mini-thoracotomies, such as
pneumothorax through lung-fistula as well as local and pleural
infections, must also be considered with this approach.
Additionally, the need for sufficient analgesia must be considered
for bedside applications. If studies on safety aspects indeed
indicate that bedside applications of this technique are safe, this
could help to extend this procedure to other non-surgical
disciplines. Extending this technique to other fields could assist
with the collection of important clinical data to investigate its
efficacy. Since current clinical data on PIPAC present promising
results, similar results are expected for ITC treatment in patients
with PM with or without MPE. However, further studies are required,
and the ITC approach requires further evaluation to be used in PM
and MPE treatment.
Acknowledgements
Not applicable.
Funding
The present study was funded by institutional funds
from the Departments of Biochemistry and Molecular Biology, Faculty
of Veterinary Medicine, Wroclaw University of Environmental and
Life Sciences.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
VK, MP, KN and JK performed the statistical
analysis, acquired the data and drafted the manuscript. AM designed
the study, acquired the data and drafted the manuscript. RP
designed and supervised the study, interpreted the data and
critically revised the manuscript for important intellectual
content. MA and TK substantially contributed to the conception of
the study design, supervised the study, and drafted and critically
revised the manuscript for important intellectual content. TK
moreover performed data interpretation and gave final approval for
publication. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Approval for the study was provided by the Ethical
and Veterinarian boards at Wroclaw University of Life and
Environmental Sciences (Wroclaw, Poland; approval no.
11/2018/P1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hak CC, Sivakumar P and Ahmed L: Safety of
indwelling pleural catheter use in patients undergoing
chemotherapy: A five-year retrospective evaluation. BMC Pulm Med.
16(41)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Suzuki K, Servais EL, Rizk NP, Solomon SB,
Sima CS, Park BJ, Kachala SS, Zlobinsky M, Rusch VW and Adusumilli
PS: Palliation and pleurodesis in malignant pleural effusion: The
role for tunneled pleural catheters. J Thorac Oncol. 6:762–767.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Van Meter ME, McKee KY and Kohlwes RJ:
Efficacy and safety of tunneled pleural catheters in adults with
malignant pleural effusions: A systematic review. J Gen Intern Med.
26:70–76. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Akulian J and Feller-Kopman D: The past,
current and future of diagnosis and management of pleural disease.
J Thorac Dis. 7 (Suppl 4):S329–S338. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
DeBiasi E and Puchalski J: Pleural
effusions as markers of mortality and disease severity: A
state-of-the-art review. Curr Opin Pulm Med. 22:386–391.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jiang L, Li P, Gong Z, Hu B, Ma J, Wang J,
Chu H, Zhang L, Sun P and Chen J: Effective treatment for malignant
pleural effusion and ascites with combined therapy of bevacizumab
and cisplatin. Anticancer Res. 36:1313–1318. 2016.PubMed/NCBI
|
|
7
|
Giger-Pabst U, Demtröder C, Falkenstein
TA, Ouaissi M, Götze TO, Rezniczek GA and Tempfer CB: Pressurized
intraperitoneal aerosol chemotherapy (PIPAC) for the treatment of
malignant mesothelioma. BMC Cancer. 18(442)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Khosrawipour V, Mikolajczyk A, Schubert J
and Khosrawipour T: Pressurized intra-peritoneal aerosol
chemotherapy (PIPAC) via endoscopical microcatheter system.
Anticancer Res. 38:3447–3452. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Göhler D, Große S, Bellendorf A,
Falkenstein TA, Ouaissi M, Zieren J, Stintz M and Giger-Pabst U:
Hyperthermic intracavitary nanoaerosol therapy (HINAT) as an
improved approach for pressurised intraperitoneal aerosol
chemotherapy (PIPAC): Technical description, experimental
validation and first proof of concept. Beilstein J Nanotechnol.
8:2729–2740. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Khosrawipour V, Khosrawipour T, Kern AJ,
Osma A, Kabakci B, Diaz-Carballo D, Förster E, Zieren J and
Fakhrian K: Distribution pattern and penetration depth of
doxorubicin after pressurized intraperitoneal aerosol chemotherapy
(PIPAC) in a postmortem swine model. J Cancer Res Clin Oncol.
142:2275–2280. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Khosrawipour V, Khosrawipour T,
Diaz-Carballo D, Förster E, Zieren J and Giger-Pabst U: Exploring
the spatial drug distribution pattern of pressurized
intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol.
23:1220–1224. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mikolajczyk A, Khosrawipour V, Schubert J,
Grzesiak J, Chaudhry H, Pigazzi A and Khosrawipour T: Effect of
liposomal doxorubicin in pressurized intra-peritoneal aerosol
chemotherapy (PIPAC). J Cancer. 9:4301–4305. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schubert J, Khosrawipour V, Chaudhry H,
Arafkas M, Knoefel WT, Pigazzi A and Khosrawipour T: Comparing the
cytotoxicity of taurolidine, mitomycin C, and oxaliplatin on the
proliferation of in vitro colon carcinoma cells following
pressurized intra-peritoneal aerosol chemotherapy (PIPAC). World J
Surg Oncol. 17(93)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Muir WW, Hubbell JAE and Bednarski RM:
Handbook of veterinary anesthesia 4th edition. Polish Edition by
Elsevier Urban & Partner, Wroclaw, pp320-323, 2008.
|
|
15
|
Noszczyk-Nowak A, Pasławska U, Gajek J,
Janiszewski A, Pasławski R, Zyśko D and Nicpoń J: Ventricular
effective refraction period and ventricular repolarization analysis
in experimental tachycardiomyopathy in swine. Adv Clin Exp Med.
25:409–414. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Göhler D, Khosrawipour V, Khosrawipour T,
Diaz-Carballo D, Falkenstein TA, Zieren J, Stintz M and Giger-Pabst
U: Technical description of the microinjection pump
(MIP®) and granulometric characterization of the aerosol
applied for pressurized intraperitoneal aerosol chemotherapy
(PIPAC). Surg Endosc. 31:1778–1784. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mikolajczyk A, Khosrawipour V, Schubert J,
Chaudhry H, Pigazzi A and Khosrawipour T: Particle stability during
pressurized intra-peritoneal aerosol chemotherapy (PIPAC).
Anticancer Res. 38:4645–4649. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bellendorf A, Khosrawipour V, Khosrawipour
T, Siebigteroth S, Cohnen J, Diaz-Carballo D, Bockisch A, Zieren J
and Giger-Pabst U: Scintigraphic peritoneography reveals a
non-uniform 99mTc-Pertechnetat aerosol distribution
pattern for pressurized intra-peritoneal aerosol chemotherapy
(PIPAC) in a swine model. Surg Endosc. 32:166–174. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Khosrawipour T, Khosrawipour V and
Giger-Pabst U: Pressurized intra peritoneal aerosol chemotherapy in
patients suffering from peritoneal carcinomatosis of pancreatic
adenocarcinoma. PLoS One. 12(e0186709)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mikolajczyk A, Khosrawipour V, Schubert J,
Plociennik M, Nowak K, Fahr C, Chaudhry H and Khosrawipour T:
Feasibility and characteristics of pressurized aerosol chemotherapy
(PAC) in the bladder as a therapeutical option in early-stage
urinary bladder cancer. In Vivo. 32:1369–1372. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Khosrawipour V, Bellendorf A, Khosrawipour
C, Hedayat-Pour Y, Diaz-Carballo D, Förster E, Mücke R, Kabakci B,
Adamietz IA and Fakhrian K: Irradiation does not increase the
penetration depth of doxorubicin in normal tissue after pressurized
intra-peritoneal aerosol chemotherapy (PIPAC) in an ex vivo model.
In Vivo. 30:593–597. 2016.PubMed/NCBI
|
|
22
|
Khosrawipour V, Khosrawipour T,
Hedayat-Pour Y, Diaz-Carballo D, Bellendorf A, Böse-Ribeiro H,
Mücke R, Mohanaraja N, Adamietz IA and Fakhrian K: Effect of
whole-abdominal irradiation on penetration depth of doxorubicin in
normal tissue after pressurized intraperitoneal aerosol
chemotherapy (PIPAC) in a post-mortem swine model. Anticancer Res.
37:1677–1680. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Khosrawipour V, Giger-Pabst U,
Khosrawipour T, Pour YH, Diaz-Carballo D, Förster E, Böse-Ribeiro
H, Adamietz IA, Zieren J and Fakhrian K: Effect of irradiation on
tissue penetration depth of doxorubicin after pressurized
intra-peritoneal aerosol chemotherapy (PIPAC) in a novel ex-vivo
model. J Cancer. 7:910–914. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Khosrawipour V, Diaz-Carballo D, Acikelli
AH, Khosrawipour T, Falkenstein TA, Wu D, Zieren J and Giger-Pabst
U: Cytotoxic effect of different treatment parameters in
pressurized intraperitoneal aerosol chemotherapy (PIPAC) on the in
vitro proliferation of human colonic cancer cells. World J Surg
Oncol. 15(43)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Khosrawipour V, Khosrawipour T,
Falkenstein TA, Diaz-Carballo D, Förster E, Osma A, Adamietz IA,
Zieren J and Fakhrian K: Evaluating the effect of micropump©
position, internal pressure and doxorubicin dosage on efficacy of
pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in an ex
vivo model. Anticancer Res. 36:4595–4600. 2016.PubMed/NCBI View Article : Google Scholar
|