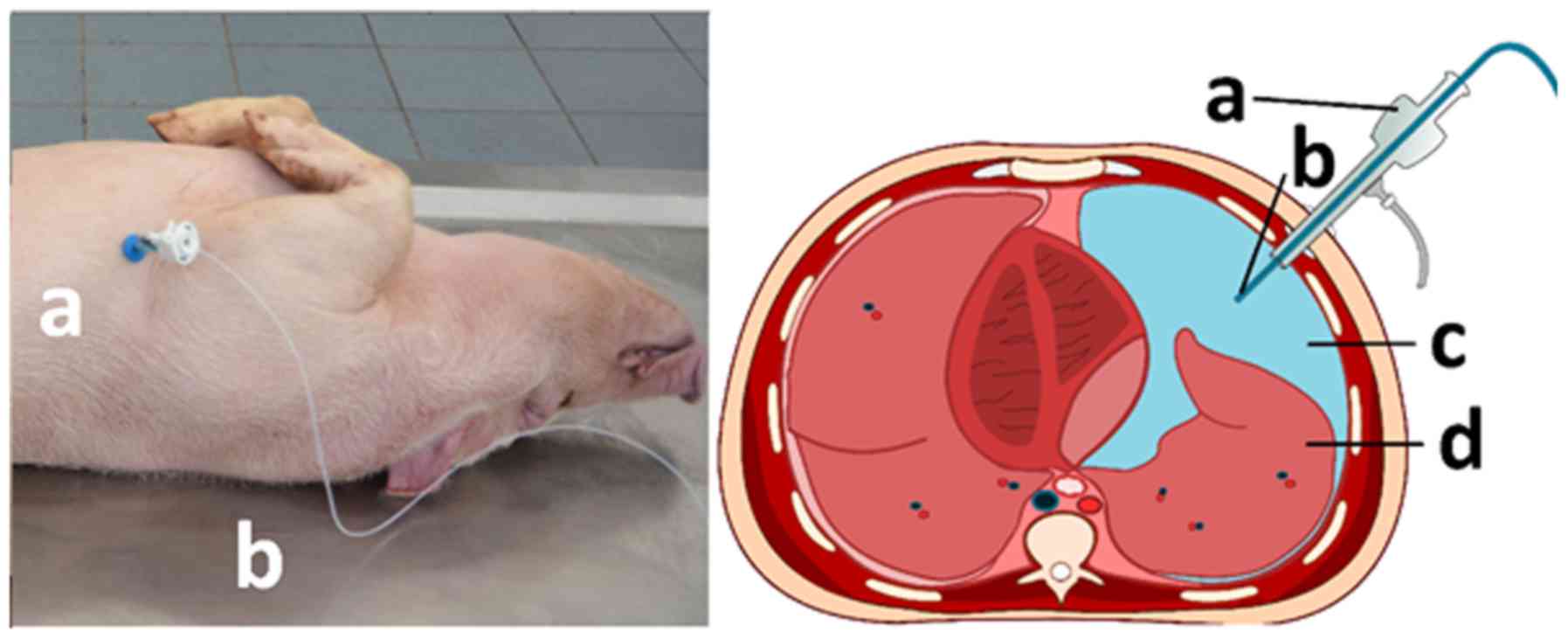
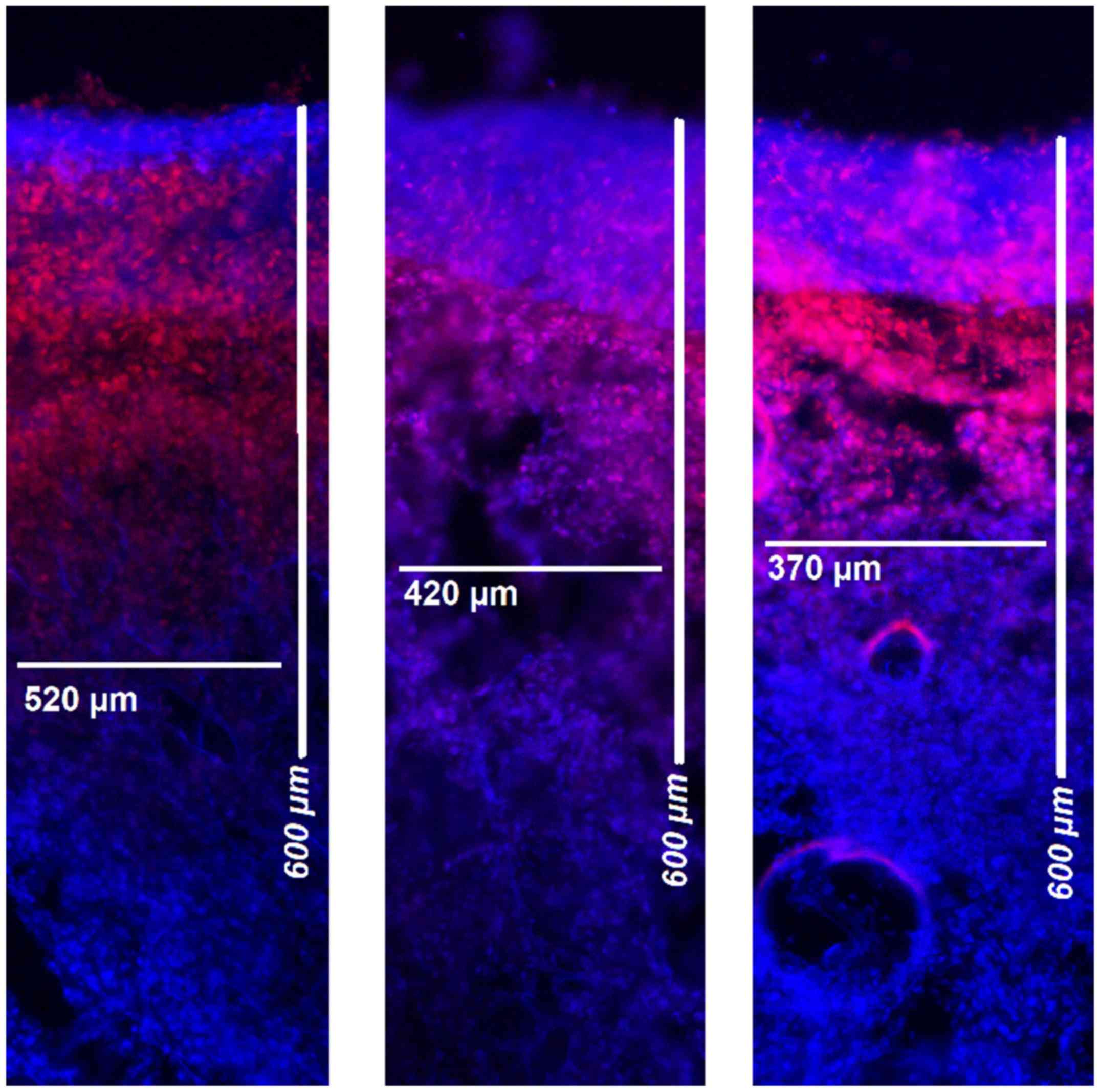
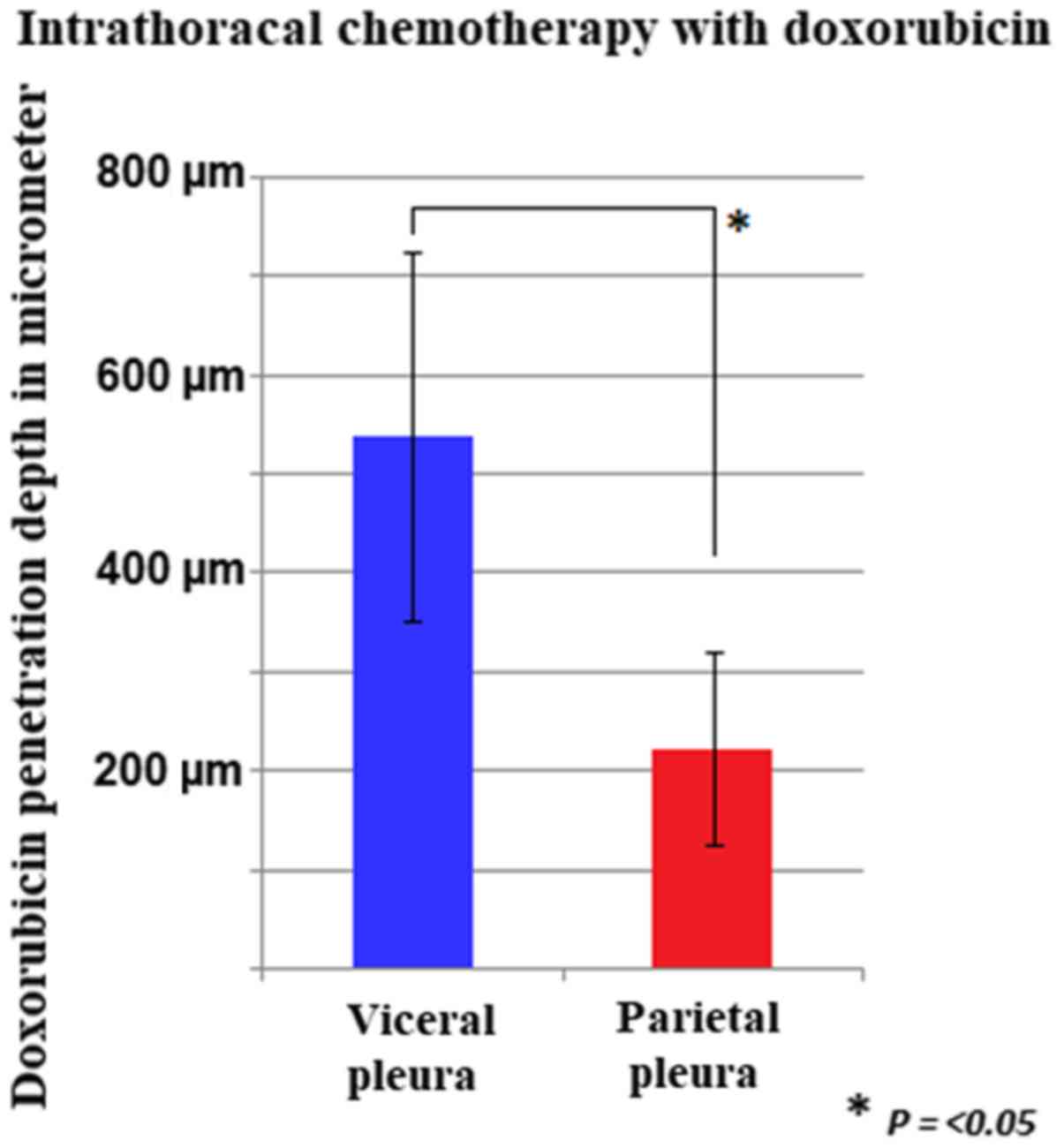
|
1
|
Hak CC, Sivakumar P and Ahmed L: Safety of
indwelling pleural catheter use in patients undergoing
chemotherapy: A five-year retrospective evaluation. BMC Pulm Med.
16(41)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Suzuki K, Servais EL, Rizk NP, Solomon SB,
Sima CS, Park BJ, Kachala SS, Zlobinsky M, Rusch VW and Adusumilli
PS: Palliation and pleurodesis in malignant pleural effusion: The
role for tunneled pleural catheters. J Thorac Oncol. 6:762–767.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Van Meter ME, McKee KY and Kohlwes RJ:
Efficacy and safety of tunneled pleural catheters in adults with
malignant pleural effusions: A systematic review. J Gen Intern Med.
26:70–76. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Akulian J and Feller-Kopman D: The past,
current and future of diagnosis and management of pleural disease.
J Thorac Dis. 7 (Suppl 4):S329–S338. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
DeBiasi E and Puchalski J: Pleural
effusions as markers of mortality and disease severity: A
state-of-the-art review. Curr Opin Pulm Med. 22:386–391.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jiang L, Li P, Gong Z, Hu B, Ma J, Wang J,
Chu H, Zhang L, Sun P and Chen J: Effective treatment for malignant
pleural effusion and ascites with combined therapy of bevacizumab
and cisplatin. Anticancer Res. 36:1313–1318. 2016.PubMed/NCBI
|
|
7
|
Giger-Pabst U, Demtröder C, Falkenstein
TA, Ouaissi M, Götze TO, Rezniczek GA and Tempfer CB: Pressurized
intraperitoneal aerosol chemotherapy (PIPAC) for the treatment of
malignant mesothelioma. BMC Cancer. 18(442)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Khosrawipour V, Mikolajczyk A, Schubert J
and Khosrawipour T: Pressurized intra-peritoneal aerosol
chemotherapy (PIPAC) via endoscopical microcatheter system.
Anticancer Res. 38:3447–3452. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Göhler D, Große S, Bellendorf A,
Falkenstein TA, Ouaissi M, Zieren J, Stintz M and Giger-Pabst U:
Hyperthermic intracavitary nanoaerosol therapy (HINAT) as an
improved approach for pressurised intraperitoneal aerosol
chemotherapy (PIPAC): Technical description, experimental
validation and first proof of concept. Beilstein J Nanotechnol.
8:2729–2740. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Khosrawipour V, Khosrawipour T, Kern AJ,
Osma A, Kabakci B, Diaz-Carballo D, Förster E, Zieren J and
Fakhrian K: Distribution pattern and penetration depth of
doxorubicin after pressurized intraperitoneal aerosol chemotherapy
(PIPAC) in a postmortem swine model. J Cancer Res Clin Oncol.
142:2275–2280. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Khosrawipour V, Khosrawipour T,
Diaz-Carballo D, Förster E, Zieren J and Giger-Pabst U: Exploring
the spatial drug distribution pattern of pressurized
intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol.
23:1220–1224. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mikolajczyk A, Khosrawipour V, Schubert J,
Grzesiak J, Chaudhry H, Pigazzi A and Khosrawipour T: Effect of
liposomal doxorubicin in pressurized intra-peritoneal aerosol
chemotherapy (PIPAC). J Cancer. 9:4301–4305. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schubert J, Khosrawipour V, Chaudhry H,
Arafkas M, Knoefel WT, Pigazzi A and Khosrawipour T: Comparing the
cytotoxicity of taurolidine, mitomycin C, and oxaliplatin on the
proliferation of in vitro colon carcinoma cells following
pressurized intra-peritoneal aerosol chemotherapy (PIPAC). World J
Surg Oncol. 17(93)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Muir WW, Hubbell JAE and Bednarski RM:
Handbook of veterinary anesthesia 4th edition. Polish Edition by
Elsevier Urban & Partner, Wroclaw, pp320-323, 2008.
|
|
15
|
Noszczyk-Nowak A, Pasławska U, Gajek J,
Janiszewski A, Pasławski R, Zyśko D and Nicpoń J: Ventricular
effective refraction period and ventricular repolarization analysis
in experimental tachycardiomyopathy in swine. Adv Clin Exp Med.
25:409–414. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Göhler D, Khosrawipour V, Khosrawipour T,
Diaz-Carballo D, Falkenstein TA, Zieren J, Stintz M and Giger-Pabst
U: Technical description of the microinjection pump
(MIP®) and granulometric characterization of the aerosol
applied for pressurized intraperitoneal aerosol chemotherapy
(PIPAC). Surg Endosc. 31:1778–1784. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mikolajczyk A, Khosrawipour V, Schubert J,
Chaudhry H, Pigazzi A and Khosrawipour T: Particle stability during
pressurized intra-peritoneal aerosol chemotherapy (PIPAC).
Anticancer Res. 38:4645–4649. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bellendorf A, Khosrawipour V, Khosrawipour
T, Siebigteroth S, Cohnen J, Diaz-Carballo D, Bockisch A, Zieren J
and Giger-Pabst U: Scintigraphic peritoneography reveals a
non-uniform 99mTc-Pertechnetat aerosol distribution
pattern for pressurized intra-peritoneal aerosol chemotherapy
(PIPAC) in a swine model. Surg Endosc. 32:166–174. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Khosrawipour T, Khosrawipour V and
Giger-Pabst U: Pressurized intra peritoneal aerosol chemotherapy in
patients suffering from peritoneal carcinomatosis of pancreatic
adenocarcinoma. PLoS One. 12(e0186709)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mikolajczyk A, Khosrawipour V, Schubert J,
Plociennik M, Nowak K, Fahr C, Chaudhry H and Khosrawipour T:
Feasibility and characteristics of pressurized aerosol chemotherapy
(PAC) in the bladder as a therapeutical option in early-stage
urinary bladder cancer. In Vivo. 32:1369–1372. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Khosrawipour V, Bellendorf A, Khosrawipour
C, Hedayat-Pour Y, Diaz-Carballo D, Förster E, Mücke R, Kabakci B,
Adamietz IA and Fakhrian K: Irradiation does not increase the
penetration depth of doxorubicin in normal tissue after pressurized
intra-peritoneal aerosol chemotherapy (PIPAC) in an ex vivo model.
In Vivo. 30:593–597. 2016.PubMed/NCBI
|
|
22
|
Khosrawipour V, Khosrawipour T,
Hedayat-Pour Y, Diaz-Carballo D, Bellendorf A, Böse-Ribeiro H,
Mücke R, Mohanaraja N, Adamietz IA and Fakhrian K: Effect of
whole-abdominal irradiation on penetration depth of doxorubicin in
normal tissue after pressurized intraperitoneal aerosol
chemotherapy (PIPAC) in a post-mortem swine model. Anticancer Res.
37:1677–1680. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Khosrawipour V, Giger-Pabst U,
Khosrawipour T, Pour YH, Diaz-Carballo D, Förster E, Böse-Ribeiro
H, Adamietz IA, Zieren J and Fakhrian K: Effect of irradiation on
tissue penetration depth of doxorubicin after pressurized
intra-peritoneal aerosol chemotherapy (PIPAC) in a novel ex-vivo
model. J Cancer. 7:910–914. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Khosrawipour V, Diaz-Carballo D, Acikelli
AH, Khosrawipour T, Falkenstein TA, Wu D, Zieren J and Giger-Pabst
U: Cytotoxic effect of different treatment parameters in
pressurized intraperitoneal aerosol chemotherapy (PIPAC) on the in
vitro proliferation of human colonic cancer cells. World J Surg
Oncol. 15(43)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Khosrawipour V, Khosrawipour T,
Falkenstein TA, Diaz-Carballo D, Förster E, Osma A, Adamietz IA,
Zieren J and Fakhrian K: Evaluating the effect of micropump©
position, internal pressure and doxorubicin dosage on efficacy of
pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in an ex
vivo model. Anticancer Res. 36:4595–4600. 2016.PubMed/NCBI View Article : Google Scholar
|