Introduction
Breast cancer is the most commonly diagnosed cancer
and the leading cause of cancer death among females (1). A multidisciplinary approach involving
surgical, radiation, and systemic treatments has contributed to a
reduction in breast cancer mortality in recent years. Probably, the
reduction is mainly due to better breast cancer screening and
adjuvant therapy (2).
The early diagnosis of breast cancer is vital to
increase survival rates, decrease morbidity, and reduce the
likelihood of recurrence of disease (3). When breast cancer is diagnosed at an
early stage, treatments are more successful than when the initial
tumor burden is advanced (4).
Breast cancer diagnosis involves identification of a
suspected lesion with radiological screening, and a confirmatory
biopsy (3). Conventional screening
with physical examination and mammography has less-than-desirable
sensitivity (54%) and specificity (77%) (5). Breast biopsy and histopathology studies
are the reference standard for diagnosis, but the procedure is
invasive and carries a risk of morbidity (6).
In normal secretory epithelial cells, MUC1 localizes
in the apical membrane and provides protection to the underlying
epithelia in healthy tissues, maintaining homeostasis, and
promoting cell survival in variable conditions (7). In contrast, tumor-associated MUC1
participates in intracellular signal transduction pathways and
regulates the expression of its target genes at both the
transcriptional and posttranscriptional levels (8).
Cancer antigen 15-3 (CA15-3) is a soluble form of
MUC1. CA15-3 corresponds to an immunodominant epitope in the
extracellular portion of the protein that is shed into the
bloodstream and can be detected by several monoclonal antibodies
(9). CA15-3 is the most widely used
serum marker to detect recurrent breast cancer and monitor
treatment of patients with advanced disease (10).
Saliva offers several benefits over traditional
blood-based biochemical analyses for clinical diagnostics:
Non-invasiveness and stress-free sample collection; easy and
multiple sampling opportunities; reduced need for sample
pre-processing; minimal risk of contracting infectious organisms
(11). Saliva has recently emerged
as a source of biochemical data to detect chronic diseases, as it
may contain real-time information describing the overall
physiological condition (12).
Enzyme-linked immunosorbent assay (ELISA),
electrochemiluminescence (ECLIA), and chemiluminescence (CLIA) are
the methods typically used to assess serum levels of CA15-3
(13,14). For evaluating salivary levels of
CA15-3, the most frequently reported method is ELISA (13) and there are no reports of the
application of CLIA or ECLIA. There is a need to evaluate
alternative methods for detecting salivary levels of CA15-3.
In this study, ECLIA was performed to assess serum
levels of CA15-3 in all subjects. In addition, ELISA, CLIA, and
ECLIA were used to quantify and compare CA15-3 levels in the saliva
of breast cancer patients and in healthy controls. Because serum
CA15-3 is the most commonly used serum marker in breast cancer
patients, the search for salivary CA15-3 measurement techniques
becomes interesting, because it is less invasive in patients who
usually have many venipuncture exams.
Patients and methods
Subjects
Subject recruitment and sample collection followed
the guidelines of the Institutional Review Board of the oncology
recruiting centers: Hospital Universitário de Brasília (HUB),
Hospital de Base do Distrito Federal (HBDF), Hospital Sírio Libanês
and Cettro. The cohort study was approved by the Research Ethics
Committee of the Faculty of Health Sciences, University of Brasilia
(Brasilia, Brazil; Plataforma Brasil protocol
57449716.5.0000.0030), and was conducted according to the
Declaration of Helsinki principles. Written informed consent was
obtained from each subject before participation in the study.
Patients and healthy controls were recruited between October 2017
and April 2018.
The inclusion criteria for the breast cancer
patients group were as follows: i) Capable of giving informed
consent; ii) not pregnant or lactating; iii) no active oral/dental
disease; iv) no prior neoplasia (except for non-melanomatous skin
cancers and carcinoma in situ of the cervix, or benign tumors such
as adenomas), and no alterations of renal function, congestive
heart failure, active infection hepatitis or HIV; and v) a proven
histopathologic diagnosis of breast cancer. These patients were
enrolled prior to excision of the primary tumor and systemic
treatment (neo-adjuvant chemotherapy or palliative endocrine/or
chemotherapy). We excluded patients and healthy controls if they
showed signs of morbidity and health problems such as autoimmune
disease, HIV, impaired renal function, congestive heart failure,
active infection and hepatitis. All patients were recruited by
convenience after appointment at an oncology center. The control
subjects were healthy female volunteers recruited from the general
population, for whom breast cancer was ruled out by physical
examination and radiological breast images. None of the
participants in the control group were knowingly suffering or being
treated for a malignancy. The study was not blinded.
Specimen collection, transportation,
and preparation
Venous blood and saliva samples were collected on
the same day for each participant. All participants abstained from
eating, drinking, smoking, and performing oral hygiene procedures
for at least 1 h prior to collection of saliva. Participants were
instructed to chew on a cotton swab (Salivette®;
SARSTEDT AG & Co.) for a period of 2 min. Each swab containing
saliva was returned to a separate plastic container and then
packaged in styrofoam with recyclable ice sheets. Within 4 h, the
material was transported to the laboratory for processing. The
saliva sample was centrifuged at 3,600 g x 5 min at 8̊C. After
centrifugation, the sample was transferred to a clean Eppendorf
tube and frozen at -80̊C until processing. The saliva samples were
thawed at room temperature for CA15-3 analysis. Typically, patients
donated 5-10 ml of saliva.
Blood samples were obtained by venipuncture and were
collected in serum tubes with separator gel. Blood was centrifuged
at 3,500-5,000 rpm for 5 min and the total volume obtained was
separated into 2 Eppendorf tubes and frozen at -20̊C prior to
analysis.
ECLIA for detection of serum and
salivary CA15-3
Measurement of serum CA15-3 was performed by ECLIA
on a fully automated Roche Cobas 8000 analyzer with an e801 module
(Roche Diagnostics) according to the manufacturer's instructions,
and reported in U/ml. The limit of blank, limit of detection, and
limit of quantification for measuring CA15-3 in serum with the
Cobas e801 module are 1.0, 1.5 and 3 U/ml, respectively (Elecsys
CA15-3 II Label, cat. no. 07027001500V2.0; Roche Diagnostics).
Measurement of salivary CA15-3 was performed as
described above for serum; however, saliva is an off-label specimen
for the applied assay.
CLIA for detection of salivary
CA15-3
Measurement of salivary CA15-3 was performed
according to the manufacturer's instructions using a sandwich CLIA
with the BR-MA 15-3 reagent kit in an IMMULITE 1000®
system (Siemens Healthcare Diagnostics Inc.). The kit for serum
assay was used for salivary assay; however, saliva is an off-label
specimen for the applied assay. There is no kit designed for use
with saliva.
The microtiter plates were pre-coated with an
antibody specific for the analyte. Standards or samples were added
to the appropriate microtiter plate wells, where the analyte
present in the standards and samples would bind to the immobilized
antibody. Next, biotin-conjugated antibody was added and bound to
the analyte on the plate. The complex of two antibodies and the
analyte in the wells forms a ‘sandwich’ structure. After any
unbound biotin-conjugated antibody was removed by washing,
avidin-conjugated horseradish peroxidase was added to each
microplate well. After incubation at 25̊C for 20 min, luminol was
added into the wells. Relative luminescence intensity was
determined using a photomultiplier, in relative light units (RLU),
being proportional to the amount of CA15-3 present in the sample,
and the results were expressed as U/ml. The assay limit of
detection for CA15-3 is 1.0 U/ml (14).
ELISA for detection of salivary
CA15-3
ELISA reactions were performed using the CA15-3
AccuBind™ reagent kit (Lake Forest, California, United
States of America) for use in BEST 2000® equipment
(Biokit S.A.), according to the manufacturer's instructions. The
kit used for saliva was the same as that used for serum; however,
saliva is an off-label specimen. The absorbance was read at 450 nm
using a spectrophotometer and concentrations were calculated from
standard curves constructed from known concentrations of the
ligand.
For the calculation of the results, a
standard-logarithmic curve was obtained by plotting the measured
values of the 6 calibrators by the corresponding units
(linear/log). The analysis was performed in duplicate, and the mean
of the two values obtained was calculated. The results were
expressed as U/ml.
For this assay, the limit of blank and functional
limit of detection for measuring CA15-3 in serum are 0.2 U/ml and
1.25 U/ml, respectively (AccuBind™ reagent kit,
Revision: 3, Date: 072611, cat. no. 5625-300, DCO:0504; Monobind
Inc.).
TNM (tumor, node, metastasis staging
system) and molecular profile of breast cancer
TNM staging was performed according to the 7th
edition of the AJCC (15), and the
molecular profile classification was determined in accordance with
the immunohistochemical definitions of the Saint Gallen consensus
(16). The mean levels of serum and
salivary CA15-3, detected by ECLIA, CLIA, and ELISA, were
determined for each patient based on TNM and molecular profile.
Statistical analysis
Statistical analysis was performed using SAS 9.4
version 9.4 (SAS Institute Inc.). Student's t tests and
Chi-square/Fisher's exact tests were applied to demographic and
clinical characteristics. A Mann-Whitney U test was used to
determine differences between mean values of serum and salivary
CA15-3 among controls and breast cancer patients. A Kruskal-Wallis
test was used to compare mean values of salivary CA15-3 and serum
CA15-3 among molecular subtypes and stages, and, when P≤0.05,
multiple comparisons were implemented using the Dwass, Steel,
Critchlow-Fligner (DSCF) method. Correlations between serum and
salivary CA15-3 in controls and breast cancer patients were
assessed using Pearson correlation coefficients. Values of
P<0.05 were considered statistically significant.
Results
Table I summarizes
the characteristics of the 28 control subjects and 26 breast cancer
patients. The mean age of the controls was lower than breast cancer
patients (37.64+/-13.57 years vs. 48.23+/-11.51 years, P=0.0033).
There were more postmenopausal women among breast cancer patients
than in the controls (11 vs. 4, P=0.0216). There was no significant
difference between the healthy controls and cancer patients
regarding tobacco use, medication use, and presence of systemic
disorders (P=0.1842, P=0.5541, and P=0.8473, respectively). Mean
body mass index was significantly higher in breast cancer patients
than in controls (P=0.0184).
 | Table IDemographic data based on participants
records. |
Table I
Demographic data based on participants
records.
|
Characteristicsa | Healthy control
(n=28) | Breast cancer
(n=26) | P-valueb |
|---|
| Age | 37.64±13.57 | 48.23±11.51 | <0.01 |
| Body mass index | 22.93±3.14 | 25.39±4.25 | 0.02 |
| Menopause status | | | 0.02 |
|
Pre-menopause | 24 (85.71) | 15 (57.69) | |
|
Menopause | 4 (14.29) | 11 (42.31) | |
| Tobacco use | | | 0.18 |
|
No | 27 (96.43) | 19 (84.62) | |
|
Yes | 1 (3.57) | 4 (15.38) | |
| Use of
medication | | | 0.55 |
|
No | 15 (53.57) | 16 (61.54) | |
|
Yes | 13 (46.43) | 10 (38.46) | |
| Systemic disease | | | 0.85 |
|
No | 19 (67.86) | 17 (65.38) | |
|
Yes | 9 (32.14) | 9 (34.62) | |
There were two cases at stage I (7%), ten (39%) at
stage IIa, three (12%) at stage IIb, four (15%) at IIIb, one (3%)
at stage IIIc and five (23%) at stage IV breast cancer cases. There
were three (11.5%) luminal B-like HER2 negative, seven (27%)
luminal A-like, five (19%) HER2 positive (nonluminal), four (15%)
luminal B-like HER2 positive, and five (19%) triple negative breast
cancer cases. There was no information for TNM in one patient and
for the molecular profile in two patients. The complete subject
information is listed in Table
SI.
Serum (ECLIA) and salivary (CLIA and ELISA) values
of CA15-3 for each subject are listed in Table SI. Salivary CA15-3 was undetected by
ECLIA. Mean serum CA15-3 by ECLIA was 134±369.00 U/ml in breast
cancer patients and 15.73±6.18 U/ml in healthy controls, P=0.06
(Table II). Although ECLIA is
considered the reference test to evaluate CA15-3 in blood, it
showed a wide variation in measurements between patients,
generating a large standard deviation.
 | Table IISerum and salivary CA15-3 mean
concentration ± standard deviation for healthy controls and
patients with breast cancer. |
Table II
Serum and salivary CA15-3 mean
concentration ± standard deviation for healthy controls and
patients with breast cancer.
| Method | Healthy control
(n=28) | Breast cancer
(n=26) |
P-valuea |
|---|
| ECLIA Serum
(U/ml) | 15.73±6.18 | 134±369 | 0.06 |
| CLIA Salivary
(U/ml) | 6.51±7.18 | 4.73±5.74 | 0.19 |
| ELISA Salivary
(U/ml) | 1.83±2.09 | 1.77±1.08 | 0.56 |
Mean salivary CA15-3 by CLIA was 4.73±5.74 U/ml in
breast cancer patients and 6.51±7.18 U/ml in healthy controls,
P=0.18. Mean salivary CA15-3 measured by ELISA was 1.77±1.08 U/ml
in breast cancer patients and 1.83±2.09 U/ml in healthy controls,
results confirm that serum CA15-3 values are higher in breast
cancer patients results confirm that serum CA15-3 values are higher
in breast cancer patients. Either the ECLIA assay was not able
detect the CA15-3 protein in saliva or the CA15-3 levels in saliva
were below the ECLIA limit of detection (1.5 U/ml). The CLIA and
ELISA limits of detection were 1.0 U/ml and 1.25 U/ml,
respectively, hence ECLIA was the least sensitive among the tested
assays. There was no significant difference between serum CA15-3
levels in breast cancer patients and healthy women (P=0.06). There
was no difference in salivary CA15-3 concentration between breast
cancer cases and controls when measured by CLIA (P=0.18) and ELISA
(P=0.55).
Table III shows the
CA15-3 concentrations in saliva and serum according to breast
cancer molecular subtypes. The analysis was performed in 24
patients with a known molecular profile. There was no difference in
mean concentration values for serum CA15-3 measured by ECLIA
(P=0.20), salivary CA15-3 measured by ELISA (P=0.70) and CLIA
(P=0.78) according to molecular subtype. ECLIA for serum CA15-3
revealed the highest values for luminal A subtype, with
269.47±659.97 U/ml CA15-3 concentration. The highest values for
CA15-3 mean concentration in luminal B HER2+ subtype were 2.58±1.83
U/ml with ELISA and 10.57±11.74 U/ml with CLIA.
 | Table IIICA15-3 mean concentration values ±
standard deviation according to breast cancer molecular
subtype. |
Table III
CA15-3 mean concentration values ±
standard deviation according to breast cancer molecular
subtype.
| Method | Luminal A | Luminal B
HER2+ | Luminal B
HER2- | HER2 positive | Triple
negative |
P-valuea |
|---|
| ECLIA Serum
(U/ml) | 269.47±659.97 | 141.60±183.22 | 196.90±186.53 | 18.04±7.98 | 14.98±6.87 | 0.20 |
| ELISA Salivary
(U/ml) | 1.71±1.11 | 2.58±1.83 | 1.61±0.33 | 1.97±1.27 | 1.28±0.26 | 0.70 |
| CLIA Salivary
(U/ml) | 4.13±5.25 | 10.57±11.74 | 3.43±3.03 | 2.72±1.14 | 5.65±5.44 | 0.78 |
Table IV shows the
CA15-3 mean concentrations in saliva and serum according to TNM
stages. The analysis was performed in 25 patients with known TNM
stage. There was no difference in salivary CA15-3 by ELISA (P=0.44)
and CLIA (P=0.40) among different breast cancer stages. Serum
CA15-3 levels were significantly different in at least two stages
of breast cancer (P=0.01). The DSCF multiple comparison test
revealed differences in serum CA15-3 concentrations among stage IV
and stage IIa cases. The mean serum CA15-3 value for stage IV cases
(508.20±718.32 U/ml) was higher than that for stage IIa (17.18±9.14
U/ml) (P=0.03). There were no other significant differences in mean
serum CA15-3 values between stages. In all analyses in both serum
and saliva, the TNM stage IV disease cases showed the highest mean
CA15-3 concentration: 508.20±718.32 U/ml with ECLIA in serum,
2.73±1.82 U/ml with ELISA in saliva, and 7.78±9.70 U/ml with CLIA
in saliva.
 | Table IVMean CA15-3 concentration values ±
standard deviation according to TNM stage. |
Table IV
Mean CA15-3 concentration values ±
standard deviation according to TNM stage.
| Method | I | II aa | II ba | III ba | IVa |
P-valuea |
|---|
| ECLIA Serum
(U/ml) | 16.50±11.31 | 17.18±9.14 | 15.00±5.43 | 97.13±100.13 | 508.20±718.32 | 0.01 |
| ELISA Salivary
(U/ml) | 1.41±0.19 | 1.77±0.98 | 1.46±0.22 | 1.28±0.13 | 2.73±1.82 | 0.45 |
| CLIA Salivary
(U/ml) | - | 4.00±3.18 | 3.50±1.56 | 1.25±0.21 | 7.78±9.70 | 0.40 |
Among breast cancer cases, there was a significant
positive correlation of serum CA15-3 and salivary CA15-3 with ELISA
(r=0.56; P=<0.01); however no significant correlation of
salivary CA15-3 and serum CA15-3 was observed with CLIA (P=0.19)
Among healthy controls, the correlations of salivary CA15-3 with
serum CA15-3 according to CLIA and ELISA were not significant
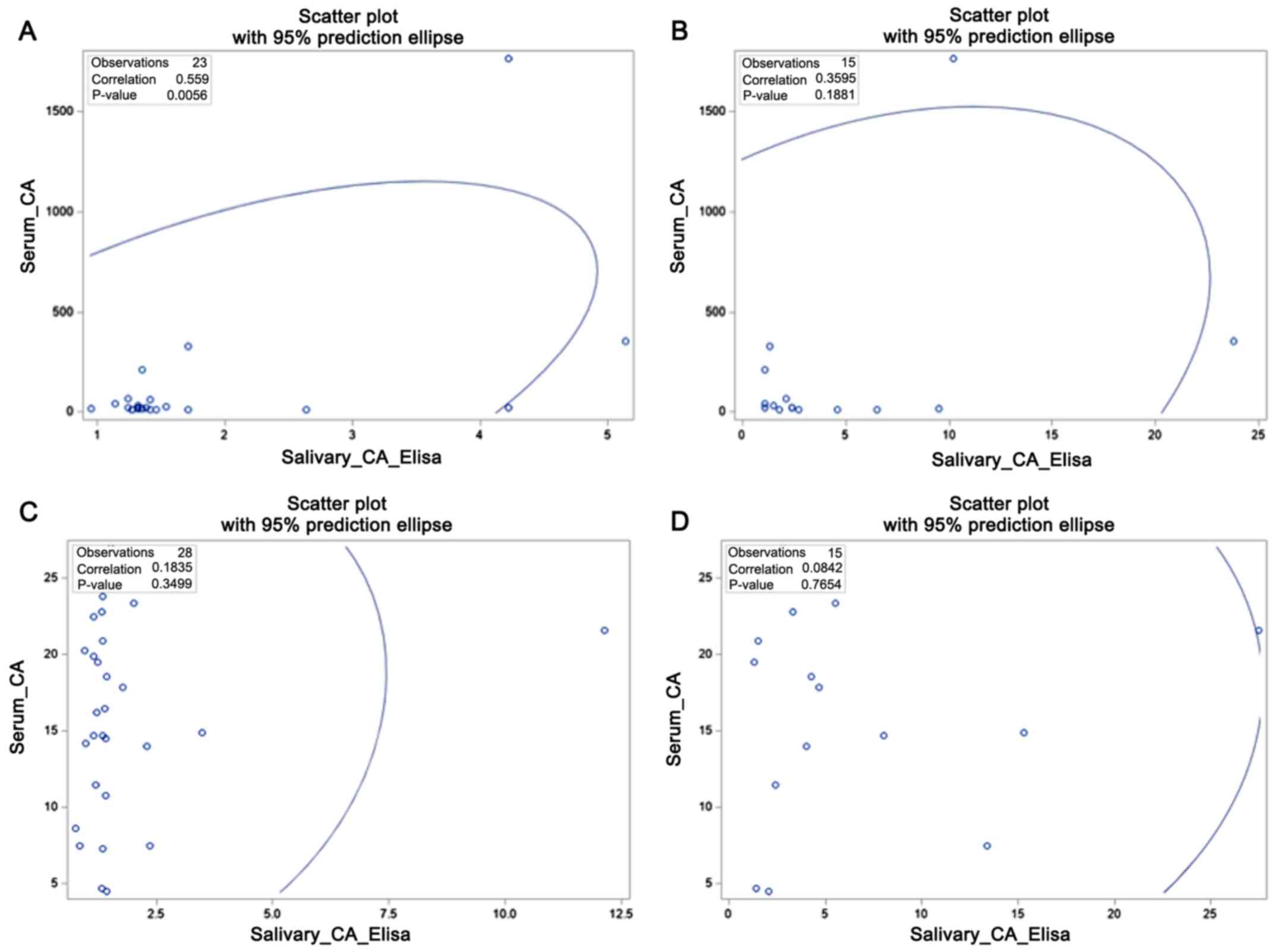
(P=0.77 and P=0.35 respectively). All correlations are shown in
Fig. 1. Fig. 1 shows that in breast cancer patients
(Fig. 1A and B), there is a growing linear relationship,
with statistical difference with ELISA (Fig. 1A). Otherwise, in the control group
(Fig. 1C and D), there is random distribution of points
without linear relationship.
Discussion
Previous studies have reported detection of salivary
CA15-3 with ELISA but not with ECLIA and CLIA (13). We chose to evaluate detection of
CA15-3 using CLIA and ECLIA because these techniques are used
routinely in clinical exams for evaluation of serum tumor markers
and serology of viral infectious agents (17). Recently, CLIA was used to evaluate
proteins in liquor, demonstrating that the method can be used to
analyze different fluids, such as saliva (17). ECLIA and CLIA do not require long
incubations or the addition of stopping reagents, so they have
superior low-end sensitivity, and are faster than conventional
colorimetric assays such as ELISA.
Several hypothetical mechanisms have been suggested
to explain the presence of large molecules such as CA15-3 in
saliva. The proposed hypothesis is that active transport of
proteins into saliva by the salivary glandular epithelium could
explain the presence of membrane-bound proteins such as CA15-3. In
breast cancer patients, there would be an overabundance of various
bioactive proteins associated with the rapid, abnormal growth of
the neoplasm, which in turn could produce a response in the
salivary glands (18). Further
studies are necessary to better understand the regulatory
mechanisms of elevated salivary CA15-3 in breast cancer
patients.
Luminal subtype breast cancer shows a higher
expression of MUC1 genes and a positive relationship between MUC1
and estrogen receptor (ER) gene expression has been reported
(19). Park et al reported
higher values for CA15-3 in luminal subtypes of tumor than in other
subtypes (20). Our results showed
the highest values for serum and salivary CA15-3 for luminal
subtypes of breast cancer. As shown in Table III, the molecular subtype of breast
cancer luminal A presented the highest standard deviation. This
result may be due to the inclusion of a patient with metastatic
breast cancer with serum CA15-3 of 1,766.0 U/ml. This patient also
had CA15-3 concentrations in the saliva of 10.2 U/ml according to
CLIA and 4.22 U/ml according to ELISA.
In many tumor types, MUC1 expression correlates with
aggressive, metastatic disease, poor response to therapy, and poor
survival (21). MUC1 expression is
seen in all subtypes of breast cancer, including luminal, HER2, and
basal, although in each of these cancer types, expression is
highest in tumors that have metastasized (21). The detection of CA15-3 in patient
sera is currently used as a marker of response to therapy and as a
prognostic indicator for survival, with high CA15-3 levels
correlating with higher grade tumors, lymph node involvement, and
presence of distant metastases in breast cancer (22). Emens et al showed that the
concentration of serum CA15-3 increases with increasing TNM stage,
with 9% of stage I, 19% of stage II, 38% of stage III, and 75% of
stage IV cases showing abnormal serum CA15-3 concentrations
(23). In our samples, stage IV
disease was related to the greatest mean values of CA15-3 in serum
and saliva when compared with the earlier stages of disease
(1-3).
In the present study, a moderate association was
found between serum and salivary CA15-3 in breast cancer patients
using ELISA (r=0.56; P=<0.01). Agha-Hosseini et al found
that salivary and serum levels of CA15-3 were significantly higher
in cancer patients, with a significant positive correlation between
serum and saliva CA15-3 concentrations (24). Streckfus et al also reported a
moderate correlation between salivary and serum CA15-3
concentration with ELISA (18).
Serum CA15-3 is an invasive exam requiring
venipuncture in patients who usually have fragile veins due to
previous chemotherapy and excessive routine blood tests. Salivary
methods for protein detection would allow evaluation without pain
and discomfort to the patient and could therefore provide a more
convenient alternative to CA15-3 serum assays (25). Salivary CA15-3 could also be an
interesting test for cancer screening in general population,
specially for the luminal subtypes of breast cancer because of the
relationship of MUC-1 and estrogen receptor. The possibility of
biomarkers using cancer derived saliva exosomes is attractive
because of the stability of vesicles in blood and fluids (26). Saliva has already been widely used in
genetic testing (27) owing to its
better transport stability compared to that of blood (28). In addition, knowledge regarding
whether proteins and tumor DNA are present in other fluids aids in
our understanding of the biological behavior of the disease.
Although there was no statistical difference, there
is a tendency for higher serum CA15-3 values in breast cancer
patients. There is a tendency for higher salivary CA15-3 values in
controls compared to breast cancer patients. The main limitations
of the study are: Sample size, unbalanced age groups and menopausal
status. Reduced sample size and lack of standardized kits for
saliva CA15-3 evaluation may have contributed to inconsistent
salivary CA15-3 results in controls. ECLIA was not a good method to
detect salivary CA15-3, although it is the reference standard for
detecting serum CA15-3. Importantly, the CA15-3 kit used for ECLIA
of saliva is standardized for blood; this may have contributed to
the poor detection of CA15-3 in saliva, as these fluids have
different conductivities, and antibodies may have different
sensitivities and affinities in different body fluids. In breast
cancer patients, we observed a correlation between serum and
salivary CA15-3 detected by ELISA. CA15-3 concentrations were
highest in stage IV and luminal breast cancer subtypes. The study
shows that CA15-3 was detected in saliva with ELISA and CLIA, but
not with the ECLIA reference technique for detecting serum CA15-3.
New studies evaluating the detection of CA15-3 in saliva may allow
this marker to be used in the follow up of breast cancer patients,
who often have their venous network compromised due to successive
puncture exams. Further investigations are needed to confirm the
capability of detection of salivary CA15-3 and its correlation to
serum CA15-3.
Supplementary Material
Subject characteristics.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the
Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF,
Brasília, Brazil). Grant no. 00193.00001319/2018-33.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DXA, ECPM, ACA, ENSG and HC conceived or designed
the study. DXA, AGCN, ECPM, ACA, ENSG, and AC performed the
research. DXA, ENSG, GBB, YKMN, RP and AC analyzed the data. GBB
and YKMN contributed with new methods or models. DXA, ACA, ENSG,
ECPM and AGCN wrote the paper. ACA, ENSG supervised the research.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Research Ethics
Committee of the Faculty of Health Sciences, University of Brasilia
(Brasilia, Brazil; Plataforma Brasil protocol
57449716.5.0000.0030), and was conducted according to the
Declaration of Helsinki principles. Written informed consent was
obtained from each subject before participation in the study.
Patient consent to publication
Written informed consent was obtained from each
subject before participation in the study, the informed consent
included consent for publication of the data and clinical
information.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:94–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Narod SA, Iqbal J and Miller AB: Why have
breast cancer mortality rates declined? J Cancer Policy. 5:8–17.
2015.
|
|
3
|
Arellano M, Jiang J, Zhou X, Zhang L, Ye
H, Wong DT and Hu S: Current advances in identification of cancer
biomarkers in Saliva. Front Biosci (Schol Ed). 1:296–303.
2009.PubMed/NCBI
|
|
4
|
Giuliano AE, Connolly JL, Edge SB,
Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ and
Hortobagyi GN: Breast cancer-major changes in the American joint
committee on cancer eighth edition cancer staging manual. CA Cancer
J Clin. 67:290–303. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Berg WA, Gutierrez L, NessAiver MS, Carter
WB, Bhargavan M, Lewis RS and Ioffe OB: Diagnostic accuracy of
mammography, clinical examination, US, and MR imaging in
preoperative assessment of breast cancer. Radiology. 233:830–849.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang YJ, Wei L, Li J, Zheng YQ and Li XR:
Status quo and development trend of breast biopsy technology. Gland
Surg. 2:15–24. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hollingsworth MA and Swanson BJ: Mucins in
cancer: Protection and control of the cell surface. Nat Rev Cancer.
4:45–60. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Nath S and Mukherjee P: MUC1: A
multifaceted oncoprotein with a key role in cancer progression.
Trends Mol Med. 20:332–342. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Duffy MJ, Shering S, Sherry F, McDermott E
and O'Higgins N: CA 15-3: A prognostic marker in breast cancer. Int
J Biol Markers. 15:330–333. 2000.PubMed/NCBI
|
|
10
|
Harris LN, Ismaila N, McShane LM, Andre F,
Collyar DE, Gonzalez-Angulo AM, Hammond EH, Kuderer NM, Liu MC,
Mennel RG, et al: Use of biomarkers to guide decisions on adjuvant
systemic therapy for women with early-stage invasive breast cancer:
American society of clinical oncology clinical practice guideline.
J Clin Oncol. 34:1134–1150. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Punyadeera C and Slowey PD: Saliva as an
emerging biofluid for clinical diagnosis and applications of
MEMS/NEMS in salivary diagnostics. Nanobiomat Clin Dent, pp453-473,
2012.
|
|
12
|
Schafer CA, Schafer JJ, Yakob M, Lima P,
Camargo P and Wong DT: Saliva diagnostics: Utilizing oral fluids to
determine health status. Monogr Oral Sci. 24:88–98. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bigler LR, Streckfus CF, Copeland L, Burns
R, Dai X, Kuhn M, Martin P and Bigler SA: The potential use of
saliva to detect recurrence of disease in women with breast
carcinoma. J Oral Pathol Med. 31:421–431. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li P, Ye H, Liu J, Jin H, Lin Y, Yan S, Yu
Y, Gao L, Xu F and Zhang Z: Evaluation of a newly developed
quantitative determination kit for tumor marker CA15-3 with
chemiluminescent assay. J Clin Lab Anal 32, 2018.
|
|
15
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M (eds): AJCC Cancer Staging Manual.
7th edition, Sringer, 2017. https://cancerstaging.org/Pages/default.aspx.
|
|
16
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ: Panel members.
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Victer TN, Dos Santos CS, Báo SN and
Sampaio TL: Deceased tissue donor serology and molecular testing
for HIV, hepatitis B and hepatitis C viruses: A lack of cadaveric
validated tests. Cell Tissue Bank. 17:543–553. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Streckfus CF, Bigler L, Edwards C,
Guajardo-Streckfus C and Bigler SA: Using saliva secretions to
model disease progression. Adv Saliva Diagn, pp187-198, 2015.
|
|
19
|
Atoum M, Nimer N, Abdeldayem S and Nasr H:
Relationships among serum CA15-3 tumor marker, TNM staging, and
estrogen and progesterone receptor expression in benign and
malignant breast lesions. Asian Pacific J Cancer Prev. 13:857–860.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Park S, Ahn HK, Park LC, Hwang DW, Ji JH,
Maeng CH, Cho SH, Lee JY, Park KT, Ahn JS, et al: Implications of
different CA 15-3 levels according to breast cancer subtype at
initial diagnosis of recurrent or metastatic breast cancer.
Oncology. 82:180–187. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Horm TM and Schroeder JA: MUC1 and
metastatic cancer: Expression, function and therapeutic targeting.
Cell Adhes Migr. 7:187–198. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Harris L, Fritsche H, Mennel R, Norton L,
Ravdin P, Taube S, Somerfield MR, Hayes DF and Bast RC Jr: American
Society of Clinical Oncology. American society of clinical oncology
2007 update of recommendations for the use of tumor markers in
breast cancer. J Clin Oncol. 25:5287–5312. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Emens LA and Davidson NE: The follow-up of
breast cancer. Semin Oncol. 30:338–348. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Agha-Hosseini F, Mirzaii-Dizgah I, Rahimi
A and Seilanian-Toosi M: Correlation of serum and salivary CA125
levels in patients with breast cancer. J Contemp Dent Pract.
10:E001–E008. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kaczor-Urbanowicz KE, Martin
Carreras-Presas C, Aro K, Tu M, Garcia-Godoy F and Wong DT: Saliva
diagnostics-current views and directions. Exp Biol Med (Maywood).
242:459–472. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dawes C and Wong DTW: Role of saliva and
salivary diagnostics in the advancement of oral health. J Dent Res.
98:133–141. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Meghnani V, Mohammed N, Giauque C, Nahire
R and David T: Performance characterization and validation of
saliva as an alternative specimen source for detecting hereditary
breast cancer mutations by next generation sequencing. Int J
Genomics. 2016(2059041)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cascella R, Stocchi L, Strafella C,
Mezzaroma I, Mannazzu M, Vullo V, Montella F, Parruti G, Borgiani
P, Sangiuolo F, et al: Comparative analysis between saliva and
buccal swabs as source of DNA: Lesson from HLA-B*57:01 testing.
Pharmacogenomics. 16:1039–1046. 2015.PubMed/NCBI View Article : Google Scholar
|