Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common subtype of non-Hodgkin's lymphoma (NHL), representing 30% of
all lymphoma cases (1). The
combination of rituximab, cyclophosphamide, doxorubicin,
vincristine, and prednisone is the first line immunochemotherapy
used in the treatment of DLBCL, with cure rates of 60-70% (2-4).
However, 30-40% of these patients will experience a relapse or
refractory disease within the first 2-3 years following
immunochemotherapy, thus exhibiting a poor prognosis (5,6). Early
relapses (≤1 year) and late relapses (>5 years) may also occur,
with incidence rates of 10-15 and 3%, respectively (5,7).
High-dose immunotherapy followed by autologous stem
cell transplantation (ASCT) is the standard treatment for patients
with relapsed/refractory (RR) DLBCL that are <65 years and
without major comorbidities; however, >60% of patients are
ineligible for transplant, presenting a therapeutic challenge
(8).
Promising immunotherapy approaches, including
chimeric antigen receptor (CAR) T-cell therapy, have boosted the
possibility of novel treatment options for patients with DLBCL
(2). CAR T-cells are a form of
immunotherapy in which immune cells are genetically engineered to
target an antigen present on tumor cells so that they seek out
those cells specifically; these T-cells then initiate an active and
sustained immune response against the target cells (9).
Following years of research and development, the
Food and Drug Administration (FDA) has already approved two CAR
T-cell products. In October 2017, axicabtagene ciloleucel, marketed
as Yescarta, became the first CAR T-cell therapy to be approved for
patients with R/R NHL (10).
Findings from phase II of the ZUMA-1 study revealed that the
highest objective response rate (ORR) achieved using the therapy
was 82%, and the highest complete remission (CR) rate was 54%
(11). On a 12-month follow-up, the
durable ORR was found to be 42%, and the durable CR rate was 40%.
In May 2018, tisagenlecleucel was also approved for the treatment
of large B-cell lymphoma, based on the phase II JULIET study; in
the study, the highest reported ORR and CR rate were 52 and 40%,
respectively (12,13). Based on a European Hematology
Association presentation, the durable ORR and CR rate are
postulated to be 34 and 29%, respectively (14). A third CAR T-cell therapy,
lisocabtagene maraleucel has also shown promise in a phase II
study, which is also expected to lead to FDA approval (15). In the phase II TRANSCEND study, at
the dose level being explored for FDA submission, the highest ORR
and CR rate were 80 and 59%, respectively; at 6 months, the durable
ORR was 47% and the durable CR rate was 41% (15).
CAR T cells have thus shown promising efficacy in
patients with DLBCL, including those with R/R disease; however,
this therapy is also associated with unexpected toxicities that can
be life-threatening, including cytokine release syndrome (CRS) and
neurotoxicity (16). Therefore, the
challenges in DLBCL management are to reduce toxicity, prolong
disease-free survival and determine factors that can predict
relapse of DLBCL following CAR T-cell therapy.
The aim of the present study was to evaluate the
general outcomes of CAR T-cell therapy in B-cell NHL, including the
ORR and CR rate, progression-free survival (PFS), overall survival
(OS) and adverse effects.
Materials and methods
Meta-analysis
The meta-analysis was designed in accordance with
the principles set by the PRISMA checklist (17). Inclusion criteria specified all
clinical studies between 2010 and 2018 in which adult patients with
DLBCL received the second generation of anti-CD19 or anti-CD20 CAR
T-cell therapy. Ongoing clinical trials without reported outcomes
and clinical trials with first-generation CAR T-cell therapy were
excluded.
The literature search was performed using the
following electronic medical bibliographic databases: PubMed
(https://pubmed.ncbi.nlm.nih.gov/),
Scopus (https://www.scopus.com), and Web of Science (https://www.webofknowledge.com). Relevant
oncology conference proceedings were also searched. Terms used
included ‘anti-CD19’, ‘anti-CD20’, ‘diffuse large B-cell lymphoma’,
‘DLBCL’, ‘CAR T-cells’ and ‘chimeric antigen receptor T-cells’. The
references of the retrieved articles and previous review articles
were reviewed manually to obtain additional articles. Two
investigators independently screened the retrieved titles and
abstracts; the full texts were screened if the articles met the
inclusion criteria. The full texts of these selected articles were
obtained and evaluated by all investigators to confirm eligibility
for inclusion (Fig. 1).
Data were extracted using a structured template, and
disagreements were resolved by consensus during the processes of
screening and data extraction. For each study included, the
following information was obtained: Author and year; phase of the
study; patient population; CAR construct and signaling; dose of
infused CAR T-cells; conditioning or lymphodepleting chemotherapy;
origin type of the CAR T cells (autologous vs.
donor-derived/allogeneic); outcomes; survival; and adverse effects.
Second-generation CAR T-cell therapies in phase I and phase II
clinical trials were selected for the final analysis. The primary
outcome was ORR, while the secondary outcome was CR. Other
secondary outcomes were PFS and OS. The toxicity data were analyzed
in two main categories: Grade 3-4 CRS and severe neurotoxicity.
Statistical analysis
The meta-analysis was performed using Comprehensive
Meta-Analysis software (version 3.3.070; BioStat, Inc.) due to the
small sample size in most of the studies included (18). The pooled odds ratios (event rate)
estimates of ORR, CR and adverse events with 95% confidence
intervals (CI) were obtained using the random-effects model.
Statistical heterogeneity of the trials' results was assessed via
graphical inspections of the forest plots and by calculating a
Chi-squared (χ2) test for heterogeneity with a
significance level of P<0.10.
Results
Clinical trial and patient clinical
characteristics
The initial search identified 293 potentially
relevant studies, and from those, a total of 11 clinical trials
including 441 patients with B-cell lymphoma were included in the
final analysis. Of these, 292 (66%) patients had de novo R/R
DLBCL, 73 (17%) patients had transformed DLBCL from follicular
lymphoma (FL), and 15 (3%) had transformed from chronic lymphocytic
leukemia (CLL) or marginal zone lymphoma (MZL). Furthermore, 25
(6%) had FL, 18 (4%) had primary mediastinal large B-cell lymphoma
(PMBCL), 14 (3%) had mantle cell lymphoma (MCL), and the remaining
4 patients had other B-cell lymphomas (1%). Tables
I-III present the characteristics and clinical outcomes of CAR
T-cell therapy in the studies analyzed (11,13,15,19-30).
Efficacy
Over a median follow-up time of 19.6 months,
response data were available for 419 of the patients with B-cell
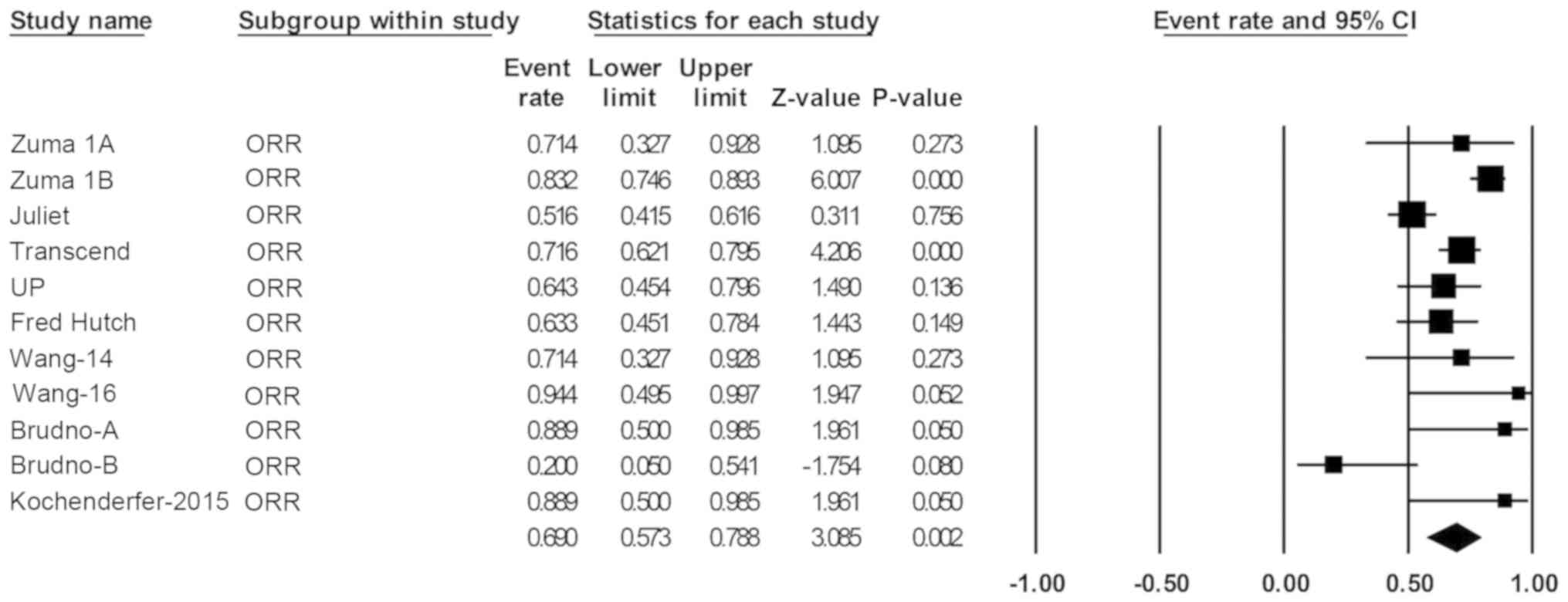
NHL. The pooled ORR (95% CI) was 69% (57-79%; Fig. 2), and the pooled CR rate (95% CI)
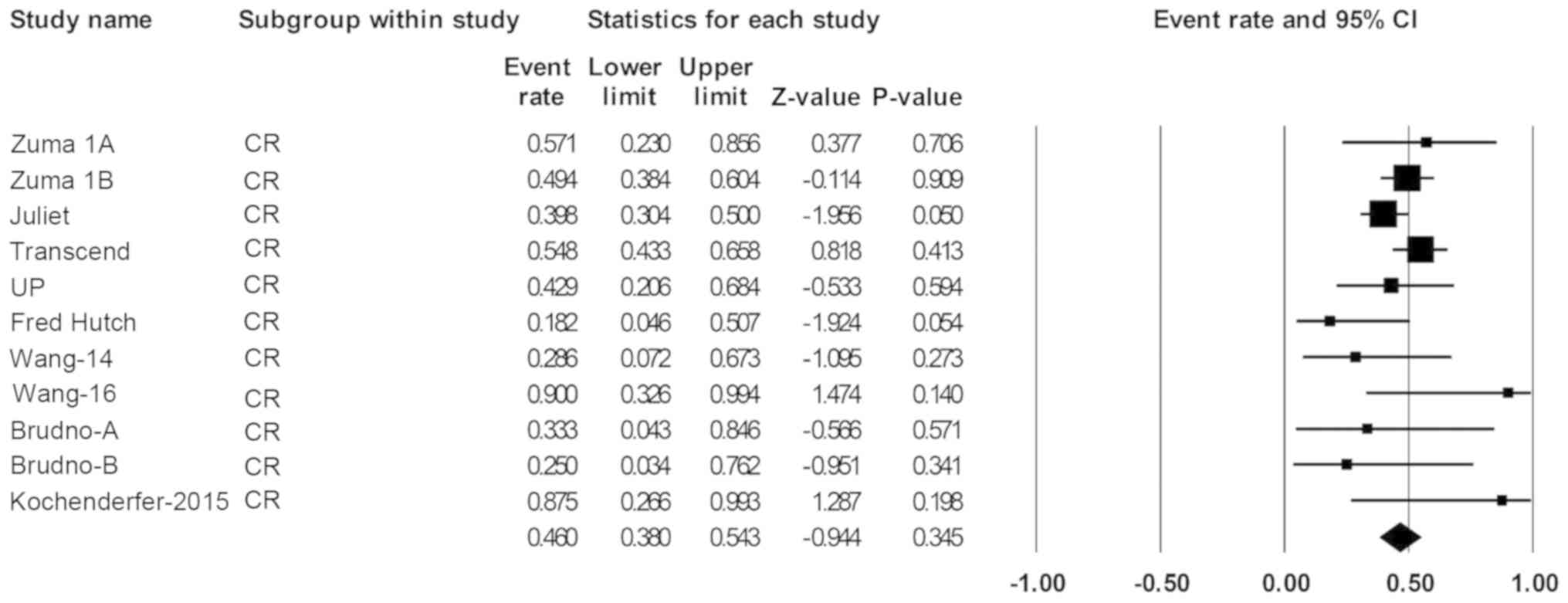
was 49% (44-52%; Fig. 3).
A total of 306 patients with de novo or
transformed DLBCL were eligible for response rate evaluation. The
ORR was 68% (55-79%; Fig. 4) and
the CR rate was 46% (38-54%; Fig.
5).
The PFS was reported for 234 patients with B-cell
lymphoma from the 11 clinical trials, and at 12 months, the PFS was
43% (95% CI, 35-75%). The median and mean PFS durations were 4.5
and 4.1 months (95% CI, 1.5-5.9 months), respectively (data not
shown).
The OS was reported for 317 patients, and at 12
months, it was 58% (95% CI, 49-60%). The median and mean OS
durations were 13.2 and 14.2 months (95% CI, 8.3-22.2 months),
respectively (data not shown).
Safety
Safety was evaluated for 421 patients (Table III). The most frequently reported
grade ≥3 adverse effects were anemia in 34% of patients (95% CI,
25-45%), thrombocytopenia at 30% (95% CI, 18-46%), and febrile
neutropenia at 19% (95% CI, 9-36%). The risks of grade ≥3 CRS and
neurotoxicity in patients were 18% (95% CI, 11-27%) and 19% (95%
CI, 12-28%), respectively (Fig.
6).
 | Table IIIChimeric antigen receptor T-cell
clinical trial toxicity profile. |
Table III
Chimeric antigen receptor T-cell
clinical trial toxicity profile.
| Author, year | Seq. no. | Project name | Toxicity: CRS and
neurotoxicity | Other toxicities,
grade 3 or higher | (Refs.) |
|---|
| Locke et al,
2017 | 1 | ZUMA 1 Trial
(a) | Grade 3-4 CRS, 11%
(n=12/108) Neurotoxicity, 32% (n=35/108) | Febrile
neutropenia: Grade 3, 31% (n=33/108); grade 4, 2% (n=2/108)
Neutropenia: Grade 3, 9% (n=10/108); grade 4, 30% (n=32/108)
Anemia: Grade 3, 43% (n=46/108); grade 4, 3% (n=3/108)
Thrombocytopenia: Grade 3, 10% (n=11/108); grade 4, 14% (n=15/108)
Intracranial hemorrhage: Grade 3, 30%; grade 4, 0%; grade 5, 14%
(n=1/7) Hypocalcemia: Grade 3, 6% (n=7/108); grade 4, 0%
Hyponatremia: Grade 3, 11% (n=12/108); Grade 4, 0%
Hypophosphatemia: Grade 3, 17% (n=18/108); Grade 4, 1% (n=1/108)
Hypotension: Grade 3, 13% (n=14/108); Grade 4, 0% Fatigue: Grade 3,
3% (n=3/108); Grade 4, 0% Treatment-related death, 2%
(n=2/108) | (19) |
| Neelapu et
al, 2017 | 2 | ZUMA 1 Trial
(b) | Grade 3-4 CRS, 22%
Neurotoxicity, 12% | Febrile
neutropenia, 15% Infection, 20% Cytopenia, 22% Tumor lysis
syndrome, 1% | (11) |
| Schuster et
al, 2017 and 2019 | 3 | JULIET | Grade 3-4, 1% Grade
3-4 neurotoxicity, 12% | Not reported | (13,20,21) |
| Abramson et
al, 2017 and 2018 | 4 | TRANSCEND | Grade 3-4, 18%
(n=5/28) Grade 3-4 neurotoxicity, 11% (n=3/28) | Febrile
neutropenia, 11% (n=3/28) Anemia, 11% (n=3/28) Atrial fibrillation,
4% (1/28) Intra-abdominal hemorrhage, 4% (n=1/28) Hypotension, 11%
(n=3/28) Hypocalcemia, 4% (n=1/28) Hypercalcemia, 4% (n=1/28)
Hyponatremia, 0% Hypomagnesemia, 0% | (15,22) |
| Schuster et
al, 2017 | 5 | University of
Pennsylvania | Severe CRS, 13%
(n=4/32) Grade 3-4 neurotoxicity, 28% (n=9/32) | Not reported | (23) |
| Turtle et
al, 2016 | 6 | Fred Hutch | Grade 3 CRS, 14%
(n=1/7), Grade 4 CRS, 0% Grade 3-4 neurotoxicity, 0% Grade 3-4
alimentary tract hemorrhage, 29% (n=2/7) | Grade 4 infusion
associated acute toxicities, 14% (n=1/7) Tumor lysis syndrome, 14%
(n=1/7) Lung dysfunction, 14% (n=1/7) Serous cavity effusion, 14%
(n=1/7) | (24) |
| Wang et al,
2014 | 7 | NA | Grade 3-4 CRS, 0%
(n=0/8) Neurotoxicity, 0% (n=0/8) | Hematological
toxicities G4, 100% (n=8/8) Non hematological toxicity G3, 88%
(n=7/8) | (25) |
| Wang et al,
2016 | 8 | NHL2 | Grade 3-4 CRS, 38%
(n=3/8) Neurotoxicity, 13% (n=1/8) | Not reported | (26) |
| Brudno et
al, 2016 | 9 | NA | Grade 3-4 CRS, 25%
(n=1/4) | For NHL patients
excluding leukemia patients: Grade 3-4 anemia, 10% (n=1/10) Grade
3-4 neutropenia, 20% (n=2/10) Grade 3-4 thrombocytopenia, 10%
(n=1/10) Grade 3-4 AST/ALT elevation, 10% (n=1/10) | (27) |
| Brudno et
al, 2016 | 10 | NA | Grade 3-4 CRS, 40%
(n=6/15) Grade 3-4 neurotoxicity, 40% (n=6/15) | Hypotension, 27%
(n=4/15) Infection, 53% (n=8/15) Acute renal failure, 7% | (28) |
| Kochenderfer et
al, 2015 | 11 | NA | CRS
Neurotoxicity | Previously
reported | (29) |
| Kochenderfer et
al, 2017 | 12 | NA | Grade 3-4 CRS, 11%
(n=12/108) Neurotoxicity, 32% (n=35/108) | Febrile
neutropenia: Grade 3, 31% (n=33/108); grade 4, 2% (n=2/108)
Neutropenia: Grade 3, 9% (n=10/108); grade 4, 30% (n=32/108)
Anemia: Grade 3, 43% (n=46/108); grade 4 3% (n=3/108)
Thrombocytopenia: Grade 3, 10% (n=11/108); grade 4, 14% (n=15/108)
Intracranial hemorrhage: Grade 3, 30%; grade 4, 0%; grade 5, 14%
(n=1/7) Hypocalcemia: Grade 3, 6% (n=7/108); grade 4, 0%
Hyponatremia: Grade 3, 11% (n=12/108); grade 4, 0%
Hypophosphatemia: Grade 3, 17% (n=18/108); grade 4, 1% (n=1/108)
Hypotension: Grade 3, 13% (n=14/108); grade 4 0% Fatigue: Grade 3,
3% (n=3/108); grade 4, 0% Treatment-related death, 2%
(n=2/108) | (30) |
Heterogeneity
Statistical heterogeneity was observed among the 11
clinical trials in several outcomes, including ORR for patients
with B-cell NHL (P=0.002; Fig. 2),
ORR for patients with DLBCL (P=0.007; Fig. 4), and adverse events such as CRS
(P=0.000), neurotoxicity (P=0.000), febrile neutropenia (P=0.001),
anemia (P=0.003) and thrombocytopenia (P=0.016; Fig. 6).
Discussion
The efficacy of CAR T-cell immunotherapy has
improved notably over the last decade. To date, three generations
of CAR T-cells have been constructed; of these, the second and
third generations of CAR T-cells show superior clinical outcomes
relative to the first generation (31). It has been reported that
first-generation CAR T-cells show decreased immune activation,
limited efficacy and short duration of persistence, providing no
evidence of clinical benefit for the treatment of B-cell NHL
(32-34).
The present meta-analysis showed highly favorable
clinical outcomes in patients with B-cell NHL that were treated
with second-generation CAR T-cells. The results for 419 patients in
11 trials showed an ORR and CR rate mean estimate of 69% (95% CI,
57-79%) and 49% (95% CI, 44-52%), respectively. The response rates
to CAR T-cells varied between different types of B-cell NHL. In 306
patients with R/R DLBCL eligible for rate evaluation, the ORR and
CR rate mean estimates were 68% (95% CI, 55-79%) and 46% (95% CI,
38-54%), respectively; these results are comparable to the results
reported on patients analyzed in the SCHOLAR-1 study, which showed
an ORR of 26% and a CR rate of 7% with standard systemic therapy
(35). Thus, the present findings
suggested that CAR T-cell immunotherapy has significantly improved
treatment outcomes for patients with R/R DLBCL, as well as other
B-cell NHL subtypes. Comparisons between the reported outcomes in
clinical trials included in the present study are difficult due to
the clinical heterogeneity in the variables between clinical
trials, including differences in patient populations, B-cell NHL
subtypes disease specific variables, CAR T-cell methods, follow-up
times and duration. Additionally, it has been suggested that the
differences in clinical outcome could be due to clinical factors
such as the CAR construct and signaling, conditioning or
lymphodepleting chemotherapy, prior ASCT, prior treatments or other
dissimilarities that will require further investigation (36-39).
Given the consequences of clinical heterogeneity or methodological
dissimilarities among CAR T-cell clinical trials included in this
study, statistical heterogeneity was also observed for several
outcomes, such as ORR and adverse events. Thus, a systematic review
of literature is warranted following the present meta-analysis to
summarize the evidence of relevant clinical factors that may have
clinical utility in predicting CAR T-cell therapy clinical
outcomes. Furthermore, with an increased number of clinical
studies, detailed associations between clinical factors and
clinical outcomes with CAR T-cell therapy will be uncovered further
in the future.
The high response rates from second-generation CAR
T-cells observed in the present analysis come with challenges posed
by adverse events and toxicities of treatment. Evidence suggests
that these adverse events tend to occur rapidly within the first
few weeks of treatment and can cause potentially life-threatening
complications (28,29). In 419 patients with B-cell NHL
evaluated for safety, it was observed that grade ≥3 anemia (34%;
95% CI, 25-45%) and thrombocytopenia (30%; 95% CI, 18-46%) were the
most common adverse effects of CAR T-cell therapy. Additionally,
grade ≥3 CRS and neurotoxicity were estimated in 18% (95% CI,
11-27%) and 19% (95% CI, 12-28%) of the patients, respectively. In
the present analysis, incidence of CRS and neurotoxicity varied
greatly in trials. The study by Kochenderfer et al (29) reported the highest rates of grade 3
or higher CRS and neurotoxicity, which was 40% (95% CI, 19-65%).
Based on a previous report, administration of interleukin (IL)-2 is
associated with significant neurotoxicity in patients treated with
CAR T-cells (40). Although IL-2
was not administered to patients in their study, neurological
toxicity still occurred in certain patients. A potential factor to
consider is that all patients had received cyclophosphamide and
fludarabine lymphodepletion. Of note, all patients recovered
completely from their neurological toxicities (29). In the Fred Hutchinson Cancer
Research Center CAR T-cell clinical trial, grade ≥3 CRS and
neurotoxicity were observed in 13% (95% CI, 5-29%) and 28% (95% CI,
15-46%) of patients, respectively, and these were predominantly
observed in patients who had received cyclophosphamide and
fludarabine lymphodepletion and higher CAR T-cell dose (24). A reduction in the CAR T-cell dose in
subsequent patients achieved ORR and CR rates of 82 and 64%,
respectively. In TRANSCEND trial, however, dose level was not
associated with CRS or neurotoxicity (39). Of note, the relatively high CRS and
neurotoxicity rates observed in single center studies are due to
relatively small sample size; additionally, two of the trials are
allogeneic CAR T-cells in origin (24,27,28).
Following the expansion of CAR T-cell clinical
trials, the therapeutic procedures and treatment outcomes markedly
improved. In the analysis of three front-running multi-center CAR
T-cell clinical studies, highly comparable rates of grade ≥3 CRS
and neurotoxicity were observed. In the ZUMA-1 trial, grade ≥3 CRS
and neurotoxicity were observed in 11 and 32% of patients,
respectively; despite the high rate of grade ≥3 neurotoxicity,
patients were effectively managed and with extended follow-up,
there were no new unexpected serious adverse events and no
new-onset neurological events associated with the CAR T-cells
(11,19). In the JULIET trial, grade ≥3 CRS and
neurotoxicity were observed in 22 and 12% of patients,
respectively; all cases of severe CRS were reversible, and no
deaths were reported (13,20,21).
In the analysis of the TRANSCEND trial, lower rates of toxicities
were observed, with grade ≥3 CRS occurring in only 1% of patients,
whereas neurotoxicity presented in 13%; additionally, no deaths
from CRS or neurotoxicity were reported in this trial (15,22).
In conclusion, the present meta-analysis reported on a large number
of patients with B-cell NHL treated with second-generation CAR
T-cells. The study showed a high clinical response rate to CAR
T-cell therapy among patients with B-cell NHL, particularly with
DLBCL, compared with standard chemotherapy regimens. Incidence of
CRS and neurotoxicity associated with CAR T-cell therapy were
effectively managed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this article.
Authors' contributions
MAM was involved in the conception and design of the
study, conducted data collection, analysis and interpretation, and
drafted and critically revised the manuscript, assuming general
responsibility and guaranteeing the scientific integrity of the
study. MAF was involved in drafting the study, conducting data
collection, analysis and interpretation, and critically revising
the manuscript. EI participated in statistical analysis and
interpretation, critical revision, and helped to draft and finalize
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jaffe ES, Harris NL, Stein H and Isaacson
PG: Classification of lymphoid neoplasms: The microscope as a tool
for disease discovery. Blood. 112:4384–4399. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sehn LH, Donaldson J, Chhanabhai M,
Fitzgerald C, Gill K, Klasa R, MacPherson N, O'Reilly S, Spinelli
JJ, Sutherland J, et al: Introduction of combined CHOP plus
rituximab therapy dramatically improved outcome of diffuse large
B-cell lymphoma in British Columbia. J Clin Oncol. 223:5027–5033.
2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Coiffier B: Rituximab in the treatment of
diffuse large B-cell lymphomas. Semin Oncol. 29:30–35.
2002.PubMed/NCBI
|
|
4
|
Habermann TM, Weller EA, Morrison VA,
Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI,
Peterson BA and Horning SJ: Rituximab-CHOP versus CHOP alone or
with maintenance rituximab in older patients with diffuse large
B-cell lymphoma. J Clin Oncol. 24:3121–3127. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sarkozy C and Sehn LH: Management of
relapsed/refractory DLBCL. Best Pract Res Clin Haematol.
31:209–216. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Damaj G, Bernard M, Legouill S, Cartron G,
Le Mevel A, Dubus C, Berthou C, Colombat P, Milpied N, Marolleau
JP, et al: Late relapse of localized high-grade non-hodgkin's
lymphoma: Clinical and biological features. Blood. 112:2603.
2008.
|
|
7
|
Larouche JF, Berger F, Chassagne-Clément
C, Ffrench M, Callet-Bauchu E, Sebban C, Ghesquières H,
Broussais-Guillaumot F, Salles G and Coiffier B: Lymphoma
recurrence 5 years or later following diffuse large B-cell
lymphoma: Clinical characteristics and outcome. J Clin Oncol.
28:2094–2100. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang J, Medeiros LJ and Young KH: Cancer
immunotherapy in diffuse large B-cell lymphoma. Front Oncol.
10(351)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Martin P: The use of CAR T cells in
diffuse large B-cell lymphoma and mantle cell lymphoma. Clin Adv
Hematol Oncol. 15:247–249. 2017.PubMed/NCBI
|
|
10
|
US Food and Drug Administration: FDA
approves CAR-T cell therapy to treat adults with certain types of
large B-cell lymphoma. Accessed on November 13, 2018 at https://www.fda.gov/news-events/press-announcements/fda-approves-car-t-cell-therapy-treat-adults-certain-types-large-b-cell-lymphoma.
|
|
11
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene ciloleucel CAR T-cell therapy in
refractory large B-cell lymphoma. N Engl J Med. 377:2531–2544.
2017.PubMed/NCBI
|
|
12
|
US Food and Drug Administration: FDA
approves tisagenlecleucel for adults with relapsed or refractory
large B-cell lymphoma. Accessed on November 13, 2018 at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-adults-relapsed-or-refractory-large-b-cell-lymphoma.
|
|
13
|
Schuster SJ, Bishop MR, Tam CS, Waller EK,
Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin
JR, et al: Tisagenlecleucel in adult relapsed or refractory diffuse
large B-cell lymphoma. N Engl J Med. 380:45–56. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Borchmann P, Tam CS, Jäger U, McGuirk JP,
Holte H, Waller EK, Jaglowski SM, Bishop MR, Andreadis C, Foley SR,
et al: An updated analysis of JULIET, a global pivotal Phase
2 trial of tisagenlecleucel in adult patients with relapsed or
refractory (r/r) diffuse large b-cell lymphoma (DLBCL). Presented
at 2018 EHA Congress (abstract S799), 2018. https://library.ehaweb.org/eha/2018/stockholm/214521/peter.borchmann.an.updated.analysis.of.juliet.a.global.pivotal.phase.2.trial.html.
|
|
15
|
Abramson JS, Gordon LI, Palomba ML,
Lunning MA, Arnason JE, Forero-Torres A, Wang M, Maloney DG, Sehgal
A, Andreadis C, et al: Updated safety and long term clinical
outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene
maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol.
36(7505)2018.
|
|
16
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: Explanation and elaboration. Ann Intern Med.
151:W65–W94. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Locke FL, Neelapu SS, Bartlett NL, Siddiqi
T, Chavez JC, Hosing CM, Ghobadi A, Budde LE, Bot A, Rossi JM, et
al: Phase 1 results of ZUMA-1: A multicenter study of KTE-C19
anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol
Ther. 25:285–295. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schuster SJ, Bishop MR, Tam CS, Waller EK,
Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin
JR, et al: Primary analysis of Juliet: A global, pivotal, phase 2
trial of CTL019 in adult patients with relapsed or refractory
diffuse large B-cell lymphoma. Blood. 130(577)2017.
|
|
21
|
Schuster SJ, Bishop MR, Tam C, Waller EK,
Borchmann P, McGuirk J, Jäger U, Jaglowski S, Andreadis C, Westin
J, et al: Global pivotal phase 2 trial of the CD19-targeted therapy
CTL019 in adult patients with relapsed/refractory (R/R) diffuse
large B-cell lymphoma (DLBCL)-an interim analysis. Hematol Oncol.
35(27)2017.
|
|
22
|
Abramson JS, Palomba ML, Gordon LI,
Lunning MA, Arnason JE, Wang M, Forero A, Maloney DG, Albertson T,
Garcia J, et al: High durable CR rates in relapsed/refractory (R/R)
aggressive B-NHL treated with the CD19-directed CAR T cell product
JCAR017 (TRANSCEND NHL 001): Defined composition allows for
dose-finding and definition of pivotal cohort. Blood.
130(58)2017.
|
|
23
|
Schuster SJ, Svoboda J, Chong EA, Nasta
SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V,
Landsburg D, et al: Chimeric antigen receptor T cells in refractory
B-cell lymphomas. N Engl J Med. 377:2545–2554. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Turtle CJ, Hanafi LA, Berger C, Hudecek M,
Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, et
al: Immunotherapy of non-hodgkin's lymphoma with a defined ratio of
CD8+ and CD4+ CD19-specific chimeric antigen
receptor-modified T cells. Sci Transl Med.
8(355ra116)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang Y, Zhang Wy, Han Qw, Liu Y, Dai Hr,
Guo Yl, Bo J, Fan H, Zhang Y, Zhang Yj, et al: Effective response
and delayed toxicities of refractory advanced diffuse large B-cell
lymphoma treated by CD20-directed chimeric antigen
receptor-modified T cells. Clin Immunol. 155:160–175.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang X, Popplewell LL, Wagner JR, Naranjo
A, Blanchard MS, Mott MR, Norris AP, Wong CW, Urak RZ, Chang WC, et
al: Phase 1 studies of central memory-derived CD19 CAR T-cell
therapy following autologous HSCT in patients with B-cell NHL.
Blood. 127:2980–2990. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Brudno JN, Shi V, Stroncek D, Pittaluga S,
Kanakry JA, Curtis LM, Gea-Banacloche JC, Pavletic S, Bagheri MH,
Rose JJ, et al: T cells expressing a novel fully-human anti-CD19
chimeric antigen receptor induce remissions of advanced lymphoma in
a first-in-humans clinical trial. Blood. 128(999)2016.
|
|
28
|
Brudno JN, Somerville RP, Shi V, Rose JJ,
Halverson DC, Fowler DH, Gea-Banacloche JC, Pavletic SZ, Hickstein
DD, Lu TL, et al: Allogeneic T cells that express an anti-CD19
chimeric antigen receptor induce remissions of B-cell malignancies
that progress after allogeneic hematopoietic stem-cell
transplantation without causing graft-versus-host disease. J Clin
Oncol. 34:1112–1121. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kochenderfer JN, Dudley ME, Kassim SH,
Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ,
Hughes MS, Sherry RM, et al: Chemotherapy-Refractory diffuse large
B-cell lymphoma and indolent B-cell malignancies can be effectively
treated with autologous T cells expressing an anti-CD19 chimeric
antigen receptor. J Clin Oncol. 33:540–549. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kochenderfer JN, Somerville RPT, Lu T,
Yang JC, Sherry RM, Feldman SA, McIntyre L, Bot A, Rossi J, Lam N
and Rosenberg SA: Long-Duration complete remissions of diffuse
large B cell lymphoma after anti-CD19 chimeric antigen receptor T
cell therapy. Mol Ther. 25:2245–2253. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kochenderfer JN and Rosenberg SA: Treating
B-cell cancer with T cells expressing anti-CD19 chimeric antigen
receptors. Nat Rev Clin Oncol. 10:267–276. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jensen MC, Popplewell L, Cooper LJ,
DiGiusto D, Kalos M, Ostberg JR and Forman SJ: Antitransgene
rejection responses contribute to attenuated persistence of
adoptively transferred CD20/CD19-specific chimeric antigen receptor
redirected T cells in humans. Biol Blood Marrow Transplant.
16:1245–1256. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Savoldo B, Ramos CA, Liu E, Mims MP,
Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al:
CD28 costimulation improves expansion and persistence of chimeric
antigen receptor-modified T cells in lymphoma patients. J Clin
Invest. 121:1822–1826. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Till BG, Jensen MC, Wang J, Chen EY, Wood
BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, et al:
Adoptive immunotherapy for indolent non-hodgkin lymphoma and mantle
cell lymphoma using genetically modified autologous CD20-specific T
cells. Blood. 112:2261–2271. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Crump M, Neelapu SS, Farooq U, Van Den
Neste E, Kuruvilla J, Westin J, Link BK, Hay A, Cerhan JR, Zhu L,
et al: Outcomes in refractory diffuse large B-cell lymphoma:
Results from the international SCHOLAR-1 study. Blood.
130:1800–1808. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kawalekar OU, O'Connor RS, Fraietta JA,
Guo L, McGettigan SE, Posey AD Jr, Patel PR, Guedan S, Scholler J,
Keith B, et al: Distinct signaling of coreceptors regulates
specific metabolism pathways and impacts memory development in CAR
T cells. Immunity. 44:380–390. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhao Z, Condomines M, van der Stegen SJC,
Perna F, Kloss CC, Gunset G, Plotkin J and Sadelain M: Structural
design of engineered costimulation determines tumor rejection
kinetics and persistence of CAR T cells. Cancer Cell. 28:415–428.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Park JH and Brentjens RJ: Are all chimeric
antigen receptors created equal? J Clin Oncol. 33:651–653.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Siddiqi T, Abramson JS, Palomba ML, Gordon
LI, Lunning MA, Arnason JE, Wang M, Forero-Torres A, Maloney DG,
Heipel M, et al: Correlation of patient characteristics and
biomarkers with clinical outcomes of JCAR017 in R/R aggressive BNHL
(TRANSCEND NHL 001 study). J Clin Oncol. 36(5)2018.
|
|
40
|
Kochenderfer JN, Dudley ME, Feldman SA,
Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes
MS, Sherry RM, et al: B-cell depletion and remissions of malignancy
along with cytokine-associated toxicity in a clinical trial of
anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood.
119:2709–2720. 2012.PubMed/NCBI View Article : Google Scholar
|