Introduction
Breast cancer is one of the most frequent cancers in
women (1). Approximately 80-90% of
invasive breast cancers are invasive ductal carcinomas, and the
most common sites of breast cancer metastasis are the bones, lungs
and liver (2,3).
Invasive lobular carcinoma (ILC) of the breast is
the second most common type of invasive breast cancer, representing
≤10% of all invasive breast cancers (4). ILC mostly metastasizes to the bones,
lungs, and liver, but has been reported to be associated with
higher rates of metastasis to specific sites, such as the
gastrointestinal tract, genitourinary tract, peritoneum,
retroperitoneum, and leptomeninges when compared to invasive ductal
carcinoma (5-7).
The stomach is infrequently reported as a site of
breast cancer metastasis, accounting for 0.1 to 3.5% (8,9).
Metastasis to the stomach generally follows disease extension, and
solitary gastric metastasis is thus relatively rare (10). Due to this rarity, breast cancer
with gastric metastasis is occasionally confused with primary
cancer in the stomach. In addition, patient symptoms due to gastric
metastasis such as indigestion, anorexia, and nausea are
non-specific, and may be explained by other metastases or side
effects of treatments such as chemotherapy (10). Gastric metastases are thus often
overlooked when encountering gastrointestinal symptoms in
patients.
The present report describes a case of isolated
metastasis to the stomach from ILC as a first presentation of
metastasis, identified from computed tomography (CT) in the absence
of symptoms.
Case report
A 45-year-old woman underwent mastectomy with
axillary lymph node dissection after preoperative chemotherapy
(four courses of cyclophosphamide, epirubicin and 5-fluorouracil,
followed by four courses of docetaxel) for an estrogen receptor
(ER)-positive, HER2-negative invasive lobular cancer, T4bN2M0 stage
IIIB (11). Postoperative radiation
therapy with 50 Gy in 25 fractions to supraclavicular lymph nodes
and chest wall was performed and 5 years endocrine therapy with
tamoxifen 20 mg once daily was completed. At 52 years old, CT
performed as postoperative follow-up indicated thickening of the
stomach wall with contrast enhancement (Fig. 1). Although the patient was
asymptomatic, fold thickening with erosion was observed on the
lesser curvature of the upper to lower body on gastroscopy
(Fig. 2). A biopsy specimen
obtained from the gastric lesion contained lobular carcinoma,
morphologically similar to signet ring cell carcinoma, as
frequently seen in primary gastric cancer. Immunohistochemical
examinations (Fig. 3) revealed
positive staining for hormone receptors, mammaglobin and gross
cystic disease fluid protein-15 (GCDFP-15), and negative staining
for E-cadherin. These findings suggested that the gastric lesion
represented a metastasis from the breast cancer. Positron emission
tomography (PET)-CT showed no abnormal accumulations suggestive of
metastasis in other organs. Palbociclib and fulvestrant were
started for the gastric metastasis.
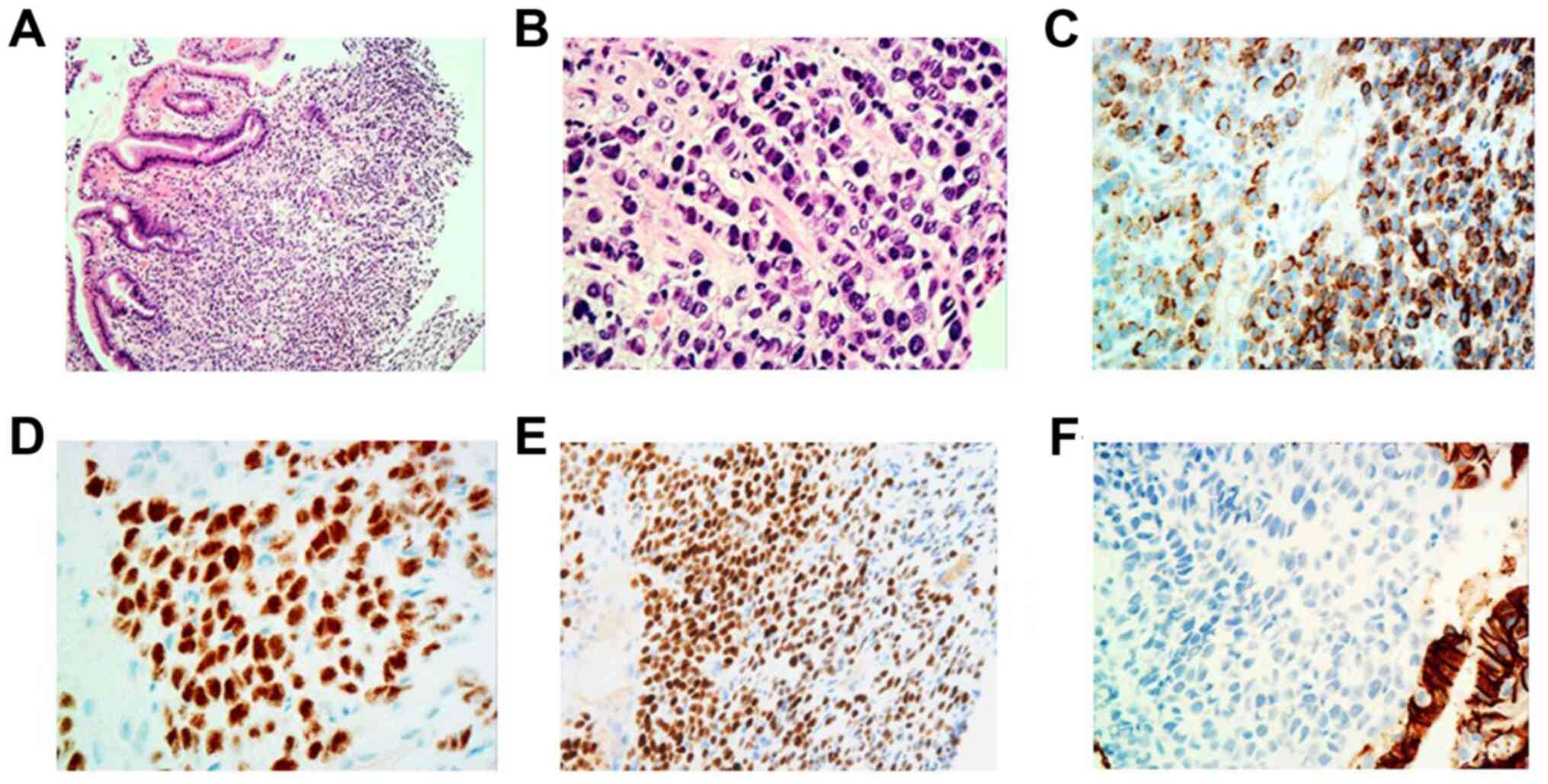 | Figure 3Gastric biopsy images showing
infiltration of undifferentiated neoplasm with a single-file linear
pattern within the gastric mucosa. (A) H&E. Magnification, x40.
(B) H&E. Magnification, x400. Immunohistochemistry revealed
positive staining for GCDFP-15, ER and PR. (C) GCDFP-15.
Magnification, x400. (D) ER, Magnification, x400. (E) PR.
Magnification, x400. Carcinoma cells were immunohistochemically
negative for E-cadherin. (F) E-cadherin. Magnification, x400. ER,
estrogen receptor; GCDFP-15, gross cystic disease fluid protein-15;
H&E, hematoxylin and eosin; PR, progesterone receptor. |
The patient provided written informed consent for
her clinical data to be published in a medical journal.
Discussion
We have presented the case of a patient with ILC in
whom gastric wall thickening was incidentally found on CT in the
absence of symptoms, resulting in a diagnosis of gastric
metastasis. This report emphasizes the importance of a clinical
suspicion of gastric metastasis when abnormal image findings are
detected for the stomach in patients with a history of ILC.
Gastric metastases from breast cancer are rare,
accounting for 0.1 to 3.5% (8,9). Among
invasive breast cancers, ILC is likely to metastasize to the
gastrointestinal tract, including the stomach (12). ILCs are characterized by small,
monomorphic, discohesive tumor cells with little nuclear atypia
(4,13,14).
The growth pattern of ILC with a single cell infiltration pattern
is due to the dysfunction of E-cadherin, a calcium-dependent
transmembrane protein that controls cell-to-cell adhesion and
suppresses tumor invasion and metastasis (15,16).
The lack E-cadherin expression is thus a hallmark of ILC.
Gastric involvement of metastatic breast cancer has
been reported at a mean time of 6-7 years after breast cancer
diagnosis (17). Most gastric
metastases might thus be observed among metastases to multiple
organs. The present case showed this first metastasis at 7 years
after initial treatment. The mechanism of gastric metastasis from
breast cancer with or without multiple organs remains unclear. The
molecular analysis in many cases of gastric metastasis from breast
cancer to clarify this issue should be awaited.
Gastric metastases are often asymptomatic due to
diffuse spread under the mucosal layer, and are often found
incidentally in advanced disease (18). Further, gastrointestinal symptoms
such as indigestion, anorexia, dysphagia, and dyspepsia are
non-specific for gastric metastases from breast cancer, and may be
attributed to other metastases or side effects of other treatments
such as chemotherapy (10,19). Thus, the medical examination by
interview may be unhelpful in arousing suspicion of gastric
metastases. Indeed, our patient showed wide gastric metastasis that
would easily have been overlooked without CT examination, due to
the lack of any gastrointestinal symptoms.
Endoscopic features of gastric metastases are
generally classified into two types: Those resembling submucosal
tumor; and those resembling primary gastric cancer (20). Whereas a submucosal tumor-like
lesion is the most frequent appearance of gastric metastases from
all malignant tumors, gastric metastases from breast cancer mostly
show diffuse infiltration similar to type 4 advanced gastric
cancers (10,19,21). A
‘linitis plastica’ appearance is characterized by the presence of
diffuse infiltration to the submucosal and seromuscular layers of
the stomach by tumor cells, which can cause fibrotic reaction
leading to gastric wall thickening with reduced peristalsis
(21). Radiologically, diffuse
infiltration by tumor cells can be identified as gastric wall
thickening on CT (22). In the
present case, no abnormalities of the gastric mucosa were apparent
on previous CT findings on retrospective comparison, indicating
that CT might have captured the development of metastatic gastric
cancer. In addition, PET-CT has been reported to offer limited
sensitivity in detecting gastric tumors because of physiological
18F-fluorodeoxyglucose (FDG) uptake and involuntary
movements of the stomach (23).
Furthermore, poorly differentiated types are known to show lower
FDG uptake, which is likely affected by the low concentration of
cancer cells, leading to high false-negative rates (23,24).
Concordant with these previous reports, no abnormal uptake was
apparent on FDG-PET in the present case.
Gastric metastasis from ILC has no distinctive
features in terms of cell morphology, invasive pattern, or
endoscopic or radiologic features to facilitate differentiation
from primary diffuse-type gastric cancer (25). In this respect, immunohistochemistry
is the most important evaluation for differentiating gastric
metastases (26). In the present
case, the metastatic breast cancer showed positivity for ER,
progesterone receptor (PR), and GCDFP-15 and negativity for
E-cadherin, whereas primary gastric cancer is typically ER-, PR-,
and GCDFP-15-negative and E-cadherin-positive (27,28).
Expression of ER and PR is influential but not specific for the
diagnosis of breast cancer metastasis. ER expression is relatively
rare in primary gastric cancer. Previous reports have described
finding ER and PR expressions in 10-30% of primary gastric cancers,
but these expressions were generally focal or weak (17,29).
GCDFP-15 is known as an apocrine marker protein identified in
apocrine glands, including those in the breast, sweat glands, and
vulva (30). GCDFP-15 expression
has been found in breast cancer with apocrine metaplasia,
suggesting potential utility as a protein marker for breast cancer
(31). A previous study reported
that sensitivity reached up to 90% in ILCs with signet ring
features, whereas only 55% of breast cancers showed positivity for
GCDFP-15(31). GCDFP-15 expression
is also observed in other primary cancers such as those of the
ovaries or lungs, but adenocarcinomas of the gastrointestinal tract
are almost always negative for GCDFP-15 expression (32). Based on the results of
immunohistochemical staining for these markers, we diagnosed
gastric metastasis of breast cancer in this case.
In conclusion, consideration of gastric metastasis
is important when incidental gastric findings are seen on imaging
with or without symptoms in patients with ILC. Biopsies and
immunohistochemical examination of the gastric lesion are necessary
for accurate diagnosis and optimal treatment selection.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YKa, YKo, and MO analyzed and interpreted the
clinical patient data. YKo and MO drafted the manuscript. YKa
performed the immunohistochemistry examination of the biopsy
samples. KK formulated and designed the study. YD analysed and
interpreted the histopathological data. MO critically revised the
manuscript for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Islami F, Siegel RL, Ward EM and
Jemal A: Global cancer in women: Burden and trends. Cancer
Epidemiol Biomarkers Prev. 26:444–457. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arps DP, Healy P, Zhao L, Kleer CG and
Pang JC: Invasive ductal carcinoma with lobular features: A
comparison study to invasive ductal and invasive lobular carcinomas
of the breast. Breast Cancer Res Treat. 138:719–726.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Siegel RL, Fedewa SA, Miller KD,
Goding-Sauer A, Pinheiro PS, Martinez-Tyson D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2015. CA Cancer J Clin.
65:457–480. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
McCart Reed AE, Kutasovic JR, Lakhani SR
and Simpson PT: Invasive lobular carcinoma of the breast:
Morphology, biomarkers and 'omics. Breast Cancer Res.
17(12)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ferlicot S, Vincent-Salomon A, Médioni J,
Genin P, Rosty C, Sigal-Zafrani B, Fréneaux P, Jouve M, Thiery JP
and Sastre-Garau X: Wide metastatic spreading in infiltrating
lobular carcinoma of the breast. Eur J Cancer. 40:336–341.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Arpino G, Bardou VJ, Clark GM and Elledge
RM: Infiltrating lobular carcinoma of the breast: Tumor
characteristics and clinical outcome. Breast Cancer Res.
6:R149–R156. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
He H, Gonzalez A, Robinson E and Yang WT:
Distant metastatic disease manifestations in infiltrating lobular
carcinoma of the breast. AJR Am J Roentgenol. 202:1140–1148.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
McLemore EC, Pockaj BA, Reynolds C, Gray
RJ, Hernandez JL, Grant CS and Donohue JH: Breast cancer:
Presentation and intervention in women with gastrointestinal
metastasis and carcinomatosis. Ann Surg Oncol. 12:886–894.
2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Oda Kondo H, Yamao T, Saito D, Ono H,
Gotoda T, Yamaguchi H, Yoshida S and Shimoda T: Metastatic tumors
to the stomach: Analysis of 54 patients diagnosed at endoscopy and
347 autopsy cases. Endoscopy. 33:507–510. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Taal BG, Peterse H and Boot H: Clinical
presentation, endoscopic features, and treatment of gastric
metastases from breast carcinoma. Cancer. 89:2214–2221.
2000.PubMed/NCBI
|
|
11
|
Greene FL: Breast tumours. In: TNM
classification of malignant tumours. Sobin LH, Gospodarowicz MK and
Wittekind C (eds). 7th edition. Wiley-Blackwell, Oxford, pp181-193,
2009.
|
|
12
|
Korhonen T, Kuukasjärvi T, Huhtala H,
Alarmo EL, Holli K, Kallioniemi A and Pylkkänen L: The impact of
lobular and ductal breast cancer histology on the metastatic
behavior and long term survival of breast cancer patients. Breast.
22:1119–1124. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Christgen M and Derksen P: Lobular breast
cancer: Molecular basis, mouse and cellular models. Breast Cancer
Res. 17(16)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
de Groot JS, Ratze MA, van Amersfoort M,
Eisemann T, Vlug EJ, Niklaas MT, Chin SF, Caldas C, van Diest PJ,
Jonkers J, et al: αE-catenin is a candidate tumor suppressor for
the development of E-cadherin-expressing lobular-type breast
cancer. J Pathol. 245:456–467. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rakha EA, Patel A, Powe DG, Benhasouna A,
Green AR, Lambros MB, Reis-Filho JS and Ellis IO: Clinical and
biological significance of E-cadherin protein expression in
invasive lobular carcinoma of the breast. Am J Surg Pathol.
34:1472–1479. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Berx G, Cleton-Jansen AM, Nollet F, de
Leeuw WJ, van de Vijver M, Cornelisse C and van Roy F: E-cadherin
is a tumour/invasion suppressor gene mutated in human lobular
breast cancers. EMBO J. 14:6107–6115. 1995.PubMed/NCBI
|
|
17
|
Schwarz RE, Klimstra DS and Turnbull AD:
Metastatic breast cancer masquerading as gastrointestinal primary.
Am J Gastroenterol. 93:111–114. 1998.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ushida Y, Yoshimizu S, Horiuchi Y, Yoshio
T, Ishiyama A, Hirasawa T, Tsuchida T and Fujisaki J:
Clinicopathological features of metastatic gastric tumors
originating from breast cancer: Analysis of eleven cases. World J
Oncol. 9:104–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pectasides D, Psyrri A, Pliarchopoulou K,
Floros T, Papaxoinis G, Skondra M, Papatsibas G, Macheras A,
Athanasas G, Arapantoni-Datioti P and Economopoulos T: Gastric
metastases originating from breast cancer: Report of 8 cases and
review of the literature. Anticancer Res. 29:4759–4763.
2009.PubMed/NCBI
|
|
20
|
Kim GH, Ahn JY, Jung HY, Park YS, Kim MJ,
Choi KD, Lee JH, Choi KS, Kim DH, Lim H, et al: Clinical and
endoscopic features of metastatic tumors in the stomach. Gut Liver.
9:615–622. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
D'Angelo F, Rampini A, Cardella S,
Antolino L, Nigri G, Valabrega S, Aurello P and Ramacciato P:
Breast cancer metastasis to the stomach. J Cancer Metastasis Treat.
5(30)2019.
|
|
22
|
Insko EK, Levine MS, Birnbaum BA and
Jacobs JE: Benign and malignant lesions of the stomach: Evaluation
of CT criteria for differentiation. Radiology. 228:166–171.
2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shimada H, Okazumi S, Koyama M and
Murakami K: Japanese gastric cancer association task force for
research promotion: Clinical utility of 18F-fluoro-2-deoxyglucose
positron emission tomography in gastric cancer. A systematic review
of the literature. Gastric Cancer. 14:13–21. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yun M: Imaging of gastric cancer
metabolism using 18 F-FDG PET/CT. J Gastric Cancer. 14:1–6.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pera M, Riera E, Lopez R, Vinolas N,
Romagosa C and Miquel R: Metastatic carcinoma of the breast
resembling early gastric carcinoma. Mayo Clin Proc. 76:205–207.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Eo WK: Breast cancer metastasis to the
stomach resembling early gastric cancer. Cancer Res Treat.
40:207–210. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
O'Connell FP, Wang HH and Odze RD: Utility
of immunohistochemistry in distinguishing primary adenocarcinomas
from metastatic breast carcinomas in the gastrointestinal tract.
Arch Pathol Lab Med. 129:338–347. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
van Velthuysen ML, Taal BG, van der Hoeven
JJ and Peterse JL: Expression of oestrogen receptor and loss of
E-cadherin are diagnostic for gastric metastasis of breast
carcinoma. Histopathology. 46:153–157. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Koike K, Kitahara K, Higaki M, Urata M,
Yamazaki F and Noshiro H: Clinicopathological features of gastric
metastasis from breast cancer in three cases. Breast Cancer.
21:629–634. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Viacava P, Naccarato AG and Bevilacqua G:
Spectrum of GCDFP-15 expression in human fetal and adult normal
tissues. Virchows Arch. 432:255–260. 1998.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mazoujian G, Bodian C, Haagensen DE Jr and
Haagensen CD: Expression of GCDFP-15 in breast carcinomas.
Relationship to pathologic and clinical factors. Cancer.
63:2156–2161. 1989.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gown AM, Fulton RS and Kandalaft PL:
Markers of metastatic carcinoma of breast origin. Histopathology.
68:86–95. 2016.PubMed/NCBI View Article : Google Scholar
|

















