Introduction
Granulocyte colony-stimulating factor
(G-CSF)-producing tumors of the lungs and head and neck region are
rare, and they are associated with a poor prognosis (1,2);
however, the systemic complications due to the cytokines produced
by the tumor are not well known. The adverse events of recombinant
human G-CSF (rhG-CSF) administered to mobilize peripheral blood
stem cells include spinal bone pain and osteoporosis (with
vertebral fractures in severe cases), myocardial infarction, stroke
and splenomegaly (with splenic rupture in severe cases). It has
been reported that G-CSF signaling induces osteoporosis by
activating osteoclasts through suppression of osteoblast activity
(3-6).
Furthermore, the abnormal accumulation of fluorodeoxyglucose (FDG)
in the red bone marrow on positron emission tomography/computed
tomography (PET/CT) is attributed to the enhancement of glucose
metabolism by the increased hematopoietic capacity of the
granulocyte system of the bone marrow induced by G-CSF (7-9).
Although several cases of solid cancers, such as lung cancer and
head and neck cancer, that produce G-CSF with abnormal FDG
accumulation on PET/CT have been published, no systemic
complications have been reported to date (10,11). A
case of a suspected pathological fracture of a lumbar vertebra and
splenomegaly associated with G-CSF cytokine production by maxillary
sinus squamous cell carcinoma (SCC) is described herein. This
mechanism is similar to the clinical conditions when high-dose
rhG-CSF is administered. Therefore, the aim of the present study
was to further examine the hypothesis that cytokines produced by
solid tumors may induce vertebral fractures and splenomegaly.
Case report
A 73-year-old Japanese woman was referred to the
Kochi Medical School Hospital (Kochi, Japan) in August 2018, with a
complaint of painful swelling on the right side of the maxillary
gingiva for the last month. The patient's history included
osteoporosis, for which she received conservative treatment
following a fracture at the 3rd lumbar vertebra (L3) in 2008, and
had been receiving risedronate hydrate (17.5 mg/week) since 2014.
Her family history was unremarkable. On a regular medical
examination by a local internal medicine specialist, the patient
was found to have an increased white blood cell (WBC) count
(50,100/µl; normal range: 3,300-8,600/µl) and elevated C-reactive
protein (CRP) levels (3.98 mg/dl; normal value: ≤0.14 mg/dl).
The initial examination revealed a painful swelling
with redness extending from the gingival-cheek junction on the
right side of the maxilla to the palate, and maxillary CT
examination revealed a uniform low-density area occupying the right
maxillary sinus (Fig. 1A and
B). Biopsy was performed under the
provisional diagnosis of a maxillary sinus tumor. The
histopathological diagnosis was poorly differentiated SCC, and
immunohistochemical staining for anti-G-CSF antibody was positive
(Fig. 1C and D). Based on these results, the patient was
diagnosed with a G-CSF-producing SCC of the maxillary sinus (TNM
classification: cT3N0Mx). Chemoradiotherapy was selected as the
treatment strategy, avoiding extended maxillary resection. After
the biopsy, the WBC count decreased (9,800/µl), but it increased
again 1 month later (125,300/µl), so concomitant superselective
intra-arterial infusion chemoradiotherapy (CCRT) was scheduled
(Fig. 2; Table I). FDG-PET/CT conducted at the same
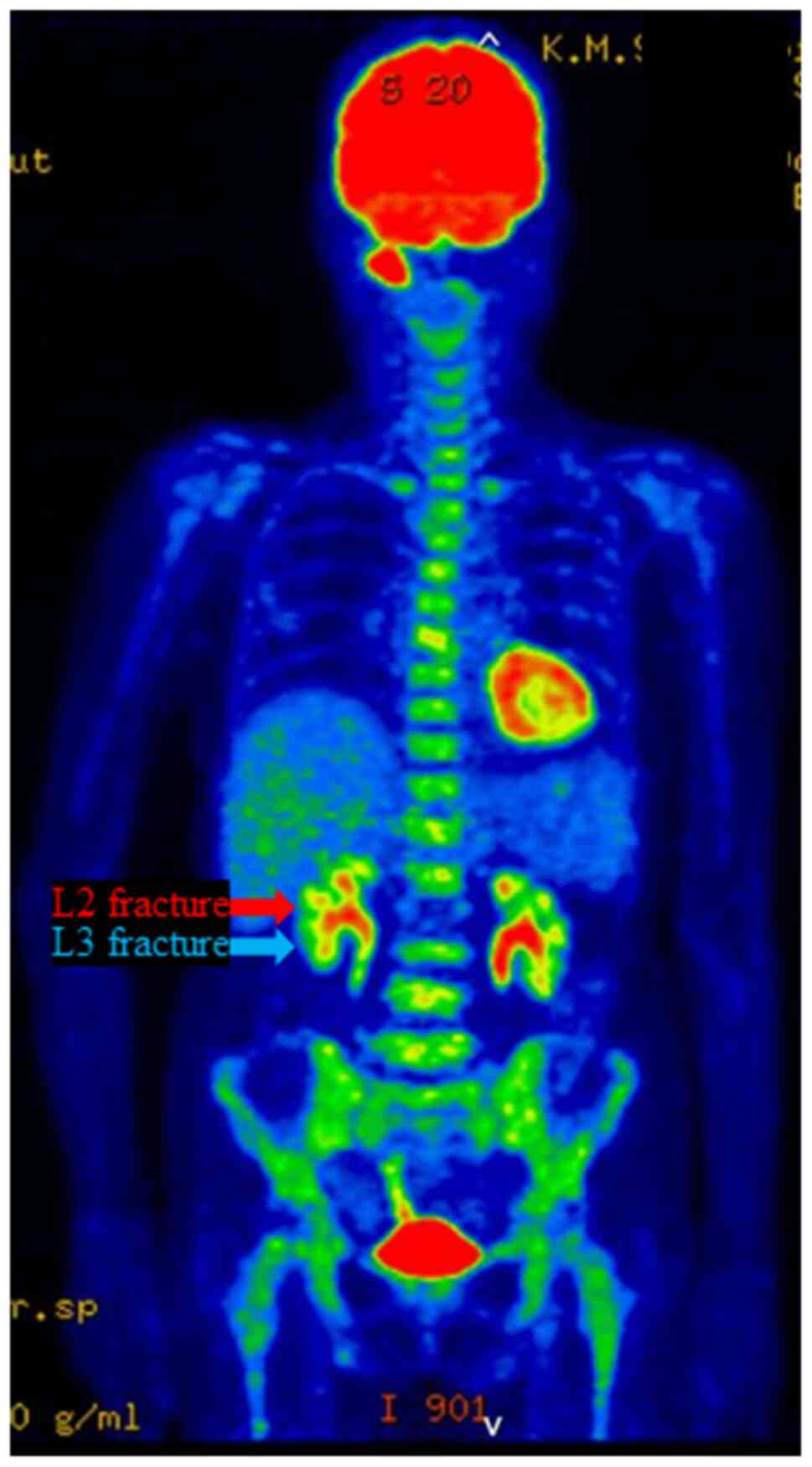
time showed no evidence of distant metastasis in the lungs or
liver, but revealed diffuse abnormal accumulation of FDG in the red
bone marrow of the whole body, including the spine (Fig. 3). Three days later, the patient
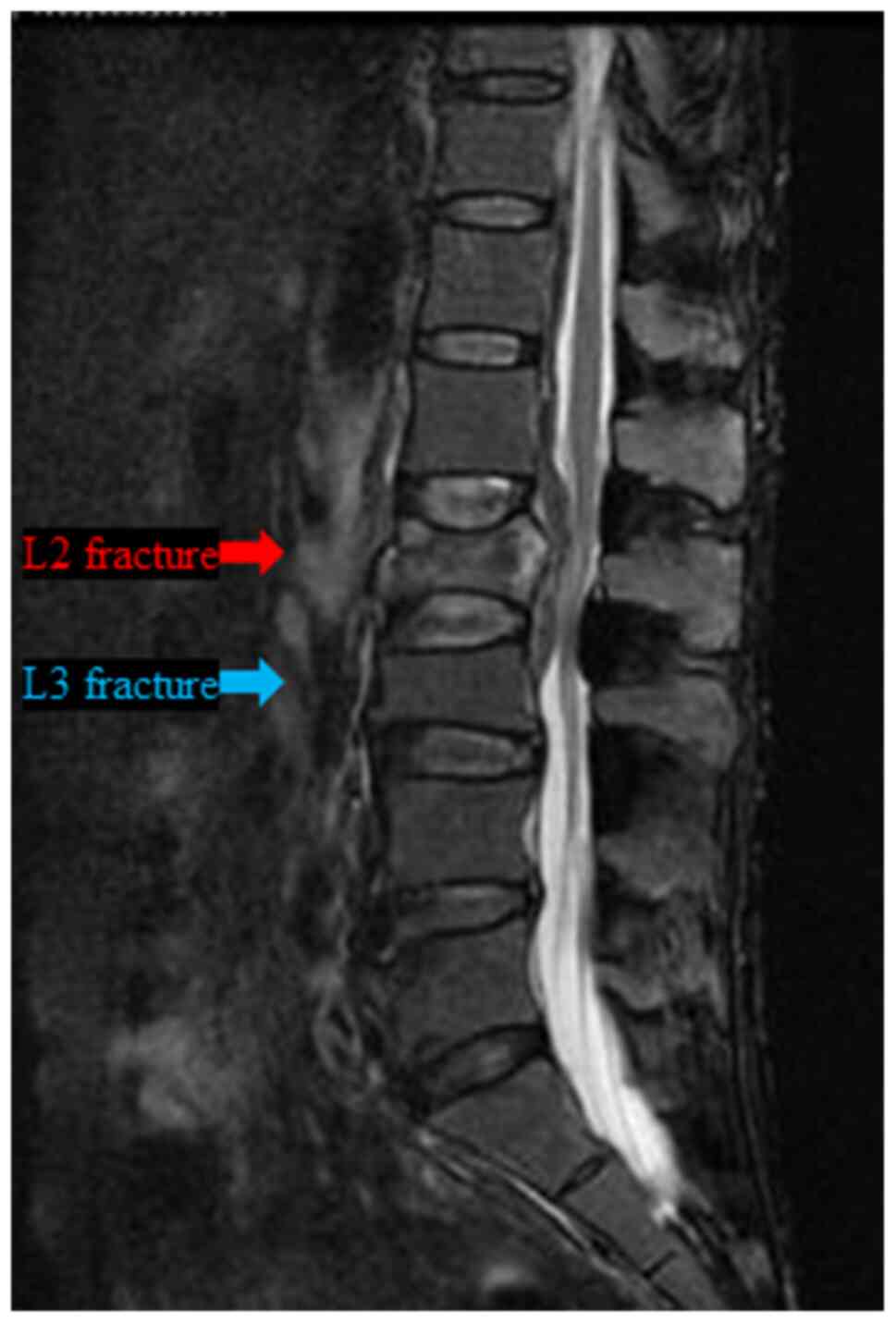
suddenly complained of severe lower back pain, and lumbar MRI
examination revealed a burst fracture of the L2 vertebra, but no
obvious distant bone metastasis was observed (Fig. 4). Subsequently, the patient was
treated conservatively with a hard brace for 1 month, while CCRT
(cisplatin: Total 650 mg; RT: 60 Gy) was performed for the
maxillary sinus cancer, and the WBC count decreased significantly
(4,400/µl at 1.5 months post-CCRT).
 | Table ILaboratory findings. |
Table I
Laboratory findings.
| | Year |
|---|
| | 2018 | 2019 | 2020 |
|---|
| Findings | September
(Pre-biopsy) | October
(Post-biopsy) | November
(Pre-CCRT) | December | January
(Post-CCRT) | September
(Pre-BNCT) | October
(Post-BNCT) | September (BSC) |
|---|
| WBC count
(x1,000/µl) | 71.1 | 9.8 | 125.3 | 64.1 | 5.3 | 22.6 | 8.6 | 131.7 |
| G-CSF (pg/ml) | 1,250 | 149 | 2,290 | 1,330 | 65.8 | 480 | 48.7 | 1,010 |
| ALP (U/l) | 1,097 | 483 | 2,339 | 1,571 | 449 | 546 | 334 | 1,232 |
| CRP (mg/dl) | 6.5 | 2.37 | 8.91 | 5.45 | 0.68 | 4.46 | 1.19 | 13.92 |
| SCC Ag (ng/ml) | NA | 4.8 | 25.8 | 3.2 | 1.6 | 2.4 | 1.7 | 30.9 |
| BAP (µg/ml) | NA | 17.3 | 75 | 30.7 | NA | NA | NA | NA |
| Ca (mg/dl) | 9 | 7.9 | 8.9 | 9 | 8.9 | 9.2 | 8.5 | 8.8 |
No evidence of spinal metastasis was found for ~2
years, from the time of the L2 fracture to the diagnosis of
terminal disease (Fig. 5). During
that period, the patient underwent boron-neutron capture therapy
(BNCT) for recurrence of the primary lesion, selective neck
dissection for late neck lymph node metastasis, and nivolumab
immunotherapy for distant lung metastasis for ~6 months (Fig. 2). However, due to the elevated WBC
count (131,700/µl; Fig. 2; Table I), onset of splenomegaly (Fig. 6), decreased performance status,
uncontrolled primary tumor and the patient's wishes, she switched
to best supportive care (BSC) and she succumbed to the disease 2
years and 2 months after the initial visit.
Discussion
G-CSF-producing tumors are associated with a poor
prognosis, with a mean survival of <1 year (1,2). In
the present case, however, the patient survived for >2 years, as
she was able to maintain stable disease with CCRT, BNCT, neck
dissection and nivolumab immunotherapy. The patient was also
diagnosed with metastatic cervical lymph nodes and distant lung
metastases, complained of severe pain at the site of cervical
metastasis and requested resection. Therefore, selective neck
dissection was performed prior to nivolumab immunotherapy. For head
and neck cancer, when distant metastases are identified, various
treatment strategies, including BSC, may be selected, taking into
consideration the patient's quality of life (12). Selective neck dissection prior to
nivolumab immunotherapy has helped maintain the quality of life of
the patients, and our approach was among the treatments considered
as standard. During the treatment period, the patient's WBC count,
serum G-CSF and alkaline phosphatase (ALP) levels followed the same
trend, and extremely high values were associated with the
occurrence of two systemic complications, namely vertebral fracture
and splenomegaly.
The pathological burst fracture of a lumbar vertebra
was inferred to be the consequence of G-CSF produced by the
maxillary sinus SCC in this elderly patient with osteoporosis,
rather than bone metastasis, for the following reasons: First,
lumbar MRI of the vertebral fracture (Fig. 4) and FDG-PET/CT examination
performed 8 months after the L2 vertebral fracture (upon recurrence
of the primary lesion; Fig. 5)
revealed no findings suggesting bone metastasis in L2. Furthermore,
the L2 vertebral fracture was not of the compression, but rather of
the burst type (Fig. 4). Second,
FDG-PET/CT performed a few days before the L2 vertebral fracture
revealed abnormal diffuse accumulation of FDG in the red bone
marrow of the whole body (Fig. 3).
These findings are often seen in cases of hematopoietic tumors such
as leukemia, malignant lymphoma and multiple myeloma, which are
associated with complications such as bone pain and vertebral
fractures (7-9).
Finally, the patient's WBC count, serum G-CSF, ALP, CRP levels, SCC
antigen titer and bone ALP increased sharply at the time of the L2
vertebral fracture (Fig. 2;
Table I).
Although the serum Ca level was slightly low (7.9
mg/dl; normal range: 8.8-10.1 mg/dl) after tumor extirpation (prior
to the L2 vertebral fracture), no significant changes were observed
throughout the treatment period (Table
I).
The mechanism by which G-CSF causes fractures is
known to be activation of osteoclasts through suppression of
osteoblast activity, and there are some reports that bone mineral
loss is caused in pediatric patients with congenital neutropenia
and hematopoietic tumors (3-6).
Furthermore, the abnormal accumulation of FDG in the red bone
marrow of the whole body on PET/CT is considered to be due to the
enhancement of glucose metabolism caused by the increased
hematopoietic activity of the granulocyte system of the bone marrow
induced by G-CSF. These FDG-PET findings have been reported in
several cases of solid tumors, such as lung cancer and head and
neck cancer, that produce G-CSF (10,11),
but no spine-related complications have been reported to date. In a
case previously reported by Kuroshima et al (11), FDG-PET/CT revealed an L4 vertebral
fracture during the treatment period, although there has been no
mention of this adverse event in the literature to date; this may
have been a vertebral fracture that occurred through the same
mechanism as that described in the present study. However, a search
through the literature using the PubMed engine revealed no other
reports suggesting that G-CSF produced by solid tumors was
associated with vertebral fractures.
On the other hand, splenomegaly and splenic
infarction have been reported to occur in association with
hematological malignancies, infective endocarditis and atrial
fibrillation. It is considered that the administration of rhG-CSF
preparations may cause splenic rupture or infarction, as platelet
aggregation through the recruitment of hematopoietic stem cells
into the peripheral blood causes infarction of the intra-splenic
vessels (13,14). According to Khinji and Linenberger
(14), of 2,992 cases of adverse
events linked to rhG-CSF preparations, 15 (0.5%) included
splenomegaly; in addition, Aubrey-Bassler and Sowers (15) reported that, of 613 cases of splenic
rupture, 10 (1.6%) were associated with rhG-CSF preparations. There
are several case reports of splenic rupture and infarction when
rhG-CSF preparations were administered to patients with cancer for
the purpose of treating or preventing myelosuppression by
chemotherapy and to healthy donors prior to stem cell
transplantation (16-19).
However, the PubMed search revealed no reports of splenomegaly
hypothetically associated with G-CSF produced by solid tumors,
other than the present case and a previously reported case by de
Wolff et al (20). The study
of de Wolff et al did not report the serum G-CSF levels over
time, and there was no evidence that metastatic melanoma produced
G-CSF. The authors suspected that melanoma was producing G-CSF as
the cause of leukocytosis. Moreover, in their study, the findings
of chest and abdominal CT examination suggested lung, liver and
spleen metastases; therefore, they did not associate
melanoma-produced G-CSF with splenomegaly, unlike our report.
In addition, other previous reports indicated that
G-CSF can upregulate the expression of MMPs and can induce cancer
cell invasion and metastasis (21-23).
Therefore, in the present case, G-CSF-related complications may
include not only vertebral fracture and splenomegaly, but also lung
metastasis.
Finally, the findings of this case report indicated
that G-CSF produced by head and neck cancer may promote the
occurrence of vertebral fracture and splenomegaly. Therefore, serum
G-CSF produced by tumor cells may be associated with various
systemic complications.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NK, SS, KK and KN treated the patient, analyzed the
raw data and wrote the manuscript; ES, MD and TY reviewed and
edited the manuscript. All the authors were involved in the
preparation of the manuscript and have approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by our institutional
Ethics Committee and written informed consent was obtained from the
patient.
Patient consent for publication
The patient and her family have provided their
written informed consent to the publication of the case details and
associated images, provided the patient's anonymity is
protected.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaneko N, Kawano S, Matsubara R, Goto Y,
Jinno T, Maruse Y, Sakamoto T, Hashiguchi Y, Iida M and Nakamura S:
Tongue squamous cell carcinoma producing both parathyroid
hormone-related protein and granulocyte colony-stimulating factor:
A case report and literature review. World J Surg Oncol.
14(161)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Asano S, Urabe A, Okabe T, Sato N and
Kondo Y: Demonstration of granulopoietic factor(s) in the plasma of
nude mice transplanted with a human lung cancer and in the tumor
tissue. Blood. 49:845–852. 1977.PubMed/NCBI
|
|
3
|
Yakisan E, Schirg E, Zeidler C, Bishop NJ,
Reiter A, Hirt A, Riehm H and Welte K: High incidence of
significant bone loss in patients with severe congenital
neutropenia (Kostmann's syndrome). J Pediatr. 131:592–597.
1997.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Takamatsu Y, Simmons PJ, Moore RJ, Morris
HA, To LB and Lévesque JP: Osteoclast-mediated bone resorption is
stimulated during short-term administration of granulocyte
colony-stimulating factor but is not responsible for hematopoietic
progenitor cell mobilization. Blood. 92:3465–3473. 1998.PubMed/NCBI
|
|
5
|
Sidan L, Tianshou L, Yongbing C, Yinchao
N, Changhong L, Lanting L, Qiaochuan L and Lugui Q: Granulocyte
colony-stimulating factor induces osteoblast inhibition by B
lymphocytes and osteoclast activation by T lymphocytes during
hematopoietic stem/progenitor cell mobilization. Biol Blood Marrow
Transplant. 21:1384–1391. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Turhan AB, Binay C, Bor O and Simsek E:
The effects of short-term use of granulocyte colony-stimulating
factor on bone metabolism in child cancer patients. Norh Clin
Istanb. 5:277–281. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Goshen E, Tima Davidson T, Yeshurun M and
Zwas ST: Combined increased and decreased skeletal uptake of F-18
FDG. Clin Nucl Med. 31:520–522. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Christopher MJ and Link DC: Granulocyte
colony-stimulating factor induces osteoblast apoptosis and inhibits
osteoblast differentiation. J Bone Miner Res. 23:1765–1774.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kusumahstuti KP, Watabe T, Kitamura N and
Yamamoto T: Diffuse bone marrow uptake related to granulocyte
colony-stimulating factor-producing maxillary sinus carcinoma on
4-borono-2-18F-fluoro-L-phenylalanine positron emission
tomography/computed tomography. World J Nucl Med. 20:188–191.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Morooka M, Kubota K, Murata Y, Shibuya H,
Ito K, Mochizuki M, Akashi T, Chiba T, Nomura T, Ito H and Morita
T: (18)F-FDG-PET/CT findings of granulocyte colony stimulating
factor (G-CSF)-producing lung tumors. Ann Nucl Med. 22:635–639.
2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kuroshima T, Wada M, Sato T, Takano M and
Makino S: G-CSF producing oral carcinoma with diffuse uptake of FDG
in the bone marrow: A case report. Oncol Lett. 15:1241–1245.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pfister DG, Spencer S, Adelstein D, Adkins
D, Anzai Y, Brizel DM, Bruce JY, Busse PM, Caudell JJ, Cmelak AJ,
et al: Head and Neck Cancers, Version 2.2020, NCCN Clinical
Practice Guidelines in Oncology. J Natl Compr Canc Netw.
18:873–898. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Picardi M, Rosa GD, Selleri C, Scarpato N,
Soscia E, Martinelli V, Ciancia R and Rotoli B: Spleen enlargement
following recombinant human granulocyte colony-stimulating factor
administration for peripheral blood stem cell mobilization.
Haematologica. 88:794–800. 2003.PubMed/NCBI
|
|
14
|
Al-Khinji A and Linenberger M: Splenic
infarction and G-CSF. Transfusion. 55(708)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Aubrey-Bassler FK and Sowers N: 613 cases
of splenic rupture without risk factors or previously diagnosed
disease: A systematic review. BMC Emerg Med. 12(11)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alshamrani MA, Al-Foheidi M and Abdulrahim
AH: Granulocyte colony stimulating factor (G-CSF) induced splenic
infarction in breast cancer patient treated with dose-dense
chemotherapy regimen. Case Rep Oncol Med. 8174986:2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Stroncek D, Shawker T, Follmann D and
Leitman SF: G-CSF-induced spleen size changes in peripheral blood
progenitor cell donors. Transfusion. 43:609–613. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pitini V, Ciccolo A, Arrigo C, Aloi G,
Micali C and Torre FL: Spontaneous rupture of spleen during
periferal blood stem cell mobilization in a patient with breast
cancer. Haematologica. 85:559–560. 2000.PubMed/NCBI
|
|
19
|
Masood N, Shaikh AJ, Memon WA and Idress
R: Splenic rupture, secondary to G-CSF use for chemotherapy induced
neutropenia: A case report and review of literature. Cases J.
24(418)2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
de Wolff JF, Planken EV and den Ottolander
GJ: Extreme leucocytosis and splenomegaly in metastasized melanoma.
Neth J Med. 62:164–167. 2004.PubMed/NCBI
|
|
21
|
Tsuruta N, Yatsunami J, Takayama K,
Nakanishi Y, Ichinose Y and Hara N: Granulocyte-macrophage-colony
stimulating factor stimulates tumor invasiveness in squamous cell
lung carcinoma. Cancer. 82:2173–2183. 1998.PubMed/NCBI
|
|
22
|
Tomita T, Fujii M, Tokumaru Y, Imanishi Y,
Kanke M, Yamashita T, Ishiguro R, Kanzaki J, Kameyama K and Otani
Y: Granulocyte-macrophage colony-stimulating factor upregulates
matrix metalloproteinase-2 (MMP-2) and membrane type-1 MMP
(MT1-MMP) in human head and neck cancer cells. Cancer Lett.
156:83–91. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sugimoto C, Fujieda S, Sunaga H, Noda I,
Tanaka N, Kimura Y, Saito H and Matsukawa S: Granulocyte
colony-stimulating factor (G-CSF)-mediated signaling regulates type
IV collagenase activity in human head and neck cancer cells. Int J
Cancer. 93:42–46. 2001.PubMed/NCBI View
Article : Google Scholar
|