Introduction
The Third International Consensus Definitions for
Sepsis was released in 2016 (Sepsis 3.0) (1). Sepsis is defined as a dysfunctional
host response to infection and life-threatening organ dysfunction,
which can lead to septic shock, multiple organ failure and death.
Sepsis is a common systemic infection in intensive care units. An
international study showed that the hospital mortality rate of
sepsis was 17% and that of severe sepsis was 26%; sepsis kills ~5.3
million people/year (2). Because of
its high morbidity and mortality, this disease attracts worldwide
medical attention.
Growing research has improved understanding of the
pathogenesis of sepsis (3-5).
The pathophysiological process of sepsis is complex and includes
inflammation, immune and coagulation functions and changes in cell
function, metabolism and microcirculation (6); the most important of these is the
immune mechanism (7). Immune
regulation affects the prognosis of patients with sepsis (8). The immunoregulation mechanism serves a
key role in early hyperimmune responses, such as systemic
inflammatory response syndrome, excessive release of inflammatory
factors, late immunosuppression and T lymphocytes (9).
MicroRNAs (miRs) are important in regulation of
post-transcriptional gene expression, especially regulation of cell
apoptosis and proliferation (10).
In recent years, miRs have been shown to regulate signaling
pathways, inflammation and immune cells, such as T lymphocytes
(10,11), in sepsis. miR-126 is an important
member of the miR family and is expressed in endothelial cells of
blood vessels, as well as the heart, lung and other tissue. miR-126
inhibits the development of the T helper (Th)2-specific immune
response and regulates differentiation of T lymphocytes in the
direction of Th2 or T regulatory cells (Tregs) (12). It has also been found that miR-126
enhances activation of T lymphocytes by upregulating the insulin
receptor substrate-1 pathway (13).
miR-21 exhibits antiapoptotic effects in cancer. For example,
overexpression of miR-21 decreases 5-fluorouracil-induced apoptosis
and necrosis of non-small cell lung cancer cells (14), however, further investigation is
required to determine its effect on the immune system. Certain
studies have shown that miR-21 inhibits apoptosis of activated T
cells (15,16). miR-21 mediates the interaction
between Treg and endothelial cells via inducible T cell
co-stimulator (ICOS) and ICOS ligand in B lymphocytes (17). Similarly, a study has shown that
miR-21 affects the differentiation of CD4+ and
CD8+ T cell subsets (18).
The present study aimed to investigate the effects
of miR-126 and miR-21 on sepsis immune response, apoptosis of T
lymphocytes and release of inflammatory factors. The expression
levels of miR-126 and miR-21 and the apoptotic rate of T
lymphocytes were observed and the association between these factors
was analyzed.
Materials and methods
Sepsis model and groups
The experimental rats (weight, 200-250 g; age, 8
weeks) were provided by the Experimental Animal Center of Bengbu
Medical College (Anhui, China). Rats were maintained at 20-25˚C,
50-65% humidity, 14/10-h light/dark cycle and free access to food
and water. A total of 48 male Sprague-Dawley rats were divided into
6 groups: Normal control (NC) and sepsis (0, 12, 24, 48 and 72 h;
n=8/group). NC rats received intraperitoneal injection with 0.9%
saline (10 ml/kg); experimental rats were used to construct a model
of sepsis and received intraperitoneal injection with
lipopolysaccharide (LPS; 15 mg/kg) . Peripheral blood (100 µl) was
taken at each time point (at 0, 12, 24, 48, 72 h).
Lymphocyte isolation
Lymphocytes were separated by density gradient
centrifugation. Rat lymphocyte isolation solution (Sigma-Aldrich;
MercK KGaA) was used and the samples were centrifuged by horizontal
rotor centrifuge (4˚C, 450 x g, 25 min). The centrifuged liquid was
separated into four layers. The lymphocyte layers were carefully
collected by suction tube and centrifuged (4˚C, 300 x g, 10 min).
The lymphocyte layers were centrifuged (4˚C, 250 x g, 10 min) and
the supernatant was discarded. The lymphocyte pellet was collected
for subsequent analysis.
T cell counting
T cell apoptosis was detected by mixing lymphocytes
(1x106 cells/ml) with 400 µl Annexin V binding solution.
Then, 5 µl Annexin V-FITC (cat. no. 40302-A; Shanghai Yeasen
Biotechnology Co., Ltd.) was added to the T cell suspension, gently
mixed and incubated at 2-8˚C for 15 min After adding 5-10 µl PI dye
solution (cat. no. 40302-B; Shanghai Yeasen Biotechnology Co.,
Ltd.), gently mixing and incubating at 2-8˚C for 5 min, T cells
were counted by flow cytometry (FACS Calibur; BD Biosciences) and
analyzed by software (FlowJo V10.0; BD Biosciences). The collected
lymphocytes were resuspended in 2-5 ml cold ethanol and then fixed
with 1X Binding Buffer (cat. no. 40302-C; Shanghai Yeasen
Biotechnology Co., Ltd.) for 1 h at -20˚C. Cells were collected by
centrifugation (4˚C, 1,000 x g, 10 min). Cells were resuspended in
PBS and RNase A solution, then immersed in a water bath at 37˚C (30
min). Cells were collected by centrifugation (4˚C, 1,000 x g, 10
min). The lymphocytes were resuspended in PI dye solution and
incubated at 4˚C (30 min) without light. The results were detected
by flow cytometry (FACSCalibur; BD Biosciences).
Caspase-3 activity detection
Total protein of lymphocytes was extracted by
protein extraction kit (cat. no. SD-001; Invent Biotechnologies,
Inc.) and collected by centrifugation (4˚C, 16,000 x g, 30 sec).
After extracting total protein from the collected lymphocytes,
caspase-3 reaction buffer containing fluorescent substrates was
added into the control and sample well. The fluorescence intensity
was analyzed by fluorescence spectrophotometer (Qubit Flex; Thermo
Fisher Scientific, Inc.) immediately after adding the sample. The
fluorescence intensity was measured every 10 min. The monitoring
time was 120 min and the detection temperature was 37˚C. The
observed fluorescence intensity was caspase-3 activity.
Western blot analysis
Lymphocyte proteins were extracted from the
collected lymphocytes by protein extraction kit (cat. no. SD-001;
Invent Biotechnologies, Inc.). BCA protein assay kit was used to
determine protein concentration and 50 µg protein/lane was sampled.
A 5X SDS buffer solution was added for electrophoresis. The
starting voltage was 60 V (5% concentrated gel). When the strip ran
out of the gel concentrate, the voltage was increased to 120 V (12%
separated gel) for the transmembrane. The transmembrane current was
250 mA. After the membrane was washed with TBST (0.05% Tween-20)
for 1-2 min, the antigen was blocked. The PVDF membrane was removed
and placed in blocking solution (5% skimmed milk) and shaken gently
for 1 h on a shaker at room temperature. Primary antibodies
(diluted with 5% skimmed milk) were as follows: Caspase-3 (1:2,000;
cat. no. ab184787; Abcam)and GAPDH (1:3,000; cat. no. ab125247;
Abcam). Shock incubation at 37˚C overnight followed by incubation
at 4˚C for 2 h was performed. Then, the membrane was washed with
TBST (0.05% Tween-20) three times times on a shaker (10 min each)
and the secondary antibody [horseradish peroxidase (HRP)-conjugated
goat anti rabbit IgG; cat. no. KGAA35; Jiangsu Kaiji Biotechnology
Co., Ltd.] was added at 25˚C for 1 h. The antibody was diluted with
5% skimmed milk at 1:3,000, then added to the membrane. The
membrane was washed with TBST three times (10 min each). Finally,
the exposure was developed by chemiluminescence (G:BOX chemiXR5;
Syngene Europe) and analyzed (Gel-Pro32 software; Media
Cybernetics, Inc.).
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Then, 0.5 µg RNA was
subjected to RT using ReverTra Ace qPCR RT Master Mix with gDNA
Remover (Toyobo Life Science) as follows: 37˚C for 15 min, 85˚C for
5 sec and 4˚C. Relative RNA expression quantitation was performed
using SYBR Premix EX Taq (Takara Bio, Inc.) according to the
manufacturer's protocol and the 2-ΔΔCq method (19). Primer sequences used for RT-qPCR
were as follows: MiR-126, forward, 5'-CGCGTCGTACCGTGAGTAAT-3' and
reverse, 5'-AGTGCAGGGTCCGAGGTATT-3'; miR-21 forward,
5'-CGCAACAGCAGTCGATGG-3' and reverse, 5'-AGTGCAGGGTCCGAGGTATT-3'
and U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'. U6 was used as an internal control for
miRNA Total RNA from collected lymphocytes was extracted using an
RNA kit (cat. no. DP501; Tiangen Biotech Co., Ltd.). RNA
concentration was determined using a spectrophotometer (NanoDrop
1000). The levels of miR-126 and miR-21 was determined by using
fluorescence qPCR system (cat. no. 7900HT; Applied Biosystems;
Thermo Fisher Scientific, Inc.) with the following thermocycling
conditions: 95˚C for 5 min, followed by 40 cycles of 95˚C for 10
sec, 60˚C for 30 sec and 95˚C for 15 sec, then 60˚C for 1 min.
ELISA
The blank, standard and sample wells were selected
and the blank well was not sampled. Different concentrations of 50
µl standard product and 100 µl horseradish peroxidase-labeled TNF-α
or IL-6 antibody (cat. nos. RJ16622 and RJ15478, respectively; both
Shanghai Renjie Biological Technology Co, Ltd.) were added to
standard wells. The sample wells were filled with 10 µl sample, 40
µl sample diluent and 100 µl HRP-labeled antibody. The plate was
incubated at 37˚C (60 min), then the detergent was shaken off and
the plate was patted dry and washed five times. Following addition
of chromogenic solution, the plate was incubated at 37˚C (15 min).
Next, 50 µl terminating solution was added to terminate the
reaction. The absorbance [optical density (OD) value] of each well
was measured at 450 nm. The standard curve was drawn according to
the concentration of the standard sample and the corresponding OD
value and the concentration of samples was calculated by regression
equation according to the OD value of each sample.
Statistical analysis
Data were statistical analyzed by SPSS 24.0 (IBM
Corp.) and are presented as the mean ± SD of 3-6 independent
repeats. Comparison between two groups were performed by one-way
ANOVA with post hoc Bonferroni's correction or paired Student's
t-test. Correlation analysis was evaluated via the Pearson method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Release of inflammatory factors
increases in septic rats
Levels of TNF-α and IL-6 secreted in the NC and
sepsis groups were measured at different time points. TNF-α and
IL-6 release in the sepsis groups peaked at 12 h, then decreased.
There was statistical significance in TNF-α and IL-6 secretion
between the two groups (Fig.
1).
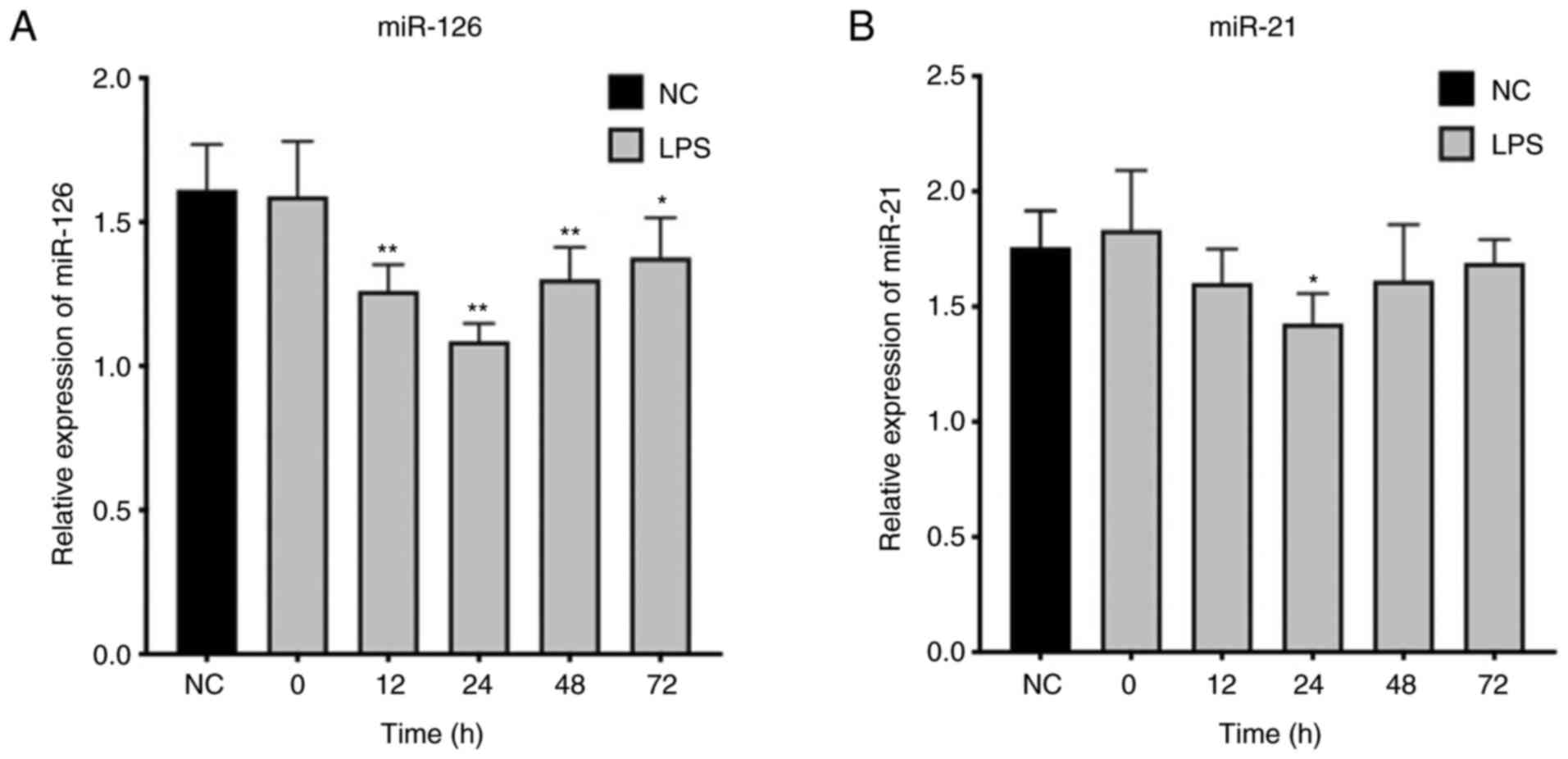
miR-126 and miR-21 expression is
downregulated in T lymphocytes of septic rats
Expression levels of miR-126 and miR-21 in T
lymphocytes in the NC and sepsis groups were compared. Expression
of miR-126 and miR-21 in the sepsis groups were significantly below
that in the NC group (Fig. 2).
Expression of miR-126 in T lymphocytes in the sepsis
groups initially decreased and then increased slowly after 24 h.
These changes were statistically significant (Fig. 3A). Similarly, expression of miR-21
initially decreased and then increased slowly after 24 h. Compared
with the NC group, miR-21 was only significantly different in the
sepsis groups at 24 h; differences were not statistically
significant at 12, 48 and 72 h (Fig.
3B).
T lymphocyte apoptosis increases in
septic rats
T cell counting showed that apoptosis of T
lymphocytes significantly increased up to 48 h, then decreased in
the sepsis groups (Fig. 4).
Expression and activity of caspase-3
in T lymphocytes is elevated in septic rats
Expression of caspase-3 in T lymphocytes in the
sepsis groups significantly increased up to 48 h and then decreased
(Fig. 5A and B). The change in caspase-3 activity was
consistent with that of its expression levels (Fig. 5C).
Expression of miR-126 is positively
correlated with that of miR-21 in T lymphocytes of septic rats
The levels of miR-126 and miR-21 in T lymphocytes of
septic rats decreased up to 24 h and then slowly increased
(Fig. 6A). There was a linear
positive correlation between expression levels of these two miRs
(Fig. 6B).
Expression of miR-126 is correlated
with inflammatory factors, apoptotic rate of T lymphocytes and
expression and activity of caspase-3 in septic rats
miR-126 expression was negatively correlated with
expression levels of inflammatory factors, the apoptotic rate of T
lymphocytes and expression and activity of caspase-3. Levels of
inflammatory factors peaked at 12 h, expression of miR-126 at 24 h
and the apoptostic rate of T lymphocytes at 48 h (Fig. 7A).
miR-126 was negatively correlated with levels of
TNF-α and IL-6 (Fig. 7E and
F), apoptotic rate of T lymphocytes
(Fig. 7B) and expression and
activity of caspase-3 (Fig. 7C and
D).
Expression of miR-21 is correlated
with inflammatory factors and the expression and activity of
caspase-3 but not T lymphocyte apoptosis in septic rats
The expression levels of miR-21 were negatively
correlated with levels of inflammatory factors, apoptotic rate of T
lymphocytes and expression and activity of caspase-3, but not
significantly correlated with apoptotic rate of T lymphocytes
(Fig. 8A).
miR-21 was negatively correlated with levels of
TNF-α and IL-6 (Fig. 8E and
F) and expression and activity of
caspase-3 (Fig. 8C and D). However, miR-21 wasnot significantly
correlated with the apoptotic rate of T lymphocytes (Fig. 8B).
Discussion
At present, the uncontrolled inflammatory response
is considered to be the basis for the pathogenesis of sepsis. The
release of inflammatory factors in the early stage of sepsis leads
to amplification of an inflammatory cascade and tissue and organ
damage (20-23).
TNF-α and IL-6 are associated with occurrence and progress of
inflammatory reactions in sepsis (24). TNF-α is the most important
proinflammatory factor in the early stage of inflammation and a key
mediator of the LPS damage effect (25). Here, levels of TNF-α and IL-6
increased gradually after LPS was injected into the abdominal
cavity of rats, peaked at 12 h, and then decreased over time.
Although the levels of TNF-α and IL-6 decreased significantly, they
were still higher than in NC rats at 72 h. This trend is consistent
with a study by Sun et al (26), which demonstrated that levels of
HMGB1, TNF-α, IL-6 in serum from patients with sepsis increased
first and then decreased.
With the aggravation of inflammatory reactions, the
body enters a state of immunosuppression in sepsis (27). T lymphocytes are important in the
pathogenesis of sepsis and immunomodulation therapy is a focus of
research (28). Numerous studies
have shown that decreased levels of T cells result in increased
apoptosis of T cells in sepsis (29-31).
Here, apoptosis of T lymphocytes in septic rats increased, which
was consistent with the aforementioned studies. The number of T
lymphocytes decreased and levels of caspase-3, which reflect
apoptosis, increased significantly. Apoptosis of T lymphocytes was
peaked at 48 h, which appeared after the peak of the inflammatory
response (TNF-α and IL-6), then gradually decreased to normal
levels.
Previous attempts to decrease mortality by
decreasing release of inflammatory factors and the inflammatory
response in sepsis have failed. Meta-analysis has shown that
high-throughput hemofiltration and other methods of eliminating
inflammatory factors do not improve the prognosis of patients with
sepsis (32). Studies have show
that the development and prognosis of sepsis is associated with
apoptosis of immune cells, particularly T lymphocytes (7,33).
Regulation of T lymphocyte apoptosis improves the prognosis of
sepsis (34). Regarding T
lymphocyte apoptosis, various caspases are activated in sepsis;
lymphocyte apoptosis is triggered by release of TNF-α,
glucocorticoids, granzymes or by the absence of IL-2(35). Animal experiments have confirmed
that use of a caspase inhibitor (VX-166) increases the survival
rate of septic mice from 40 to 92% (36,37).
Therefore, regulation of T lymphocyte apoptosis is an important
area of research. MiRNAs expression affects differentiation and
proliferation of T lymphocytes (38,39).
Here, miR-126 and miR-21 in T lymphocytes of septic rats decreased
significantly; this trend was contrary to that of inflammatory
factors and T lymphocyte apoptosis, which decreased up to 24 h,
then gradually increased. Agudo et al (40) found that miR-126 regulates the
function of plasma-like dendritic cells via the VEGFR2 axis and
participates in the innate immune response initiated by
microorganisms such as viruses. miR-126 regulates peripheral
induction of Tregs via PI3K/AKT signaling, suggesting that miR-126
is important in the immune response (41). Recently, it has been shown that
miR-126 inhibits invasion and metastasis of malignant glioma by
downregulating the proliferation of mature T lymphocytes,
suggesting that miR-126 exerts a regulatory effect on T lymphocytes
(42). The results of the present
study confirmed the aforementioned findings. There was a linear
correlation between miR-126 and levels of TNF-α and IL-6, apoptotic
rate of T lymphocytes and activity and expression of caspase-3 in T
lymphocytes in septic rats. In addition, altered expression of
miR-126, which preceded apoptotic changes, indicated that miR-126
may regulate apoptosis of T lymphocytes in septic rats. Previous
studies have shown that miR-126 is expressed primarily in T cells
and affects the activation of CD4+ T cells (43-45).
Inflammatory factors, such as IL-12, TGF-β and IFN-γ, show
increased expression in CD4+ T lymphocytes of mice with
a miR-126 gene knockout (13),
which supports the results of the present study. These results
further indicated that the apoptosis of T lymphocytes increased and
expression of miR-126 decreased in sepsis, both of which were
consistent with previous research (46-48).
It has also been found that miR-21 is universally expressed in T
lymphocytes, especially in memory phenotype T lymphocytes (49). Inhibition of miR-21 promotes
apoptosis and growth defects of memory phenotype T lymphocytes,
suggesting that the survival of this type of T lymphocyte is
associated with miR-21(49). Ruan
et al (15) found that
miR-21 regulates the TNF-α-induced protein 8-like 2 gene to inhibit
apoptosis of T lymphocytes and that NF-κB regulates expression of
miR-21. Here, levels of miR-126 and miR-21 in T lymphocytes in
septic rats were similar to those in the aforementioned studies.
Expression levels of miR-126 and miR-21 initially decreased, then
increased and were negatively correlated with release of TNF-α and
IL-6 and activity and expression of caspase-3. Moreover, the
increase in TNF-α and IL-6 occurred prior to the decrease in miR-21
and was followed by an increase in activity and expression of
caspase-3, which suggested that inflammatory factors such as TNF-α
and IL-6 may regulate expression of miR-21 and the molecular
mechanism underlying T lymphocyte apoptosis. This is consistent
with the study by Ruan et al but requires further study to
determine which signaling pathways are involved in miR-21-mediated
regulation of apoptosis and release of inflammatory factors in
sepsis.
In summary, the inflammatory response and apoptosis
of T lymphocytes are important in sepsis. miR-126 and miR-21
expression levels in T lymphocytes in sepsis were significantly
altered; the changes in miR-126 and miR-21 expression were
consistent with the inflammatory response and apoptosis, indicating
they may be associated with inflammatory factors and apoptosis of T
lymphocytes in sepsis. Further research is required to determine
whether and how miR-126 and miR-21 regulate apoptosis of T
lymphocytes in sepsis to provide novel options for the diagnosis
and treatment of sepsis.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science Research
Projects in Universities of Anhui Province (grant nos. KJ2018A0244
and KJ2019A0351).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ and CL analyzed patient data and wrote the
manuscript. QZ and MYa collected the data and performed the
experiments. MYu analyzed the experimental data. All authors read
and approved the final version of the manuscript. QZ and CL confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the animal ethics
committee of Bengbu Medical College (approval no. 2018074).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shankar-Hari M, Phillips GS, Levy ML,
Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD and
Singer M: Sepsis Definitions Task Force. Developing a new
definition and assessing new clinical criteria for septic shock:
For the third international consensus definitions for sepsis and
septic shock (Sepsis-3). JAMA. 315:775–787. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fleischmann C, Scherag A, Adhikari NK,
Hartog CS, Tsaganos T, Schlattmann P, Angus DC and Reinhart K:
International Forum of Acute Care Trialists. Assessment of global
incidence and mortality of hospital-treated sepsis. Current
estimates and limitations. Am J Respir Crit Care Med. 193:259–272.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gotts JE and Matthay MA: Sepsis:
Pathophysiology and clinical management. BMJ.
353(i1585)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
van der Poll T, van de Veerdonk FL,
Scicluna BP and Netea MG: The immunopathology of sepsis and
potential therapeutic targets. Nat Rev Immunol. 17:407–420.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huang M, Cai S and Su J: The pathogenesis
of sepsis and potential therapeutic targets. Int J Mol Sci.
20(5376)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rizzo AN and Dudek SM: Endothelial
glycocalyx repair: Building a wall to protect the lung during
sepsis. Am J Respir Cell Mol Biol. 56:687–688. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rimmele T, Payen D, Cantaluppi V, Marshall
J, Gomez H, Gomez A, Murray P and Kellum JA: ADQI XIV Workgroup.
Immune cell phenotype and function in sepsis. Shock. 45:282–291.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Delano MJ and Ward PA: The immune system's
role in sepsis progression, resolution, and long-term outcome.
Immunol Rev. 274:330–353. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hotchkiss RS, Monneret G and Payen D:
Sepsis-induced immunosuppression: From cellular dysfunctions to
immunotherapy. Nat Rev Immunol. 13:862–874. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Hart M, Walch-Ruckheim B, Krammes L, Kehl
T, Rheinheimer S, Tänzer T, Glombitza B, Sester M, Lenhof HP,
Keller A and Meese E: MiR-34a as hub of T cell regulation networks.
J Immunother Cancer. 7(187)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Deng JN, Li YQ, Liu Y, Li Q, Hu Y, Xu JQ,
Sun TY and Xie LX: Exosomes derived from plasma of septic patients
inhibit apoptosis of T lymphocytes by down-regulating bad via
hsa-miR-7-5p. Biochem Biophys Res Commun. 513:958–966.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pandey RK, Sundar S and Prajapati VK:
Differential expression of miRNA regulates t cell differentiation
and plasticity during visceral leishmaniasis infection. Front
Microbiol. 7(206)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chu F, Hu Y, Zhou Y, Guo M, Lu J, Zheng W,
Xu H, Zhao J and Xu L: MicroRNA-126 deficiency enhanced the
activation and function of CD4+ T cells by elevating
IRS-1 pathway. Clin Exp Immunol. 191:166–179. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ding S, Zheng Y, Xu Y, Zhao X and Zhong C:
MiR-21/PTEN signaling modulates the chemo-sensitivity to
5-fluorouracil in human lung adenocarcinoma A549 cells. Int J Clin
Exp Pathol. 12:2339–2352. 2019.PubMed/NCBI
|
|
15
|
Ruan Q, Wang P, Wang T, Qi J, Wei M, Wang
S, Fan T, Johnson D, Wan X, Shi W, et al: MicroRNA-21 regulates
T-cell apoptosis by directly targeting the tumor suppressor gene
Tipe2. Cell Death Dis. 5(e1095)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ando Y, Yang GX, Kenny TP, Kawata K, Zhang
W, Huang W, Leung PS, Lian ZX, Okazaki K, Ansari AA, et al:
Overexpression of microRNA-21 is associated with elevated
pro-inflammatory cytokines in dominant-negative TGF-β receptor type
II mouse. J Autoimmun. 41:111–119. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zheng Z, Xu PP, Wang L, Zhao HJ, Weng XQ,
Zhong HJ, Qu B, Xiong J, Zhao Y, Wang XF, et al: MiR21 sensitized
B-lymphoma cells to ABT-199 via ICOS/ICOSL-mediated interaction of
Treg cells with endothelial cells. J Exp Clin Cancer Res.
36(82)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Teteloshvili N, Smigielska-Czepiel K,
Kroesen BJ, Brouwer E, Kluiver J, Boots AM and van den Berg A:
T-cell activation induces dynamic changes in miRNA expression
patterns in CD4 and CD8 T-cell subsets. Microrna. 4:117–122.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Balk RA: Systemic inflammatory response
syndrome (SIRS): Where did it come from and is it still relevant
today? Virulence. 5:20–26. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Song GY, Chung CS, Chaudry IH and Ayala A:
Immune suppression in polymicrobial sepsis: Differential regulation
of Th1 and Th2 responses by p38 MAPK. J Surg Res. 91:141–146.
2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheng Z, Abrams ST, Toh J, Wang SS, Wang
Z, Yu Q, Yu W, Toh CH and Wang G: The critical roles and mechanisms
of immune cell death in sepsis. Front Immunol.
11(1918)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pool R, Gomez H and Kellum JA: Mechanisms
of organ dysfunction in sepsis. Crit Care Clin. 34:63–80.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen J, Xuan J, Gu YT, Shi KS, Xie JJ,
Chen JX, Zheng ZM, Chen Y, Chen XB, Wu YS, et al: Celastrol reduces
IL-1β induced matrix catabolism, oxidative stress and inflammation
in human nucleus pulposus cells and attenuates rat intervertebral
disc degeneration in vivo. Biomed Pharmacother. 91:208–219.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xie Z, Zhang H, Wang J, Li Z, Qiu C and
Sun K: LIN28B-AS1-IGF2BP1 association is required for LPS-induced
NFκB activation and pro-inflammatory responses in human macrophages
and monocytes. Biochem Biophys Res Commun. 519:525–532.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sun J, Shi S, Wang Q, Yu K and Wang R:
Continuous hemodiafiltration therapy reduces damage of multi-organs
by ameliorating of HMGB1/TLR4/NFκB in a dog sepsis model. Int J
Clin Exp Pathol. 8:1555–1564. 2015.PubMed/NCBI
|
|
27
|
Hamers L, Kox M and Pickkers P:
Sepsis-induced immunoparalysis: Mechanisms, markers, and treatment
options. Minerva Anestesiol. 81:426–439. 2015.PubMed/NCBI
|
|
28
|
Ono S, Tsujimoto H, Hiraki S and Aosasa S:
Mechanisms of sepsis-induced immunosuppression and immunological
modification therapies for sepsis. Ann Gastroenterol Surg.
2:351–358. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Luan YY, Yao YM, Xiao XZ and Sheng ZY:
Insights into the apoptotic death of immune cells in sepsis. J
Interferon Cytokine Res. 35:17–22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim JS, Kim SJ and Lee SM: Genipin
attenuates sepsis-induced immunosuppression through inhibition of T
lymphocyte apoptosis. Int Immunopharmacol. 27:15–23.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Atmatzidis S, Koutelidakis IM,
Chatzimavroudis G, Kotsaki A, Louis K, Pistiki A, Savva A,
Antonopoulou A, Atmatzidis K and Giamarellos-Bourboulis EJ:
Detrimental effect of apoptosis of lymphocytes at an early time
point of experimental abdominal sepsis. BMC Infect Dis.
11(321)2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yin F, Zhang F, Liu S and Ning B: The
therapeutic effect of high-volume hemofiltration on sepsis: A
systematic review and meta-analysis. Ann Transl Med.
8(488)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Luan YY, Yin CF, Qin QH, Dong N, Zhu XM,
Sheng ZY, Zhang QH and Yao YM: Effect of regulatory T cells on
promoting apoptosis of T lymphocyte and its regulatory mechanism in
sepsis. J Interferon Cytokine Res. 35:969–980. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zheng G, Pan M, Li Z, Xiang W and Jin W:
Effects of vitamin D on apoptosis of T-lymphocyte subsets in
neonatal sepsis. Exp Ther Med. 16:629–634. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Oberholzer C, Oberholzer A, Clare-Salzler
M and Moldawer LL: Apoptosis in sepsis: A new target for
therapeutic exploration. FASEB J. 15:879–892. 2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tinsley KW, Cheng SL, Buchman TG, Chang
KC, Hui JJ, Swanson PE, Karl IE and Hotchkiss RS: Caspases -2, -3,
-6, and -9, but not caspase-1, are activated in sepsis-induced
thymocyte apoptosis. Shock. 13:1–7. 2000.
|
|
37
|
Weber P, Wang P, Maddens S, Wang PSh, Wu
R, Miksa M, Dong W, Mortimore M, Golec JM and Charlton P: VX-166: A
novel potent small molecule caspase inhibitor as a potential
therapy for sepsis. Crit Care. 13(R146)2009.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
Gagnon JD, Kageyama R, Shehata HM, Fassett
MS, Mar DJ, Wigton EJ, Johansson K, Litterman AJ, Odorizzi P,
Simeonov D, et al: MiR-15/16 restrain memory T cell
differentiation, cell cycle, and survival. Cell Rep.
28:2169–2181.e4. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li B, Wang X, Choi IY, Wang YC, Liu S,
Pham AT, Moon H, Smith DJ, Rao DS, Boldin MP and Yang L: MiR-146a
modulates autoreactive Th17 cell differentiation and regulates
organ-specific autoimmunity. J Clin Invest. 127:3702–3716.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Agudo J, Ruzo A, Tung N, Salmon H, Leboeuf
M, Hashimoto D, Becker C, Garrett-Sinha LA, Baccarini A, Merad M
and Brown BD: The miR-126-VEGFR2 axis controls the innate response
to pathogen-associated nucleic acids. Nat Immunol. 15:54–62.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
41
|
Qin A, Wen Z, Zhou Y, Li Y, Li Y, Luo J,
Ren T and Xu L: MicroRNA-126 regulates the induction and function
of CD4(+) Foxp3(+) regulatory T cells through PI3K/AKT pathway. J
Cell Mol Med. 17:252–264. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Han L, Liu H, Wu J and Liu J: MiR-126
suppresses invasion and migration of malignant glioma by targeting
mature T cell proliferation 1 (MTCP1). Med Sci Monit. 24:6630–6637.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhao S, Wang Y, Liang Y, Zhao M, Long H,
Ding S, Yin H and Lu Q: MicroRNA-126 regulates DNA methylation in
CD4+ T cells and contributes to systemic lupus
erythematosus by targeting DNA methyltransferase 1. Arthritis
Rheum. 63:1376–1386. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yang G, Wu D, Zeng G, Jiang O, Yuan P,
Huang S, Zhu J, Tian J, Weng Y and Rao Z: Correlation between
miR-126 expression and DNA hypomethylation of CD4+ T
cells in rheumatoid arthritis patients. Int J Clin Exp Pathol.
8:8929–8936. 2015.PubMed/NCBIeCollection, 2015.
|
|
45
|
Tian M, Ji Y, Wang T, Zhang W, Zhou Y and
Cui Y: Changes in circulating microRNA-126 levels are associated
with immune imbalance in children with acute asthma. Int J
Immunopathol Pharmacol. 32(2058738418779243)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hotchkiss RS, Tinsley KW, Swanson PE,
Schmieg RE Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP,
Buchman TG and Karl IE: Sepsis-induced apoptosis causes progressive
profound depletion of B and CD4+ T lymphocytes in
humans. J Immunol. 166:6952–6963. 2001.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jones Buie JN, Zhou Y, Goodwin AJ, Cook
JA, Vournakis J, Demcheva M, Broome AM, Dixit S, Halushka PV and
Fan H: Application of deacetylated poly-N-acetyl glucosamine
nanoparticles for the delivery of miR-126 for the treatment of
cecal ligation and puncture-induced sepsis. Inflammation.
42:170–184. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Su J and Ding L: Upregulation of miR-126
inhibits podocyte injury in sepsis via EGFL6/DKC1 signaling
pathway. Mol Med Rep. 23(373)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Smigielska-Czepiel K, van den Berg A,
Jellema P, Slezak-Prochazka I, Maat H, van den Bos H, van der Lei
RJ, Kluiver J, Brouwer E, Boots AM and Kroesen BJ: Dual role of
miR-21 in CD4+ T-cells: Activation-induced miR-21
supports survival of memory T-cells and regulates CCR7 expression
in naive T-cells. PLoS One. 8(e76217)2013.PubMed/NCBI View Article : Google Scholar
|