Introduction
Synchronous double breast cancer (BC) and axillary
(Ax) follicular lymphoma (FL) have different treatment priorities,
depending on their respective stage. BC may be treated with
surgical resection, chemotherapy, radiation therapy and endocrine
therapy, or a combination of these modalities, according to cancer
type and stage. However, FL is mainly managed using chemotherapy or
careful observation, based on the tumour volume. The majority of
the cases of synchronous BC and malignant lymphoma (ML) of the Ax
lymph nodes (LNs) are commonly diagnosed using sentinel LN biopsy
(SLNB) or Ax LN dissection at the time of surgery for BC (1-11).
To the best of our knowledge, the case presented herein was the
first case in which synchronous Ax FL was diagnosed using core
needle biopsy (CNB) of the Ax LNs during staging examination for
BC. In the present case, the two synchronous cancers were staged to
establish the treatment priority. We herein report the case
details, along with a review of the related literature.
Case report
The patient was a 73-year-old woman who was followed
up by a local doctor for a fibroadenoma of the left breast
diagnosed by ultrasound (US) examination 4 years prior. The patient
had no subjective symptoms or specific disease-related history and
had undergone spontaneous menopause at 50 years of age. The family
history included a diagnosis of BC in the patient's maternal aunt.
The patient had been subjected to US examination in June 2020, and
a tumor was detected in the left breast. CNB of the tumor was
performed, and the patient was diagnosed with invasive ductal
carcinoma (IDC) of the left breast and was referred to Chiba Cancer
Center in September 2020.
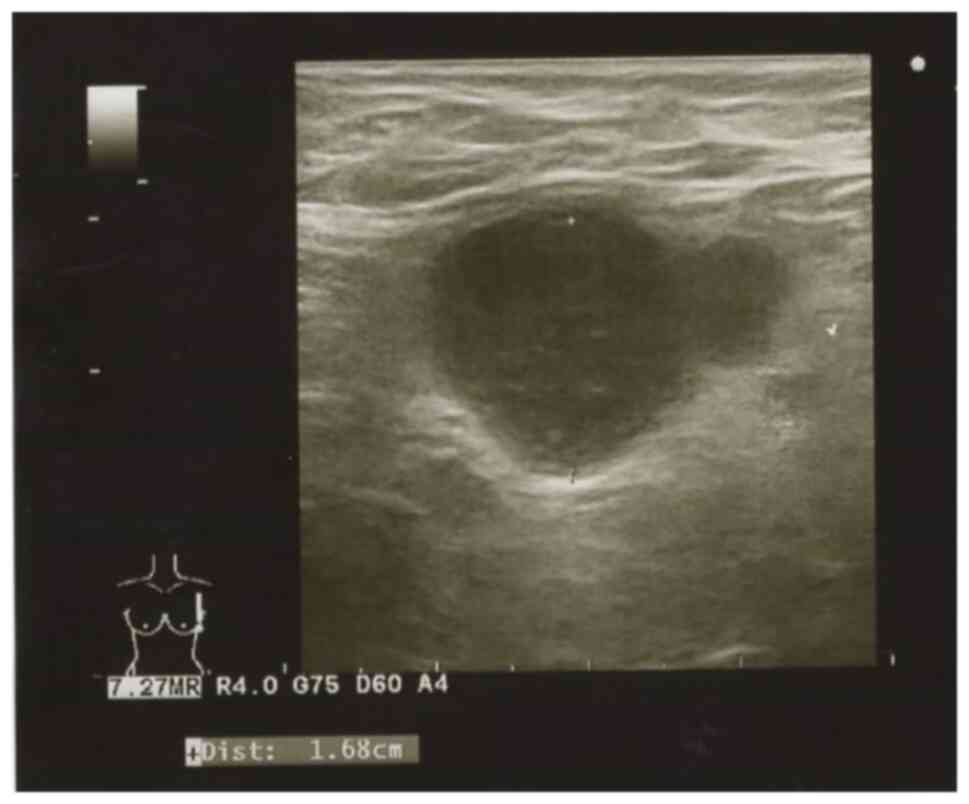
US examination of the left breast and Ax LNs
revealed a large 8-mm hypoechoic lesion with irregular margins in
the nipple areola-complex of the left breast, situated in the
middle and immediately above the nipple. The histological diagnosis
was IDC, histological grade I (tubule formation score 2, nuclear
atypia score 2, and mitotic count score 1), estrogen
receptor-positive (90%), progesterone receptor-negative (0%) and
HER2 score 0 (Fig. 1). Enlarged
left Ax LNs and loss of the medulla were detected (Fig. 2). Ax LN metastasis of IDC was
suspected; therefore, US-guided CNB was performed using a 16G 22-mm
needle, and four specimens were collected. Histopathological
examination of the Ax LN samples revealed the formation of lymphoid
follicles consisting mainly of medium-sized centrocytes with
occasional large centroblasts. Immunostaining revealed that most of
the follicular component cells were positive for CD10, CD20, Bcl-2
and Bcl-6, and negative for CD3, multiple myeloma oncogene 1 (MUM1)
and Epstein-Barr virus-encoded small RNA (EBER). The CD21-positive
dendritic cells almost coincided with the follicles, leading to the
diagnosis of FL (Fig. 3). The
tissues were fixed using 10% neutral buffered formalin at 25˚C for
24 h. After fixation, the specimen was cut into 5-7-mm sections,
which were then dehydrated using ethanol, impregnated and embedded
in paraffin in a closed automatic fixation unit during a 22-h
program. The paraffin-embedded specimen was then cut into 4-µm
sections using a sliding microtome. The sections were then placed
on glass slides, spread using a spreader, and dried in an oven at
60˚C for 20 min. The following equipment was used for
immunostaining: CD20, CD3, Bcl-2, Bcl-6 and EBER: VENTANA BenchMark
ULTRA (Roche Diagnostics K.K.; cat. no. 13B1X00201000050); and
CD10, MUM1 and CD21: Dako Omnis (Agilent Technologies Japan, Ltd.;
cat. no. 13B3X10204000004). Nikon Eclipse Ni-U Biological
Microscope (Nikon Corporation) was used for imaging at a
magnification of x20.
Blood examinations revealed that the IL-2 receptor
level was 1,859 U/ml (normal range, 122-496 U/ml); however, there
were no significant abnormalities in the blood cell count or liver
enzyme levels, and the patient had no B symptoms (fever, weight
loss or sweating).
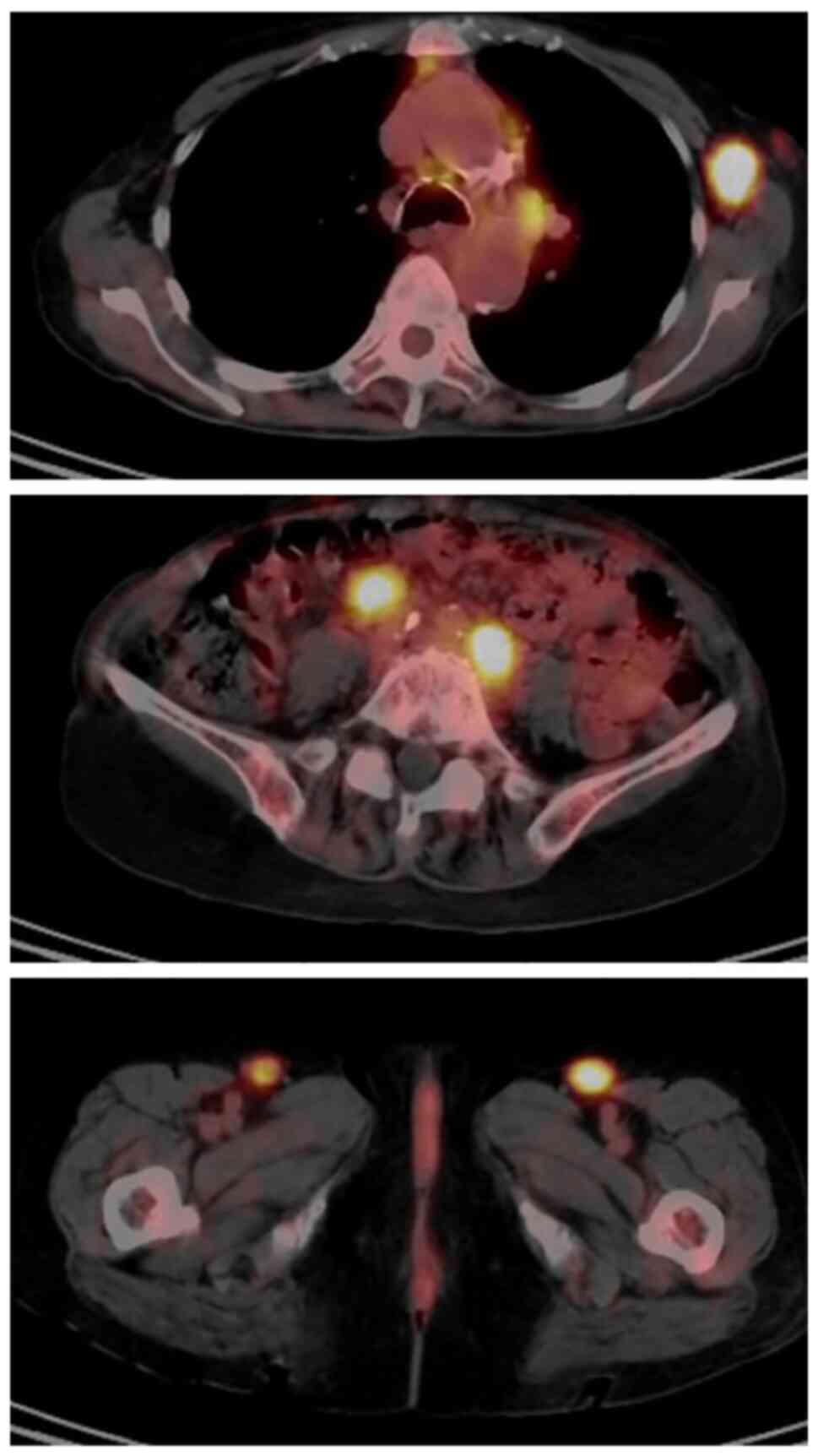
The patient was referred to the Division of
Hematology-Oncology at Chiba Cancer Center and underwent positron
emission tomography computed tomography (PET-CT) examination, which
revealed high 18F-fluorodexyglucose accumulation in the
mediastinal, bilateral axillary, abdominal para-aortic, iliac and
bilateral inguinal LNs (Fig. 4).
None of the LNs had enlarged beyond 3 cm in size, and none met the
Groupe d' Etude des Lymphomes Folliculaires high tumour volume
criteria; therefore, careful observation of the patient was decided
based on the low tumour volume (12-14).
The patient was subjected to partial left mastectomy
and SLNB [indigo carmine dye and 99mTc gamma probe and
intraoperative US-guided marking with patent blue dye and tattooing
(15)] for the IDC. In addition, an
enlarged LN (20 mm) was identified near the SLN, which was also
resected. No BC metastasis was found in the resected SLN. Large and
small follicular structures were observed in the enlarged LNs,
which extended into the adipose tissue outside the LN capsule.
Immunostaining revealed that the cells in the follicular area were
positive for CD79a, CD20, CD10 and Bcl-2, and negative for CD3 and
CD5, which was consistent with the diagnosis of FL. The pathologist
reported no differences in the diagnosis or staging between the
specimens obtained at surgery and those obtained by CNB
preoperatively. The left BC was pT1aN0M0 stage I. The patient
received oral adjuvant treatment with the aromatase inhibitor (AI)
anastrozole (1 mg/day for 5 years), as well as radiotherapy at a
total dose of 50 Gy (2 Gy/fr x 25). FL was continuously monitored
by the Department of Hematology and Oncology. The disease status
was stable disease at the 6-month postoperative follow-up.
Discussion
Synchronous BC and Ax ML is an infrequent occurrence
with only a few cases reported to date. However, to the best of our
knowledge, no case of ML diagnosed by US-guided CNB during
preoperative screening for BC has been reported to date (1-11).
In most cases of synchronous BC and Ax ML, a definitive diagnosis
is obtained by SLNB or Ax LN dissection (1-10);
this is because physical examination, incisional biopsy of Ax LNs,
PET-CT and fine-needle aspiration cytology (FNAC) are the most
commonly used methods for the assessment of Ax LNs during
preoperative staging of BC (16,17).
However, several clinical trials have reported the
usefulness of FNAC for SLNB (18,19).
The advantages of CNB for patients and surgeons are as follows: i)
It has a higher rate of diagnosis of BC metastasis compared with
FNAC; ii) it allows for differentiation from other diseases; iii)
it allows for an immunological histological diagnosis; and iv) it
is as safe and simple to perform as FNAC.
First, Nakamura et al (20) reported the usefulness of CNB and
concluded that it had a higher sensitivity for positive Ax LN
metastases compared with FNA (CNB vs. FNA, 87.5-90 vs. 64.8-76.0%,
respectively) (21). Accurate
diagnosis of metastases in the Ax LNs may help patients avoid
unnecessary SNLB and reduce the operative time and cost.
When Ax LN metastasis of BC progresses, the
lymphatic flow is altered due to the obstruction of the lymph
vessels or nodes by the cancer cells, and the lymphatic flow
increases to not only other Ax LNs, but also the parasternal LNs
(22). Loss of the original
lymphatic flow is considered to reduce the uptake of dye and
isotope particles in the SLNs and decrease the identification and
positive diagnosis rates of SLNB (23-25).
Therefore, Ax LN dissection should be initially performed in cases
with clinical evidence of Ax LN metastasis (N1-2), and the use of
SLNB to accurately diagnose Ax LN metastasis should be limited to
cases with no clinical evidence of Ax LN metastasis (N0) (26,27).
In the present case, the Ax LNs were significantly enlarged on
imaging compared with normal LNs, and IDC metastasis was suspected.
However, preoperative evaluation ruled out BC metastasis to the Ax
LNs, and SLNB was performed. If the patient had been found to have
metastasis to the Ax LNs (N1-2) on imaging and had not undergone
CNB, Ax LN dissection would have been performed (28), which would be an over-invasive
procedure for this patient. Considering that ML is not accompanied
by abnormalities in the lymphatic pathway, but may affect the
lymphatic drainage and other factors, it was determined as
reasonable to perform SLNB by carefully tracking the lymphatic
vessels.
Second, the assessment of the Ax LNs for BC may be
inconsistent with the progression of primary BC. Fortunato et
al (29) and Barone et
al (30) reported that the
frequency of Ax LN metastases in pT1a disease was 4-7.8%, and that
most of these Ax LNs were not palpable on preoperative examination.
Therefore, it is important to be proactive with the tissue
diagnosis if it is inconsistent with the progression of primary BC.
The differential diagnosis of enlarged LNs includes reactive
lymphadenopathy due to rheumatism, inflammatory diseases, as well
as ML (31).
Some reports suggest that PET-CT should be performed
as a priority in cases with enlarged LNs, regardless the tumour
size, as in the present case (32).
However, the differential diagnosis between some inflammatory and
infectious lesions and malignant lesions is difficult (33). Prior CNB can be used to
differentiate between Ax LN metastases from BC and inflammatory
disease or ML, avoiding excessive testing and surgically invasive
procedures (20,34). In the present case, due to the
discrepancy between the small size of the breast tumour and the
enlarged Ax LNs, a CNB was performed. As a result, a diagnosis of
ML was made.
Third, an immunohistological diagnosis makes it
possible to assess the hormone receptor and HER2 status of the LNs.
Recently, it was reported that the hormone receptor status may
differ between the primary tumour and LN metastases. Nakamura et
al (35) reported the
therapeutic efficacy of preoperative chemotherapy according to the
LN subtype and indicated that metastatic LNs may be an indication
for SNB if preoperative chemotherapy results in a pathological
complete response.
An immunohistological diagnosis is difficult with
FNAC. Woo et al (11)
performed FNA for preoperative diagnosis of ML and found no
atypical cells. Consequently, they reported that ML was the
definitive postoperative diagnosis by SNLB. Therefore,
immunohistological examination is particularly important for the
diagnosis of ML.
The European Society for Medical Oncology has long
considered surgical resection to be the gold standard biopsy method
for staging ML due to the need for adequate tissue samples
(36). However, in recent years, an
increasing number of reports have shown that the diagnostic
accuracy of subtype classification techniques using CNB is
comparable to that of surgical resection (37,38),
whereas CNB is superior to surgical resection in terms of the
waiting period for examination, time required for the examination
per se, invasiveness and medical costs (39,40).
Both CNB and surgical biopsy of Ax LN specimens may be used to
diagnose FL, with comparable results in terms of accuracy.
Finally, the safety and procedures of CNB and FNAC
are almost identical. Abnormal LNs are assessed by the thickness of
the cortex, the disappearance of hyperechoic areas near the LN
hilum, and the uneven distribution of blood flow within the LN
(16,41,42).
The US-guided approach to the Ax LNs prevents the needle tip from
injuring major blood vessels and nerves, thus avoiding
complications (43).
Based on the four aforementioned advantages, we
recommend aggressive CNB of abnormally large Ax LNs prior to BC
surgery. Performing CNB on abnormally large Ax LNs without
exception, even in early-stage BCs with a small tumour size,
enabled the diagnosis of synchronous BC and Ax FL with minimal
invasion. Therefore, CNB evaluation of Ax LNs may continue to play
an important role in preoperative BC screening in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RE wrote the paper. RN and NY revised the manuscript
for important intellectual content and technical details. RN and NY
can confirm the authenticity of the raw data. TM, SH, IS, HT, HH
and MO contributed to data acquisition and drafted the manuscript.
MI performed the histological examinations and was a major
contributor to writing the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roy SD, Stafford JA, Scally J and
Selvachandran SN: A rare case of breast carcinoma co-existing with
axillary mantle cell lymphoma. World J Surg Oncol.
1(27)2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Barranger E, Marpeau O, Uzan S and Antoine
M: Axillary sentinel node involvement by breast cancer coexisting
with B-cell follicular lymphoma in nonsentinel nodes. Breast J.
11:227–228. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cox J, Lunt L and Webb L: Synchronous
presentation of breast carcinoma and lymphoma in the axillary
nodes. Breast. 15:246–252. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Garg NK, Bagul NB, Rubin G and Shah EF:
Primary lymphoma of the breast involving both axillae with
bilateral breast carcinoma. World J Surg Oncol.
6(52)2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cuff KE, Dettrick AJ and Chern B:
Synchronous breast cancer and lymphoma: A case series and a review
of the literature. J Clin Pathol. 63:555–557. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Laudenschlager MD, Tyler KL, Geis MC, Koch
MR and Graham DB: A rare case of synchronous invasive ductal
carcinoma of the breast and follicular lymphoma. S D Med.
63:123–125. 2010.PubMed/NCBI
|
|
7
|
Sordi E, Cagossi K, Lazzaretti MG,
Gusolfino D, Artioli F, Santacroce G, Brandi ML and Piscitelli P:
Rare case of male breast cancer and axillary lymphoma in the same
patient: An unique case report. Case Rep Med.
2011(940803)2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miles EF and Jacimore LL: Synchronous
bilateral breast carcinoma and axillary non-hodgkin lymphoma: A
case report and review of the literature. Case Rep Oncol Med.
2012(685919)2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Arana S, Vasquez-Del-Aguila J,
Espinosa-Bravo M, Peg V and Rubio IT: Lymphatic mapping could not
be impaired in the presence of breast carcinoma and coexisting
small lymphocytic lymphoma. Am J Case Rep. 14:322–325.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tamaoki M, Nio Y, Tsuboi K, Nio M, Tamaoki
M and Maruyama R: A rare case of non-invasive ductal carcinoma of
the breast coexisting with follicular lymphoma: A case report with
a review of the literature. Oncol Lett. 7:1001–1006.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Woo EJ, Baugh AD and Ching K: Synchronous
presentation of invasive ductal carcinoma and mantle cell lymphoma:
A diagnostic challenge in menopausal patients. J Surg Case Rep.
22(rjv153)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Brice P, Bastion Y, Lepage E, Brousse N,
Haïoun C, Moreau P, Straetmans N, Tilly H, Tabah I and
Solal-Céligny P: Comparison in low-tumor-burden follicular
lymphomas between an initial no-treatment policy, prednimustine, or
interferon alfa: A randomized study from the Groupe d'Etude des
Lymphomes Folliculaires. Groupe d'Etude des Lymphomes de l'Adulte.
J Clin Oncol. 15:1110–1117. 1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Solal-Céligny P, Lepage E, Brousse N,
Tendler CL, Brice P, Haïoun C, Gabarre J, Pignon B, Tertian G,
Bouabdallah R, et al: Doxorubicin-containing regimen with or
without interferon alfa-2b for advanced follicular lymphomas: Final
analysis of survival and toxicity in the Groupe d'Etude des
Lymphomes Folliculaires 86 trial. J Clin Oncol. 16:2332–2338.
1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sebban C, Mounier N, Brousse N, Belanger
C, Brice P, Haioun C, Tilly H, Feugier P, Bouabdallah R, Doyen C,
et al: Standard chemotherapy with interferon compared with CHOP
followed by high-dose therapy with autologous stem cell
transplantation in untreated patients with advanced follicular
lymphoma: The GELF-94 randomized study from the Groupe d'Etude des
Lymphomes de l'Adulte (GELA). Blood. 108:2540–2544. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Park S, Koo JS, Kim GM, Sohn J, Kim SI,
Cho YU, Park BW, Park VY, Yoon JH, Moon HJ, et al: Feasibility of
charcoal tattooing of cytology-proven metastatic axillary lymph
node at diagnosis and sentinel lymph node biopsy after neoadjuvant
chemotherapy in breast cancer patients. Cancer Res Treat.
50:801–812. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gentilini O and Veronesi U: Abandoning
sentinel lymph node biopsy in early breast cancer? A new trial in
progress at the European institute of oncology of Milan (SOUND:
Sentinel node vs. observation after axillary UltraSouND). Breast.
21:678–681. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cyr AE, Tucker N, Ademuyiwa F,
Margenthaler JA, Aft RL, Eberlein TJ, Appleton CM, Zoberi I, Thomas
MA, Gao F and Gillanders WE: Successful completion of the pilot
phase of a randomized controlled trial comparing sentinel lymph
node biopsy to no further axillary staging in patients with
clinical T1-T2 N0 breast cancer and normal axillary ultrasound. J
Am Coll Surg. 223:399–407. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nguyen BM, Halprin C, Olimpiadi Y, Traum
P, Yeh JJ and Dauphine C: Core needle biopsy is a safe and accurate
initial diagnostic procedure for suspected lymphoma. Am J Surg.
208:1003–1008. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zinzani PL, Colecchia A, Festi D,
Magagnoli M, Larocca A, Ascani S, Bendandi M, Orcioni GF,
Gherlinzoni F, Albertini P, et al: Ultrasound-guided core-needle
biopsy is effective in the initial diagnosis of lymphoma patients.
Haematologica. 83:989–992. 1998.PubMed/NCBI
|
|
20
|
Nakamura R, Yamamoto N, Miyaki T, Itami M,
Shina N and Ohtsuka M: Impact of sentinel lymph node biopsy by
ultrasound-guided core needle biopsy for patients with suspicious
node positive breast cancer. Breast Cancer. 25:86–93.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bhandari A, Xia E, Wang Y, Sindan N, Kc R,
Guan Y, Lin YL, Wang X, Zhang X and Wang O: Impact of sentinel
lymph node biopsy in newly diagnosed invasive breast cancer
patients with suspicious node: A comparative accuracy survey of
fine-needle aspiration biopsy versus core-needle biopsy. Am J
Transl Res. 10:1860–1873. 2018.PubMed/NCBI
|
|
22
|
Sandrucci S and Mussa A: Sentinel lymph
node biopsy and axillary staging of T1-T2 N0 breast cancer: A
multicenter study. Semin Surg Oncol. 15:278–283. 1998.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gulec SA, Moffat FL, Carroll RG and Krag
DN: Gamma probe guided sentinel node biopsy in breast cancer. Q J
Nucl Med. 41:251–261. 1997.PubMed/NCBI
|
|
24
|
Sener SF, Winchester DJ, Brinkmann E,
Winchester DP, Alwawi E, Nickolov A, Perlman RM, Bilimoria M,
Barrera E and Bentrem DJ: Failure of sentinel lymph node mapping in
patients with breast cancer. J Am Coll Surg. 198:732–736.
2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kramer EL: Lymphoscintigraphy:
Radiopharmaceutical selection and methods. Int J Rad Appl Instrum
B. 17:57–63. 1990.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Albertini JJ, Lyman GH, Cox C, Yeatman T,
Balducci L, Ku N, Shivers S, Berman C, Wells K, Rapaport D, et al:
Lymphatic mapping and sentinel node biopsy in the patient with
breast cancer. JAMA. 276:1818–1822. 1996.PubMed/NCBI
|
|
27
|
Veronesi U, Paganelli G, Galimberti V,
Viale G, Zurrida S, Bedoni M, Costa A, de Cicco C, Geraghty JG,
Luini A, et al: Sentinel-node biopsy to avoid axillary dissection
in breast cancer with clinically negative lymph-nodes. Lancet.
349:1864–1867. 1997.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al:
Early breast cancer Trialists' Collaborative group (EBCTCG):
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106.
2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fortunato L, Santoni M, Drago S, Gucciardo
G, Farina M, Cesarini C, Cabassi A, Tirelli C, Terribile D, Grassi
GB, et al: Sentinel lymph node biopsy in women with pT1a or
‘microinvasive’ breast cancer. Breast. 17:395–400. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Barone JE, Tucker JB, Perez JM, Odom SR
and Ghevariya V: Evidence-based medicine applied to sentinel lymph
node biopsy in patients with breast cancer: Am. Surg. 71:66–70.
2005.PubMed/NCBI
|
|
31
|
Kim KH, Son EJ, Kim EK, Ko KH, Kang H and
Oh KK: Safety and efficiency of the ultrasound-guided large needle
core biopsy of axilla lymph nodes. Yonsei Med J. 49:249–254.
2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Brennan ME and Houssami N: Evaluation of
the evidence on staging imaging for detection of asymptomatic
distant metastases in newly diagnosed breast cancer. Breast.
21:112–123. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kawada N, Uehara H, Hosoki T, Takami M,
Shiroeda H, Arisawa T and Tomita Y: Usefulness of dual-phase
18F-FDG PET/CT for diagnosing small pancreatic tumors. Pancreas.
44:655–659. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pugliese N, Perna MD, Cozzolino I, Ciancia
G, Pettinato G, Zeppa P, Varone V, Masone S, Cerchione C, Pepa RD,
et al: Randomized comparison of power doppler
ultrasonography-guided core-needle biopsy with open surgical biopsy
for the characterization of lymphadenopathies in patients with
suspected lymphoma. Ann Hematol. 96:627–637. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nakamura R, Hayama S, Sonoda I, Miyaki T,
Itami M and Yamamoto N: Clinical impact of the biology of
synchronous axillary lymph node metastases in primary breast cancer
on preoperative treatment strategy. J Surg Oncol. 123:1513–1520.
2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tilly H, Vitolo U, Walewski J, da Silva
MG, Shpilberg O, André M, Pfreundschuh M and Dreyling M: ESMO
guidelines working group. Diffuse large B-cell lymphoma (DLBCL):
ESMO clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 23 (Suppl 7):vii78–vii82. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chatani S, Hasegawa T, Kato S, Murata S,
Sato Y, Yamaura H, Yamamoto K, Yatabe Y and Inaba Y: Image-guided
core needle biopsy in the diagnosis of malignant lymphoma:
Comparison with surgical excision biopsy. Eur J Radiol.
127(108990)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hu Q, Naushad H, Xie Q, Al-Howaidi I, Wang
M and Fu K: Needle-core biopsy in the pathologic diagnosis of
malignant lymphoma showing high reproducibility among pathologists.
Am J Clin Pathol. 140:238–247. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lachar WA, Shahab I and Saad AJ: Accuracy
and cost-effectiveness of core needle biopsy in the evaluation of
suspected lymphoma: A study of 101 cases. Arch Pathol Lab Med.
131:1033–1039. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ardeshna KM, Qian W, Smith P, Braganca N,
Lowry L, Patrick P, Warden J, Stevens L, Pocock CF, Miall F, et al:
Rituximab versus a watch-and-wait approach in patients with
advanced-stage, asymptomatic, non-bulky follicular lymphoma: An
open-label randomised phase 3 trial. Lancet Oncol. 15:424–435.
2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Feu J, Tresserra F, Fábregas R, Navarro B,
Grases PJ, Suris JC, Fernández-Cíd A and Alegret X: Metastatic
breast carcinoma in axillary lymph nodes: In vitro US detection.
Radiology. 205:831–835. 1997.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Vassallo P, Wernecke K, Roos N and Peters
PE: Differentiation of benign from malignant superficial
lymphadenopathy: The role of high-resolution US. Radiology.
183:215–220. 1992.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Abe H, Schmidt RA, Sennett CA, Shimauchi A
and Newstead GM: US-guided core needle biopsy of axillary lymph
nodes in patients with breast cancer: Why and how to do it.
Radiographics. 27 (Suppl 1):S91–S99. 2007.PubMed/NCBI View Article : Google Scholar
|