Introduction
Mucoepidermoid carcinoma (MEC) is the most common
malignancy originating in the salivary gland (1,2) and
Warthin-like MEC was recently categorized as a novel and low-grade
form of this disease (3). This
rare variant is characterized histopathologically by the presence
of prominent lymphocytic infiltration and cystic changes that
resemble Warthin's tumour (3). The
neoplastic cells comprising Warthin-like MEC are intermediate cells
and a variable number of mucinous cells may be present, similar to
that in conventional MEC; however, the bi-layered tall oncocytic
cells, which are characteristic of Warthin's tumour, are not
observed in Warthin-like MEC (3,4).
Mastermind-like transcriptional coactivator 2 (MAML2)
encodes a transcription coactivator of NOTCH proteins. The presence
of MAML2 rearrangement is characteristic of Warthin-like MEC
(3). This rearrangement is
observed in most cases of conventional MEC, particularly in
low-grade tumours (3).
Fine-needle aspiration (FNA) cytology is a useful
technique for diagnosing salivary gland tumours (5-7).
However, the cytological features of Warthin-like MEC have remained
to be fully established due to its rarity (8-11).
In the present study, the sixth cytological case of Warthin-like
MEC was reported, which occurred in a 16-year-old Japanese female.
The clinicopathological and cytological features of the present and
previously reported Warthin-like MEC cases were also reviewed and
the considerations for cytological differential diagnosis were
discussed.
Case report
A 16-year-old Japanese female visited Kansai Medical
University (Hirakata, Japan) with a painful mass on the left side
of the neck in April 2020. The patient had no notable medical or
family history. Physical examination revealed a relatively
well-circumscribed and mobile tumour in the left parotid gland; no
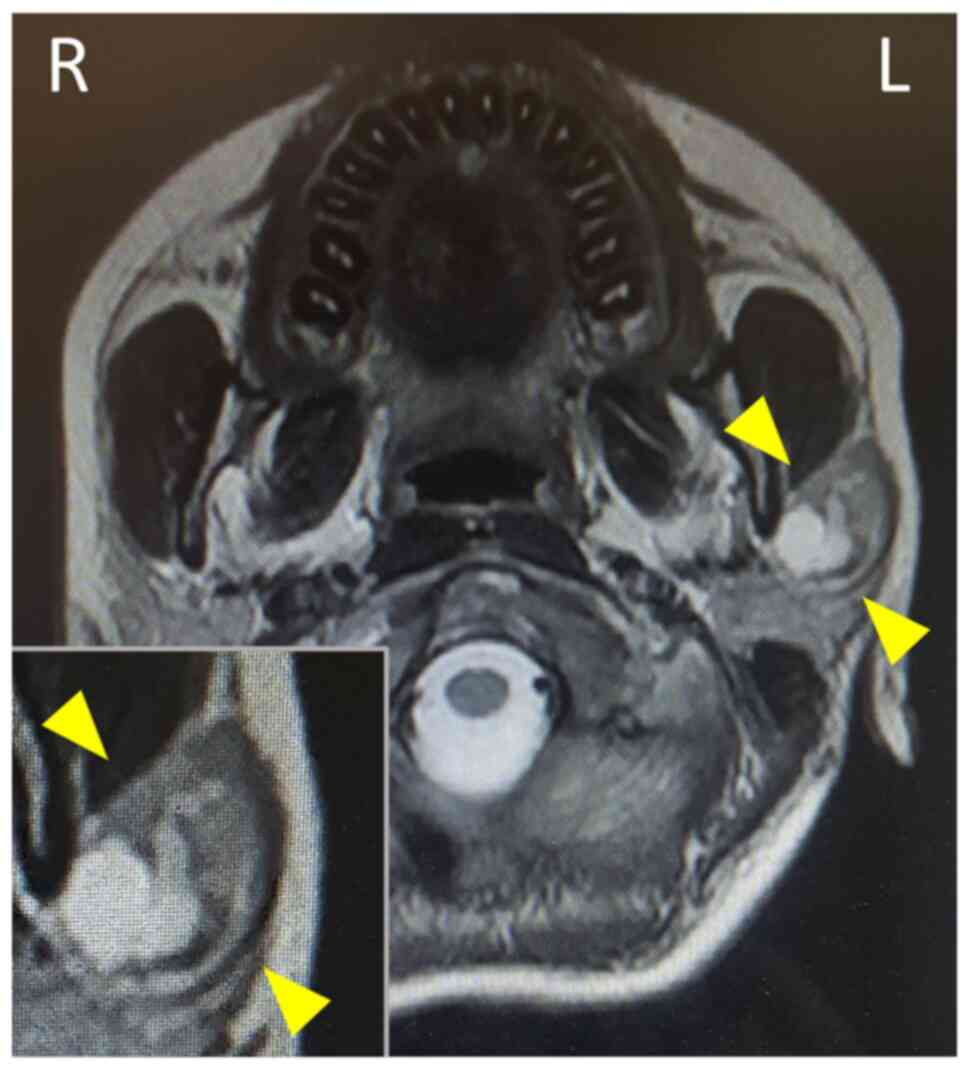
facial nerve palsy was noted. Magnetic resonance imaging revealed a
well-circumscribed tumour accompanying multiple cysts and solid
masses with intermediate intensity in the left parotid gland
(Fig. 1, inset). FNA examination
of the left parotid gland tumour was performed and the specimens
were stained by Papanicolaou stain as the same method previously
reported (6,10). The results of FNA examination were
available prior to surgery. No cell block method was applied in the
present case, as this method is not routinely performed at our
hospital. Considering the presence of the painful mass in the
parotid gland, partial parotidectomy was performed, without any
specific clinical diagnosis, as the initial FNA results were
negative for cancer. Intraoperative findings revealed a relatively
well-circumscribed mass in the left parotid gland and the mass was
not in contact with the facial nerve. The facial nerve activity was
monitored using an electromyography monitor during the operation.
After six months of post-surgery follow-up and without any
additional therapy, the patient has presented no evidence of
recurrence. This patient was subjected to standard clinical
treatment, as Warthin-like MEC is considered a low-grade malignancy
(1,3).
Results
Initial cytological features of the
parotid gland tumour
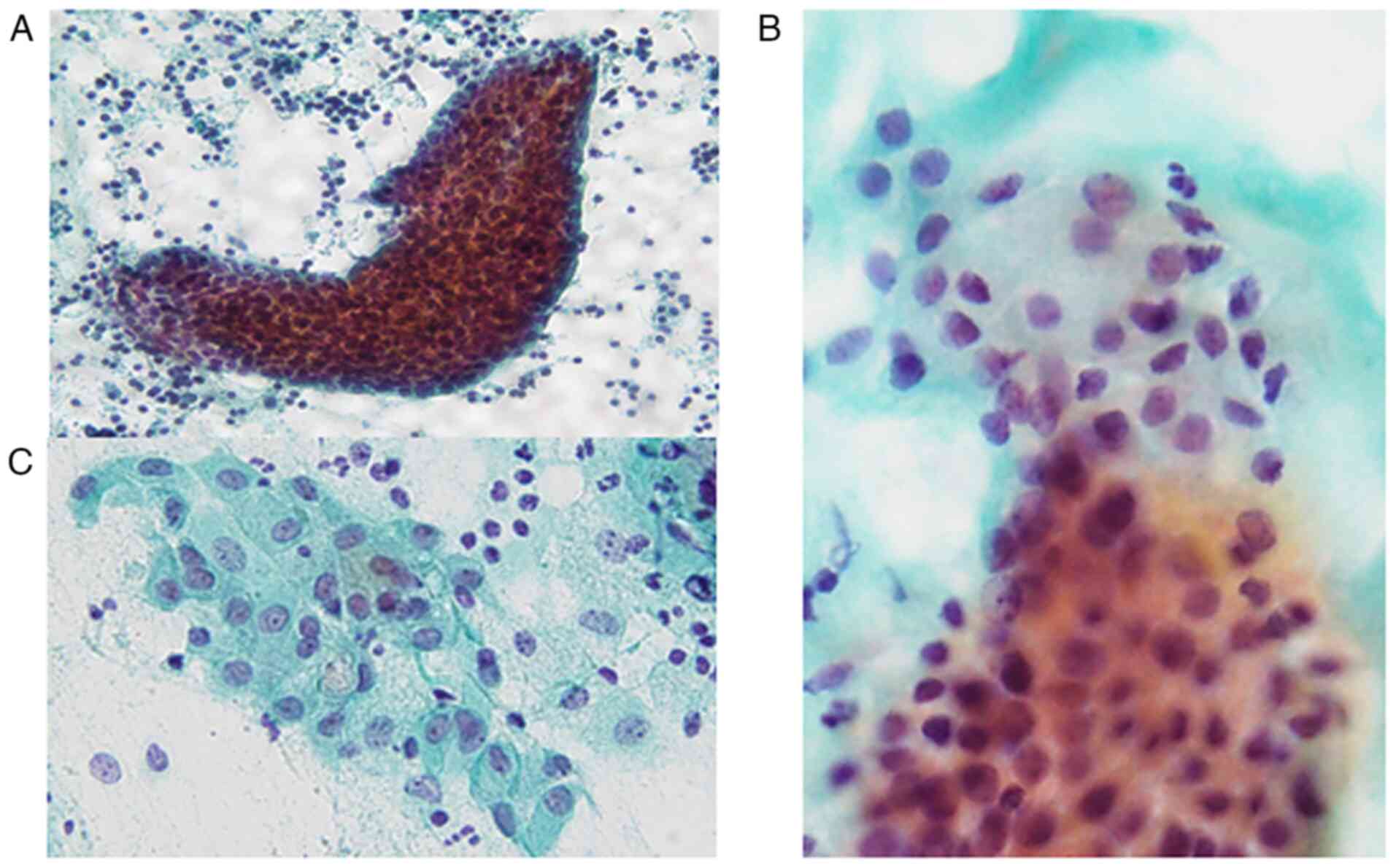
The Papanicolaou smear of the FNA specimens revealed
the presence of sheet-like, folded or scattered epithelial cell
clusters and a small number of non-neoplastic acinar cells in a
mucinous background accompanying abundant lymphocytes and scattered
macrophages (Fig. 2A). The
epithelial cell clusters comprised round cells with mildly enlarged
round to oval nuclei without nucleoli (Fig. 2B) and polygonal cells with
relatively rich cytoplasm and slightly enlarged round to oval
nuclei accompanying small nucleoli (Fig. 2B and C). Certain polygonal cells had
intracytoplasmic mucin, eccentric nuclei and lace-like cytoplasm
(Fig. 2C). No necrotic material,
keratinized cells or oncocytes were observed. Accordingly, an
initial cytological diagnosis of lymphoepithelial sialadenitis (LS)
was made.
Histopathology of parotid gland
tumour
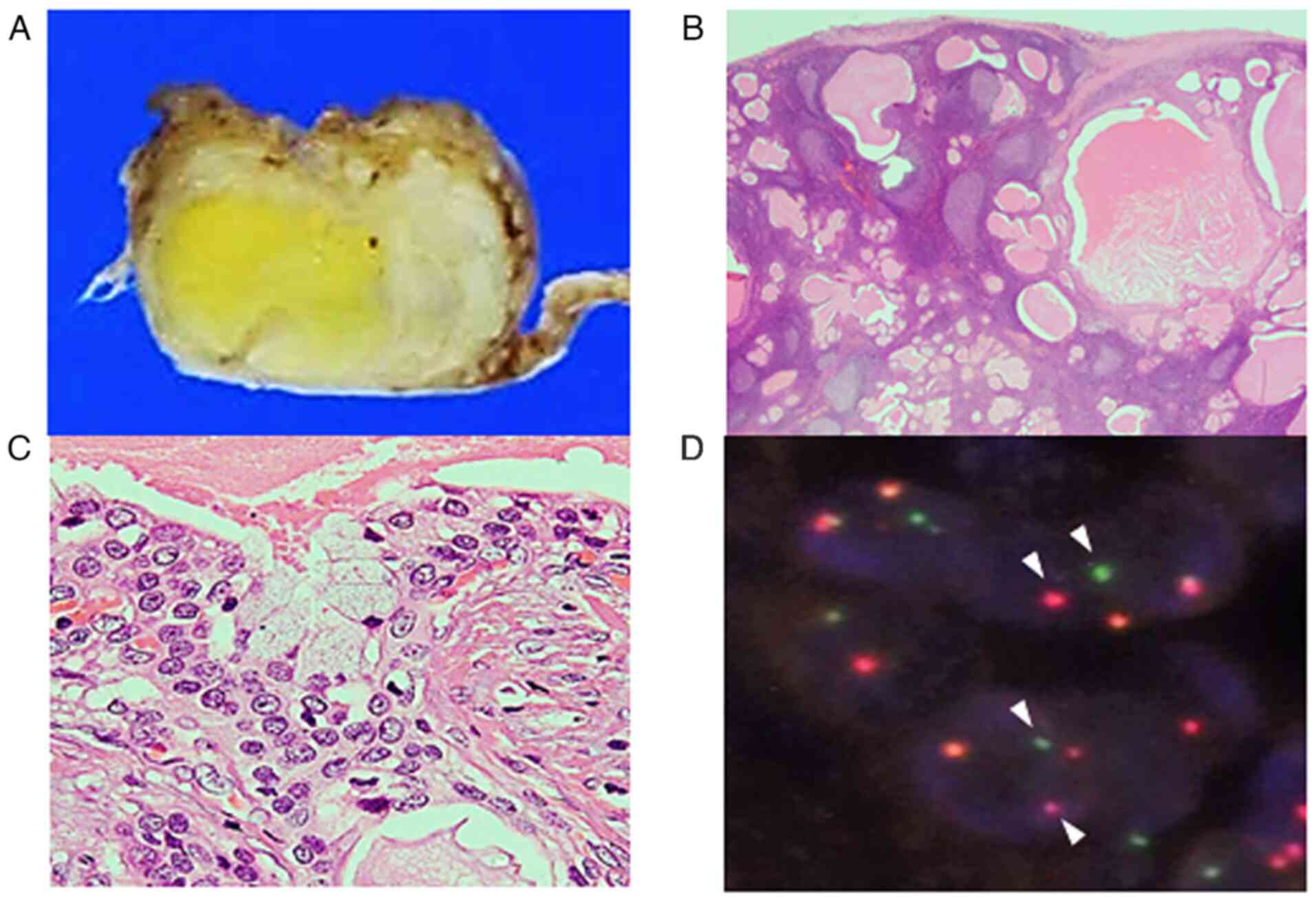
Macroscopic examination of the resected tumour
revealed a well-circumscribed tumour that was white to pale yellow
in colour (Fig. 3A).
Histopathological examination indicated multiple variable-sized
cysts with abundant lymphocytic infiltration accompanying lymphoid
follicle formation around the cysts (Fig. 3B). These cysts were lined by
intermediate cells with mildly enlarged round to oval nuclei
without conspicuous nucleoli, with interspersed mucinous cells
(Fig. 3C). No keratinization or
bi-layered oncocytes were noted. Fluorescence in situ
hybridization using the surgically resected specimen detected
MAML2 rearrangement (Fig.
3D). Accordingly, a diagnosis of Warthin-like MEC was made. The
presence of intermediate cells and mucinous cells, in addition to
the abundant lymphocytic infiltration in the histopathological
specimen, corresponded to the observations in the cytological
specimen.
Discussion
The present study reported the sixth cytological
Warthin-like MEC case. To the best of our knowledge, only 22 cases,
including the present case, have been reported since the first
report by Ishibashi et al (3) in 2015 (8-15).
Table I summarizes the
clinicopathological features of Warthin-like MEC. The most common
and chief complaint is a painless mass. All cases occur in the
parotid gland and females are preferentially affected
(females/males, 18:4). This type of tumour commonly appears in
middle-aged individuals (mean age, 44 years); however, it may also
occur in teenagers and four cases, including the present one, have
been reported, while the total age range is 13-60 years. There were
no obvious regional or nationality preferences. These clinical
characteristics are similar to those of conventional MEC, which is
more likely to occur in females and may affect the paediatric
population (1,15). Radiological features were available
for nine cases: Ultrasonography (9,11)
and computed tomography (8,10,15)
revealed a well-circumscribed tumour with multiple cysts. Magnetic
resonance imaging displayed a low- or intermediate-intensity tumour
(10-12,15).
These features are consistent with those of conventional low-grade
MEC (16).
 | Table ISummary of the clinicopathological
features of Warthin-like mucoepidermoid carcinoma. |
Table I
Summary of the clinicopathological
features of Warthin-like mucoepidermoid carcinoma.
| Authors (year) | Age, years/sex | Chief complaint | Location | Duration
(months) | Treatment | Adjuvant
(months) | Follow-up
(months) | Disease status | MAML2
fusions | Metastasis | FNA | (Refs.) |
|---|
| Ishibashi et
al (2015) | 23/F | NA | Parotid | 1 | Resection | NA | 120 | NED | Positive | No | NA | (3) |
| Ishibashi et
al (2015) | 23/F | NA | Parotid | 120 | Resection | NA | 36 | NED | Positive | No | NA | (3) |
| Ishibashi et
al (2015) | 33/F | NA | Parotid | NA | Resection | NA | 96 | NED | Positive | No | NA | (3) |
| Ishibashi et
al (2015) | 46/F | NA | Parotid | 24 | Resection | NA | 120 | NED | Positive | No | NA | (3) |
| Ishibashi et
al (2015) | 60/F | NA | Parotid | 240 | Resection | NA | 12 | NED | Positive | No | NA | (3) |
| Hang et al
(2017) | 53/F | Painlessmass | Parotid | NA | Superficial
parotidectomy | None | NA | NA | Positive | No | Done | (10) |
| Hang et al
(2017) | 53/F | Painlessmass | Parotid | 12 | Parotidectomy | None | NA | NA | Positive | No | Done | (10) |
| Heatley et
al (2018) | 17/F | Painlessmass | Parotid | NA | Resection | NA | 48 | LR | Not done | No | NA | (13) |
| Bishop et al
(2018) | 42/M, 33/F, 53/F,
51/M, 51/F, 53/F | NA | Parotid | NA | Resection (2 cases)
NA (4 cases) | NA | 7 and 20 months
(each case) | NED (2 cases) | Positive | No | NA | (14) |
| Akaev et al
(2018) | 53/F | Painlessmass | Parotid | NA | Superficial
parotidectomy | NA | 14 | NED | Positive | No | Done | (11) |
| Balasubiramaniyan
et al (2019) | 56/F | Painlessmass | Parotid | 2 | Superficial
parotidectomy | None | 12 | NED | Not done | No | Done | (9) |
| Zhang et al
(2019) | 36/M | Painlessmass | Parotid | 3 | Parotidectomy | None | 12 | NED | Positive | No | Done | (8) |
| Daoud et al
(2020) | 13/F | Painlessmass | Parotid | 12 | Resection | None | 22 | NED | Positive | No | NA | (15) |
| Daoud et al
(2020) | 14/M | Painlessmass | Parotid | 3 | Resection | Proton therapy
(4) | 30 | LR | Positive | No | NA | (15) |
| Bieńkowski et
al (2020) | 30/F | Painlessmass | Parotid | 6 | Resection | None | NA | NED | Positive | No | NA | (12) |
| Bieńkowski et
al (2020) | 51/F | Painlessmass | Parotid | 84 | Resection | None | NA | NA | Positive | No | ND | (12) |
| Present case | 16/F | Painfulmass | Parotid | 2 | Superficial
parotidectomy | None | 6 | NED | Positive | No | Done | / |
Cytological features of Warthin-like MEC have been
reported for six cases (8-11),
including the present case, although MAML2 rearrangement was
not evaluated in one case (9).
Table II summarizes the
cytological features of these cases. The characteristic cytological
features are the presence of cystic contents, including a mucin and
proteinaceous material background, and lymphocyte abundance.
Squamous, intermediate and mucinous cells were observed in three,
four and four cases, respectively. Nuclear atypia was mild in all
cases. No oncocytic cells, as observed in most of the cytological
Warthin's tumour specimens, were noted in the Warthin-like MEC
cases. Only one case was initially cytodiagnosed as MEC (9), but none as Warthin-like MEC (Table II). FNA cytology frequently fails
to obtain solid components in cases with conventional low-grade MEC
(6), which mainly involves cystic
components; therefore, it is predicted that obtaining solid
components, such as squamous, intermediate and mucinous cells, is
difficult in Warthin-like MEC. In the cytological case series of
Warthin-like MEC, only one case harboured the above-mentioned cells
(Table II). These cytological
features and the presence of lymphocyte abundance in a background
with mild nuclear atypia make the cytological diagnosis of
Warthin-like MEC difficult.
 | Table IICytological features of Warthin-like
mucoepidermoid carcinoma. |
Table II
Cytological features of Warthin-like
mucoepidermoid carcinoma.
| Authors (year) | MAML2
fusion | Initial cytological
diagnosis | Cellularity | Background | Lymphocytes | Squamous
cellsa | Intermediate
cellsb | Mucous cells | Oncocytes | Nuclear atypia | (Refs.) |
|---|
| Balasubiramaniyan
et al (2019) | Not assessed | High-grade MEC | Cellular | Cystic component,
necrosis, macrophages | + | + | + | + | - | Mild | (9) |
| Hang et al
(2017) | Positive | Low-grade MEC,
suspected but cannot exclude a non-neoplastic cyst with
reactive/metaplastic changes | Moderate | Cystic component;
mucin, histiocytes, multinucleated giant cells | + | + | + | - | - | Mild | (10) |
| Hang et al
(2017) | Positive | 1st: Pleomorphic
adenoma; 2nd: MEC, suspected | Small
amountc | Cystic component;
myxoid, proteinaceous | + | + | + | - | - | Mild | (10) |
| Akaev et al
(2018) | Positive | Insufficient
material | Small amount | Intense
inflammatory infiltrate with few duct cells | + | - | - | - | - | - | (11) |
| Zhang et al
(2019) | Positive | Warthin tumour or a
tumour with sebaceous cell component, among others | Small
amountd | Cystic contents;
macrophages, lymphoid cells | + | - | - | + | - | Mild | (8) |
| Present case | Positive | Lymphoepithelial
sialadenitis | Moderate | Cystic contents;
lymphocytes macrophages | + | - | + | + | - | Mild | / |
Cytological differential diagnoses of Warthin-like
MEC includes Warthin's tumour and LS. The characteristic
cytological features of Warthin's tumour include the combined
presence of oncocytes with or without squamous/mucinous metaplastic
cells and lymphocytes in the proteinaceous background (17). Intermediate cells are not observed
in Warthin's tumour (17). The
clinicopathological features are useful for differential diagnosis,
as Warthin's tumour frequently occurs in the bilateral parotid
glands and mainly arises in 50- to 60-year-old males with a history
of cigarette smoking (1,2), which differs from that of
Warthin-like MEC. Oncocytic metaplastic cells, as well as squamous
and mucinous metaplastic cells, may be present in LS; however,
intermediate cells and mucinous fluid have not been observed
(18). In the present case, the
presence of intermediate cells in the cytological specimens was
overlooked (as intermediate cells exhibited mild nuclear atypia,
mimicking the non-neoplastic cells, as described earlier), leading
to the initial diagnosis of LS and not Warthin-like MEC. The
presence of mucinous fluid and abundant lymphocytes in the
background, intermediate cells and lack of oncocytic cells may be
key cytological features of Warthin-like MEC, although a definitive
cytological diagnosis may be difficult to reach and the correlation
with clinicoradiological features is important.
The most critical diagnostic clue for Warthin-like
MEC is MAML2 rearrangement (3), as
observed in the present case. Cyclic adenosine monophosphate
responsive element binding protein-regulated transcription
coactivator 1/3-MAML2 fusions occur in >50% of conventional
MECs, which are specific to MEC (3,12,19)
and are correlated with low-/intermediate-grade histology and
improved prognosis (17,20). Fluorescence in situ
hybridization using cellblock specimens is used for detecting
MAML2 rearrangement in conventional MEC diagnosis (20); however, no such analysis for
Warthin-like MEC using cytological specimens has been described.
Further studies are required to clarify the usefulness of gene
rearrangement analysis using cytological specimens for the
diagnosis of Warthin-like MEC diagnosis.
In conclusion, the present study described an
additional cytological case of Warthin-like MEC and reviewed the
cytological features of this rare tumor for the first time. The
characteristic FNA cytological features of this rare tumour type
are the presence of mucinous material and abundant lymphocytes in
the background, the presence of intermediate cells and the lack of
oncocytic cells. It is crucial for cytologists and cytopathologists
to recognize these features. Clinicopathological characteristics
may help with differential diagnoses, particularly from Warthin's
tumour, and the detection of MAML2 rearrangement leads to an
accurate diagnosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
Conception and design of the study: YN and MI; data
collection and analysis: YN, MI, KO, KS, YE, CM, TF, MY, HI and KT;
confirmation of the authenticity of all raw data: YN and MI;
drafting the manuscript and figures: YN and MI. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
Declaration of Helsinki and the study protocol was approved by the
Institutional Review Board of Kansai Medical University Hospital
(approval no. 160646). Opt-out consent was obtained from the
participant of this study.
Patient consent for publication
Opt-out consent was obtained from the participant of
this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
El-Naggar AK and JKC C: World Health
Organization Classification of Head and Neck Tumours. 4th edition.
Grandis JR, Takata T, Grandis J and Slootweg P (eds). IARC, Lyon,
2017.
|
|
2
|
AFIP Atlas of Tumor Pathology, 4th Series
Fascicle: Tumors of the Salivary Glands. Ellis GL, Auclair PL
(eds). ARP, Arlington, 2007.
|
|
3
|
Ishibashi K, Ito Y, Masaki A, Fujii K,
Beppu S, Sakakibara T, Takino H, Takase H, Ijichi K, Shimozato K
and Inagaki H: Warthin-like mucoepidermoid carcinoma: A combined
study of fluorescence in situ hybridization and whole-slide
imaging. Am J Surg Pathol. 39:1479–1487. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang X, Baloch ZW, Cooper K, Zhang PJ,
Puthiyaveettil R and LiVolsi VA: The significance of mucinous
metaplasia in Warthin tumor: A frequent occurrence and potential
pitfall. Hum Pathol. 99:13–26. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sandhu VK, Sharma U, Singh N and Puri A:
Cytological spectrum of salivary gland lesions and their
correlation with epidemiological parameters. J Oral Maxillofac
Pathol. 21:203–210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Joseph TP, Joseph CP, Jayalakshmy PS and
Poothiode U: Diagnostic challenges in cytology of mucoepidermoid
carcinoma: Report of 6 cases with histopathological correlation. J
Cytol. 32:21–24. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Al-Khafaji BM, Nestok BR and Katz RL:
Fine-needle aspiration of 154 parotid masses with histologic
correlation: Ten-year experience at the University of Texas. M.D.
Anderson Cancer Center. Cancer. 84:153–159. 1998.PubMed/NCBI
|
|
8
|
Zhang D, Liao X, Tang Y, Meyer RG, Van
Dyke DL, Liu X, Islam MN and Lai J: Warthin-like mucoepidermoid
carcinoma of the parotid gland: Unusual morphology and diagnostic
pitfalls. Anticancer Res. 39:3213–3217. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Balasubiramaniyan V, Sultania M, Sable M,
Muduly D and Kar M: Warthin-like mucoepidermoid carcinoma of the
parotid gland: A diagnostic and therapeutic dilemma. Autops Case
Rep. 9(e2019122)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hang JF, Shum CH, Ali SZ and Bishop JA:
Cytological features of the Warthin-like variant of salivary
mucoepidermoid carcinoma. Diagn Cytopathol. 45:1132–1136.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Akaev I, Yeoh CC, Brennan PA and Rahimi S:
Low grade parotid mucoepidermoid carcinoma with tumour associated
lymphoid proliferation (‘Warthin-like’) and CRTC1-MAML2 fusion
transcript: Definitive diagnosis with molecular investigation only.
Oral Oncol. 80:98–99. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bieńkowski M, Kunc M, Iliszko M, Kuźniacka
A, Studniarek M and Biernat W: MAML2 rearrangement as a useful
diagnostic marker discriminating between Warthin tumour and
Warthin-like mucoepidermoid carcinoma. Virchows Arch. 477:393–400.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Heatley N, Harrington KJ and Thway K:
Warthin tumor-like mucoepidermoid carcinoma. Int J Surg Pathol.
26:31–33. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bishop JA, Cowan ML, Shum CH and Westra
WH: MAML2 rearrangements in variant forms of mucoepidermoid
carcinoma: Ancillary diagnostic testing for the ciliated and
Warthin-like variants. Am J Surg Pathol. 42:130–136.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Daoud EV, McLean-Holden AC, Pfeifer CM,
Timmons CF, Oliai BR and Bishop JA: Pediatric Warthin-like
mucoepidermoid carcinoma: Report of two cases with one
persistent/recurrent as conventional mucoepidermoid carcinoma. Head
Neck Pathol. 14:923–928. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang YQ, Mo YX, Li S, Luo RZ, Mao SY and
Shen JX: Low-grade and high-grade mucoepidermoid carcinoma of the
lung: CT findings and clinical features of 17 cases. AJR Am J
Roentgenol. 205:1160–1166. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Flezar M and Pogacnik A: Warthin's tumour:
Unusual vs. common morphological findings in fine needle aspiration
biopsies. Cytopathology. 13:232–241. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ronchi A, Montella M, Marra PM, Colella G,
Franco R and Cozzolino I: Myoepithelial sialadenitis with
metachromatic matrix: A diagnostic pitfall. A case of salivary
gland swelling in a paediatric patient evaluated by fine needle
aspiration cytology. Cytopathology. 32:257–260. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nakayama T, Miyabe S, Okabe M, Sakuma H,
Ijichi K, Hasegawa Y, Nagatsuka H, Shimozato K and Inagaki H:
Clinicopathological significance of the CRTC3-MAML2 fusion
transcript in mucoepidermoid carcinoma. Mod Pathol. 22:1575–1581.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Darras N, Mooney KL and Long SR:
Diagnostic utility of fluorescence in situ hybridization testing on
cytology cell blocks for the definitive classification of salivary
gland neoplasms. J Am Soc Cytopathol. 8:157–164. 2019.PubMed/NCBI View Article : Google Scholar
|