Introduction
Mucoepidermoid carcinoma (MEC) is one of the most
common primary salivary gland malignancies. MECs are characterised
by mucin production, and they are composed of intermediate-type and
squamoid cells exhibiting cystic and solid growth patterns
(1). The tumour commonly develops
in the three major salivary glands, including the parotid,
submandibular and sublingual glands. Moreover, it can also develop
in several minor salivary glands of the oral cavity, including
glands of the tongue (lingual glands). The lingual glands are
divided into various groups, among which the anterior lingual
glands (glands of Blandin-Nuhn) are found near the ventral surface
of the apex of the tongue. However, MECs of the anterior lingual
glands are rarely reported (2-7).
Approximately 20 and 50% of the tumours that affect
the major and minor glands, respectively, are malignant (1). The diagnosis of MECs is mainly based
on H&E staining of the tumours and a combination of both
H&E and immunohistochemistry tests, such as cytokeratin 5/6
(CK5/6) and transformation-related protein (p63) staining. To
reduce the risk of misdiagnosis, sections stained with H&E for
histopathological diagnosis require adequate biopsy material in
order to uncover the full range of morphological and cytological
characteristics. We herein present a rare case of a patient with
MEC of the anterior lingual gland, who initially underwent needle
biopsy (NB) and was misdiagnosed using the obtained specimen. An
alternative approach, namely the incisional biopsy, which was
instrumental in confirming the diagnosis of low-grade MEC of the
anterior lingual gland in the present case, is also discussed and
the relevant literature is reviewed.
Case report
Patient history
An 82-year-old woman was referred to the Department
of Oral and Maxillofacial Surgery of Ryukyu University Hospital in
April 2018 from a private dental practitioner. The patient
presented with a ~5-month history of swelling on the ventral
surface of the apex of the tongue. The swelling was insidious in
onset, had gradually increased to the current size and reportedly
interfered with the patient's chewing. The initial (primary)
assessment of the lesion was at a nearby hospital. Based on the
patient's medical records, the primary assessment resulted in a
working diagnosis of irritation fibroma following histopathological
examination of a specimen obtained via NB. The histopathological
review mentioned that the tongue mucosal tissue had thick collagen
bundles interspersed with numerous capillaries and dotted chronic
inflammatory cells under the surface of the squamous epithelium,
with no findings suggestive of malignancy (Fig. S1). However, 2 weeks prior to the
referral the patient suddenly developed pain at the tumour site,
which worsened during eating, and decided to seek a second opinion.
In addition, the patient had a history of hypertension and
osteoporosis; however, the dental, social and family history was
unremarkable. General physical examination indicated that the
patient was moderately built and well-nourished, with no pallor,
icterus, cyanosis or clubbing. The patient's face was symmetrical
and there was no local or generalised lymphadenopathy. Intraoral
examination revealed a round well-circumscribed mass, measuring
19x15 mm, in the right ventral surface of the apex of the tongue.
Although the patient's tongue movement was impaired, there was no
evidence of dysphagia (Fig. 1).
Locally, the mass was tender on palpation and hard in
consistency.
Investigations
Radiological assessment was performed, including
contrast-enhanced CT scan, contrast-enhanced MRI using the
short-tau inversion-recovery (STIR)-PROPELLER technique, and
fluorodeoxyglucose-positron emission tomography
(18F-FDG-PET)/CT, in addition to the routine
haematological and histopathological diagnostic evaluations.
Axial CT scan revealed a metallic artefact that
hindered a clear view of the right hypoglossal area. The MRI
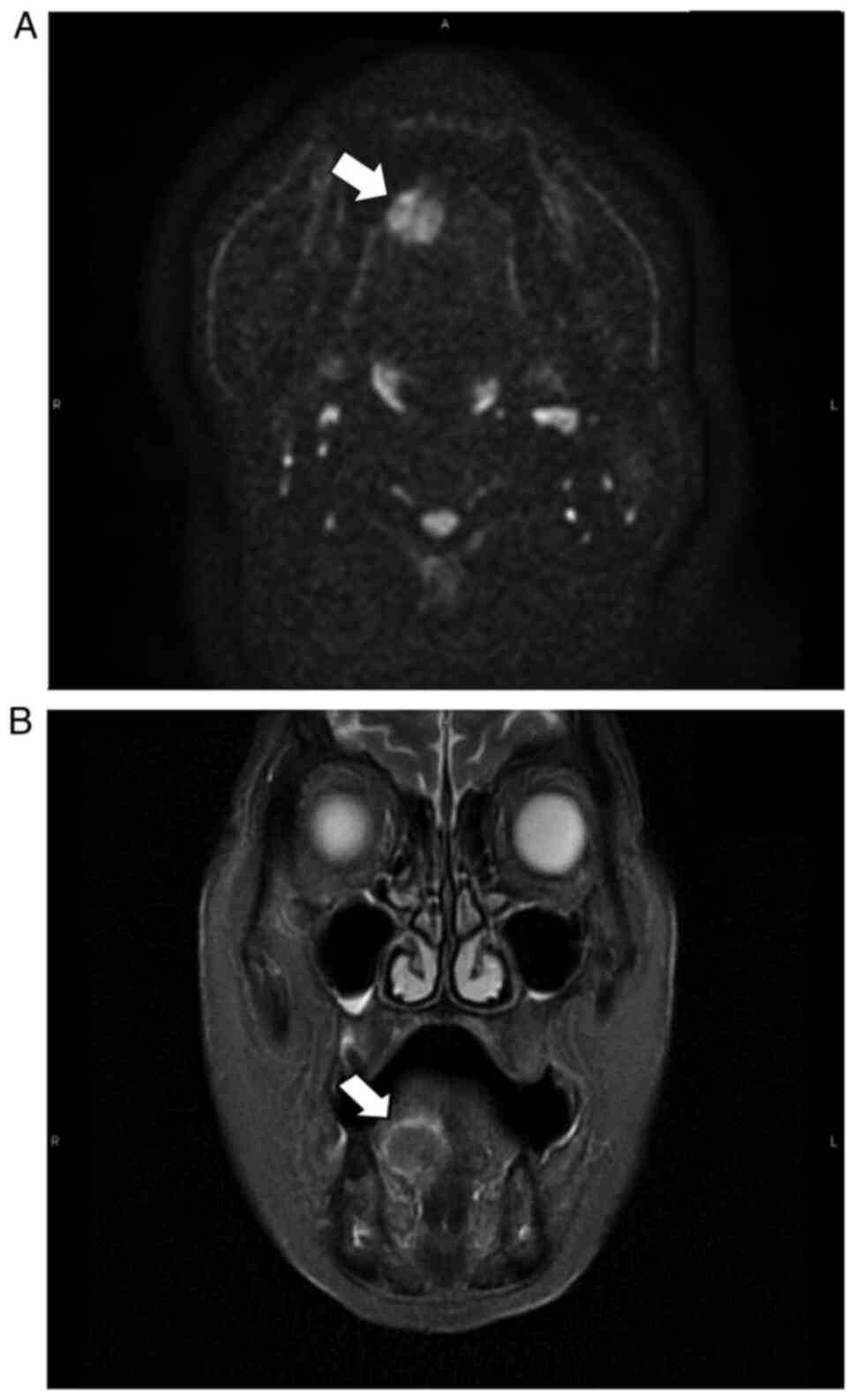
(Fig. 2A) and STIR-PROPELLER
imaging (Fig. 2B) revealed right
anterior lingual hyperintensities on diffusion-weighted imaging.
Preoperative FDG-PET/CT examination showed abnormal FDG
accumulation in the right anterior lingual margin (maximum
standardized uptake value: 6.56; Fig.
3). None of the investigative findings were indicative of
cervical lymph node or distant metastasis. The findings of the
haematological evaluations were normal. The differential diagnosis
included tongue cancer and mucous cyst.
Treatment and follow-up
The patient was locally anaesthetised without
sedation, the tongue was stabilised with a traction suture and a
perilesional (incisional) biopsy was performed to establish a
definitive diagnosis, perform histological grading and select the
appropriate treatment. On gross examination, the resected specimen
included a poorly circumscribed lesion with a whitish appearance in
the posterior region. Histopathological examination revealed that
the superficial layer comprised tissue covered with thickened,
ulcerated stratified squamous epithelium; atypical cells were
arranged in solid sheets and alveoli and proliferated
infiltratively. No obvious keratinisation was observed. On
immunohistochemistry examination, the cells arranged in alveolar
formations were positive for cytokeratin AE1/AE3 and
carcinoembryonic antigen (data not shown), and the intra-alveolar
mucus was positive for periodic acid-Schiff staining (Fig. 4A). The cells had irregular nuclei
and foamy bright endoplasmic reticulum, and they proliferated in a
vesicular nest and sheet-like pattern (Fig. 4B); furthermore, perineural invasion
was also identified (Fig. 4C).
Taken together, these findings confirmed the diagnosis of low-grade
MEC following the histopathological grading system described by
Goode et al (8). The TNM
stage of the lesion was T1N0M0.
One month later, in April 2018, partial resection of
the right anterior part of the patient's tongue was performed, with
a 10-mm safety margin around the tumour. Furthermore,
split-thickness skin grafting was performed under general
anaesthesia (Fig. 5). The
patient's healing was uneventful (Fig. S2) and she was followed-up over a
period of 3 years, with physical examinations performed monthly in
the first year, bi-monthly in the second year and every 6 months in
the third year (last follow-up visit, April 2021). An axial CT scan
of the oral cavity performed 1 year postoperatively did not
indicate any evidence of recurrence or metastasis (Fig. S3).
Discussion
First described in 1945 by Stewart et al
(9), MEC commonly affects adults
in their fifth and sixth decades of life (10), although it exhibits a predilection
for individuals in their 30s and 40s (11) and those of female sex (12). Although MEC accounts for 30% of all
cancers of the salivary glands, it comprises <5% of head and
neck cancers. An estimated 50-60% of these tumours arise in the
major salivary glands, with >80% occurring in the parotid gland,
8-13% in the submandibular gland and 2-4% in the sublingual gland.
The remaining 20% occurs in minor salivary glands, mostly in the
palate, with the tongue being the least frequently affected site
(10,13). MECs can develop in any of the
salivary glands in the three parts of the tongue: The anterior
lingual glands (glands of Blandin-Nuhn) under the ventral surface
of the apex of the tongue, the Ebner's gland in the submucosa of
the foliate and circumvallate papillae, and the posterior lingual
gland in the base of the tongue and the lingual margin. Moreover,
among the three parts of the tongue, most malignancies reportedly
occur in the base (14). In the
present case, the lesion was detected under the right ventral
surface of the apex of the tongue, near the lingual frenulum (a
site of opening of the duct of the anterior lingual glands); thus,
it was considered to have originated from the anterior lingual
salivary gland.
It has been emphasised that the histopathological
diagnosis of MEC is primarily based on morphology and ancillary
staining in combination with immunohistochemistry (15). However, it may be challenging,
particularly in high-grade tumours and intraoral MECs comprising
prominent clear cells and a significant amount of oncocytic
material (Warthin-like variant) in small biopsy specimens (15). In the present case, a NB was first
performed elsewhere, and fibrous connective tissue, collagen
bundles interspersed with numerous capillaries and chronic
inflammatory cells under the surface of squamous epithelium were
identified on histological examination. Indeed, these findings are
suggestive of an irritation fibroma. The details of the NB
procedure at the primary health care facility were not clear.
However, it may be inferred that either the NB was taken from
scarred and inflamed tissue around the tumour or the procedure was
poorly performed and an inadequate amount of tissue was collected.
Accurate preoperative diagnosis enables the formulation of a
treatment plan that is appropriate for the disease grade. The
initial symptom experienced in the present case was discomfort
during eating due to the swelling. The patient later developed pain
on chewing. This likely prompted the patient to seek a second
opinion from the private dental practitioner, who then referred her
to our hospital, leading to detection of the cancer at the
relatively early stage of T1N0M0.
Goode et al (8) evaluated tumours histologically by the
presence of specific parameters, such as <20% intracystic
component, neural invasion, necrotic foci, a mean of ≥4 mitoses per
10 high-power fields and anaplasia, and then graded them
accordingly using a score ranging from 0 to 14 as follows: 0-4,
low-grade; 5-6, intermediate-grade; and ≥7, high-grade. Treatment
recommendations can be made based on this scoring; for example,
soft tissue resection with a 1-cm mucosal margin should be
performed for T1 low-grade MEC without clinical or radiographic
signs of osseous invasion (16,17).
For the majority of the patients, wide surgical excision is usually
sufficient; however, adjuvant therapy is indicated in cases with
high-grade MEC, tumour-positive margins or evidence of lymph node
infiltration, or residual disease (18). McHugh et al (19) stated that surgical resection is the
treatment of choice for MEC, and surgical margins are considered
important for preventing recurrence. In patients with close margins
after resection, re-excision is preferred for intraoral tumours as
opposed to adjuvant irradiation, as the latter is associated with
significant morbidity (20).
Brandwein et al (21)
reported that none of the patients undergoing re-excision developed
tumour recurrence, and were thus classified as having negative
margins (>3 mm). Some authors recommend a prophylactic neck
dissection for high-grade and clinical T3 or T4 tumours (22). Elective neck irradiation may be
appropriate for patients with an increased risk of nodal metastases
(17). Spiro et al
(23) reported that, among 367
cases of MEC, 59% of high-grade, 30% of intermediate-grade and 7%
of low-grade tumours had cervical lymph node metastasis. Evans
(24) examined 69 cases and found
that 70% (14/20) of high-grade and 8% (4/49) of low-grade tumours
were associated with cervical lymph node metastasis. Hicks et
al (25), in their study on 48
MEC cases, reported lymph node metastasis in 72% of high-grade, 22%
of intermediate-grade and 0% of low-grade tumours. Based on these
results, the frequency of cervical lymph node metastasis was
closely associated with the tumour grade. MECs of the minor
salivary glands have been reported to be most commonly low-grade
and to have a better clinical course compared with MECs of the
major salivary glands (6,8,26).
In the present case, the patient was diagnosed with
low-grade MEC as per the histological classification of Goode et
al (8), in which the biopsy
scored 4 points and a safety margin of 10 mm was set for the
operation. Although no signs of recurrence were observed in this
patient 3 years postoperatively, there are reports of local
recurrence and distant metastasis that warrant the continuation of
follow-up, even after this period (8).
In conclusion, we herein presented a rare case of an
initially misdiagnosed low-grade MEC of the anterior lingual gland,
which was correctly diagnosed using an alternative approach to the
NB, the incisional biopsy. Tumours that develop in rarely affected
sites, such as the anterior lingual glands, should be carefully
investigated, and sufficient histological specimens should be
obtained to ensure early detection of malignancy, accurate tumour
grading and treatment optimization. The incisional biopsy is likely
superior to NB for obtaining adequate material for an accurate
diagnostic histopathological evaluation.
Supplementary Material
Histopathological examination of the
needle biopsy specimen obtained from the patient's previous medical
records. The pathological findings were described as tongue mucosal
tissue with thick collagen bundles interspersed with numerous
capillaries and dotted chronic inflammatory cells under the surface
squamous epithelium, suggesting a diagnosis of irritation fibroma.
H&E staining; scale bar, 250 μm.
Macroscopic appearance of the tongue
after 1 year of postoperative follow-up. The surgical site at the
right anterior tongue (arrowhead) appears to have healed
uneventfully.
Postoperative axial CT scan of the
oral cavity. A CT examination at 1 year postoperatively revealed no
pathological findings.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed for the case
report are available from the corresponding author on reasonable
request.
Authors' contributions
SG, TN, SM and FH analyzed and interpreted the
patient data; SG, AM and EHN drafted and critically revised the
manuscript for important intellectual content; YS and HN confirmed
the authenticity of all the raw data and provided final approval of
the completed article. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for both the surgical treatment and publication of the case
details and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin HH, Limesand KH and Ann DK: Current
state of knowledge on salivary gland cancers. Crit Rev Oncog.
23:139–151. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mathew L, Janardhanan M, Suresh R and
Savithri V: Mucoepidermoid carcinoma of the posterior-lateral
border of tongue: A rare presentation. BMJ Case Rep.
2017(bcr2017221521)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zahran M and Youssef A: Mucoepidermoid
carcinoma of the tongue base: A case report. OTO Open.
2(2473974x18791559)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mesolella M, Iengo M, Testa D, DI Lullo
AM, Salzano G and Salzano FA: Mucoepidermoid carcinoma of the base
of tongue. Acta Otorhinolaryngol Ital. 35:58–61. 2015.PubMed/NCBI
|
|
5
|
Goldblatt LI and Ellis GL: Salivary gland
tumors of the tongue. Analysis of 55 new cases and review of the
literature. Cancer. 60:74–81. 1987.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Auclair PL, Goode RK and Ellis GL:
Mucoepidermoid carcinoma of intraoral salivary glands. Evaluation
and application of grading criteria in 143 cases. Cancer.
69:2021–2030. 1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Foote FW Jr and Frazell EL: Tumors of the
major salivary glands. Cancer. 6:1065–1133. 1953.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Goode RK, Auclair PL and Ellis GL:
Mucoepidermoid carcinoma of the major salivary glands: Clinical and
histopathologic analysis of 234 cases with evaluation of grading
criteria. Cancer. 82:1217–1224. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Stewart FW, Foote FW and Becker WF:
Muco-epidermoid tumors of salivary glands. Ann Surg. 122:820–844.
1945.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pires FR, Chen SY, da Cruz Perez DE, de
Almeida OP and Kowalski LP: Cytokeratin expression in central
mucoepidermoid carcinoma and glandular odontogenic cyst. Oral
Oncol. 40:545–551. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chaudhry AP, Vickers RA and Gorlin RJ:
Intraoral minor salivary gland tumors. An analysis of 1,414 cases.
Oral Surg Oral Med Oral Pathol. 14:1194–1226. 1961.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gill S, Mohan A, Aggarwal S and Varshney
A: Mucoepidermoid carcinoma of hard palate. Indian J Pathol
Microbiol. 61:397–398. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Devaraju R, Gantala R, Aitha H and Gotoor
SG: Mucoepidermoid carcinoma. BMJ Case Rep.
2014(bcr-2013-202776)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Burbank PM, Dockerty MB and Devine KD: A
clinicopathologic study of 43 cases of glandular tumors of the
tongue. Surg Gynecol Obstet. 109:573–582. 1959.PubMed/NCBI
|
|
15
|
Moutasim KA and Gareth JT: Salivary gland
tumours: Update on molecular diagnostics. Diagnostic Histopathol.
26:159–164. 2020.
|
|
16
|
Yih WY, Kratochvil FJ and Stewart JC:
Intraoral minor salivary gland neoplasms: Review of 213 cases. J
Oral Maxillofac Surg. 63:805–810. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen AM, Garcia J, Lee NY, Bucci MK and
Eisele DW: Patterns of nodal relapse after surgery and
postoperative radiation therapy for carcinomas of the major and
minor salivary glands: What is the role of elective neck
irradiation? Int J Radiat Oncol Biol Phys. 67:988–994.
2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu S, Ow A, Ruan M, Yang W, Zhang C and
Wang L: Prognostic factors in primary salivary gland mucoepidermoid
carcinoma: An analysis of 376 cases in an Eastern Chinese
population. Int J Oral Maxillofac Surg. 43:667–673. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
McHugh CH, Roberts DB, El-Naggar AK, Hanna
EY, Garden AS, Kies MS, Weber RS and Kupferman ME: Prognostic
factors in mucoepidermoid carcinoma of the salivary glands. Cancer.
118:3928–3936. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yan K, Yesensky J, Hasina R and Agrawal N:
Genomics of mucoepidermoid and adenoid cystic carcinomas.
Laryngoscope Investig Otolaryngol. 3:56–61. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Brandwein MS, Ivanov K, Wallace DI, Hille
JJ, Wang B, Fahmy A, Bodian C, Urken ML, Gnepp DR, Huvos A, et al:
Mucoepidermoid carcinoma: A clinicopathologic study of 80 patients
with special reference to histological grading. Am J Surg Pathol.
25:835–845. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ellis MA, Graboyes EM, Day TA and Neskey
DM: Prognostic factors and occult nodal disease in mucoepidermoid
carcinoma of the oral cavity and oropharynx: An analysis of the
National Cancer Database. Oral Oncol. 72:174–178. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Spiro RH, Huvos AG, Berk R and Strong EW:
Mucoepidermoid carcinoma of salivary gland origin. A
clinicopathologic study of 367 cases. Am J Surg. 136:461–468.
1978.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Evans HL: Mucoepidermoid carcinoma of
salivary glands: A study of 69 cases with special attention to
histologic grading. Am J Clin Pathol. 81:696–701. 1984.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hicks MJ, el-Naggar AK, Flaitz CM, Luna MA
and Batsakis JG: Histocytologic grading of mucoepidermoid carcinoma
of major salivary glands in prognosis and survival: A
clinicopathologic and flow cytometric investigation. Head Neck.
17:89–95. 1995.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Melrose RJ, Abrams AM and Howell FV:
Mucoepidermoid tumors of the intraoral minor salivary glands: A
clinicopathologic study of 54 cases. J Oral Pathol. 2:314–325.
1973.PubMed/NCBI View Article : Google Scholar
|