Introduction
Minichromosome maintenance (MCM) proteins are DNA
helicases essential for DNA replication and participate in
eukaryotic DNA replication by binding to chromatin before the
initiation of DNA replication from the early G1 phase to the S
phase of the cell cycle (1).
Hence, MCM protein deregulation has been linked to tumor formation,
progression and malignant transformation. MCM2, a member of the MCM
family (MCM2-MCM7) that comprises 904 amino acids and has a
molecular mass of 102 kDa, has been shown to be a cell
proliferation marker (2).
MCM proteins have been shown to promote cell
proliferation in vitro and in vivo in several types
of cancer, and MCM expression is implicated in cancer cell
proliferation mainly because these proteins can enhance DNA
replication. MCM2 and MCM7, in particular, are involved in various
cellular functions in humans and other eukaryotes (3). MCM7 expression in the bronchial
brushing of patients with non-small cell lung cancer (NSCLC) has
been found to be associated with prognosis (4), whereas MCM2 is frequently expressed
during premalignant lung cell proliferation and is reportedly a
sensitive marker for the early detection of pulmonary malignant
lesions. MCM expression has also been associated with higher cell
proliferation in non-dysplastic squamous epithelium, malignant
fibrous histiocytomas and endometrial carcinoma. Furthermore, MCM2
expression has been found to be associated with a higher mitotic
index and worse survival in patients with breast cancer, colon
cancer, adrenocortical carcinoma and gliomas (5-7).
We previously identified the molecular signatures
for malignancy in small cell lung cancer (SCLC), which is
characterized by highly aggressive clinical features and poor
prognosis, and detected the following five tumor proliferation
proteins involved in SCLC malignancy: MCM2, MCM4, MCM6, MCM7 and
MutS homolog 2 (MSH2) (1). Hence,
the aim of the present study was to investigate whether MCM2
expression is of clinical and prognostic value in patients who have
undergone resection of lung adenocarcinoma.
Materials and methods
Patients
Between January 2009 and December 2010, 102 patients
with lung adenocarcinoma underwent complete pulmonary resection at
St. Marianna Medical University Hospital (Kanagawa, Japan).
Complete pulmonary resection was defined as lobectomy or a more
extensive lung resection with ipsilateral hilar and mediastinal
lymph node dissection and no evidence of residual cancer, either
macroscopically or microscopically. Patients who had received
preoperative chemotherapy, radiotherapy, or both, were excluded.
Ultimately, 73 patients with a final pathological diagnosis of lung
adenocarcinoma measuring ≥10 mm were enrolled in the present
study.
The following clinicopathological data were
collected from the hospital charts of all patients: Age, sex,
pathological T factor, histological type, surgical procedure(s),
relapse type, EGFR mutation status, overall survival (OS) and
recurrence-free survival (RFS). OS was defined as the time from the
day of the surgery until the day of death from any cause; patients
who remained alive at the end of the follow-up period were treated
as censored cases. RFS was defined as the time from the day of
surgery to the day of disease recurrence or death from any cause;
patients who remained alive with no evidence of recurrence at the
end of the follow-up period were treated as censored cases.
Preoperative evaluation included physical
examination, blood examination, chest radiography and chest and
abdominal CT, as well as brain CT or MRI and positron emission
tomography (PET)-CT if clinically indicated. Staging and
pathological findings for lung adenocarcinoma were based on the 7th
TNM Classification for Lung and Pleural Tumors (8), the World Health Organization
classification (9), and the
International Association for the Study of Lung Cancer/American
Thoracic Society/European Respiratory Society classification of
lung adenocarcinoma (10).
Following pulmonary resection, all patients were
followed up at our institute every 3 months during the first year
1, every 6 months during years 2-5 and annually thereafter as
outpatients; the follow-up continued for ≥5 years. The follow-up
included physical examination, chest radiography and blood
examination, including serum tumor markers such as carcinoembryonic
antigen. Patients also routinely underwent chest and upper
abdominal CT at every scheduled outpatient appointment. Brain MRI
and/or PET-CT examinations were performed if any symptoms or signs
of recurrence were detected. If metastasis was observed, the
metastatic site was confirmed histologically or cytologically when
clinically feasible. Additionally, EGFR-TKIs were administered to
11 patients with disease recurrence, including gefitinib in 10
cases, erlotinib in 4 cases, afatinib in 3 cases and osimertinib in
1 case.
Clinical samples were tested using cobas®
EGFR Mutation Test version 2.0 (Roche Diagnostics) or the peptide
nucleic acid-locked nucleic acid (PNA-LNA) PCR clamp method (LSI
Medience Corporation). Deletion mutations in exon 19 and point
mutations in exon 18 or exon 21(L858R) were considered to be EGFR
mutations in the present study.
MCM2 expression
All collected surgical specimens were fixed in 10%
formalin for 24 h at room temperature and then embedded in
paraffin. The representative samples were routinely stained with
hematoxylin for 20 min at room temperature and eosin for 1 min at
room temperature by experienced pathologists, followed by reviewing
to confirm the inclusion of carcinoma cells. Next, the
formalin-fixed, paraffin-embedded blocks were cut into serial 3-µm
sections for immunohistochemistry (IHC). All assays were performed
using the automated Histostainer 36 A immunostainer (Nichirei
Biosciences Inc.) according to the manufacturer's instructions. In
addition, the sections were incubated for 60 min at room
temperature with anti-MCM2 monoclonal antibody (1:2,000; cat. no.
ab4461; Abcam) and examined by IHC; specificity was confirmed by
using large-cell neuroendocrine carcinoma as a positive control
according to the expression data in the Human Protein Atlas
database (https://www.proteinatlas.org/).
To evaluate MCM2 antibody immunoreactivity, five
microscopic, high-power fields (magnification, x400) were randomly
selected using a BX51 polarizing microscope (Olympus Corporation);
each field was scored a positive rate calculated as the percentage
of positive cells per total number of cells. Considering that MCM2
is expressed in the nucleus, the number of nucleus-positive cells
was counted. Three independent evaluators, including a pathologist
certified by the Japanese Society of Pathology, assessed the cells
for immunoreactivity and calculated the average value. The
expression levels of MCM2 were assessed using the labeling index,
which was determined by counting the number of distinctly stained
malignant cells, regardless of the staining intensity, divided by
the total number of tumor cells.
Statistical analysis
The OS and PRS were estimated using the Kaplan-Meier
method, and differences in survival rates were determined by
log-rank analysis. Univariate analysis was performed among
different groups. The χ2 test and Mann-Whitney U test
were used for analyzing the categorical variables and identifying
differences between two groups, respectively. For multivariate
analysis, the Cox proportional hazards model with significant
factors identified by the univariate analysis was used to determine
the association between survival and potential prognostic factors.
All P-values were two-sided, and P<0.05 was considered to
indicate statistically significant differences.
All statistical data were analyzed using EZR
(Saitama Medical Center, Jichi Medical University, Saitama, Japan),
a graphical user interface for R, and a modified version of R
commander that adds statistical functions frequently used in
biostatistics (R Foundation for Statistical Computing, Vienna,
Austria).
Results
Association between patient
characteristics and MCM2 expression
The baseline characteristics of the 73 patients and
the association between MCM2 expression and clinicopathological
factors are presented in Table I.
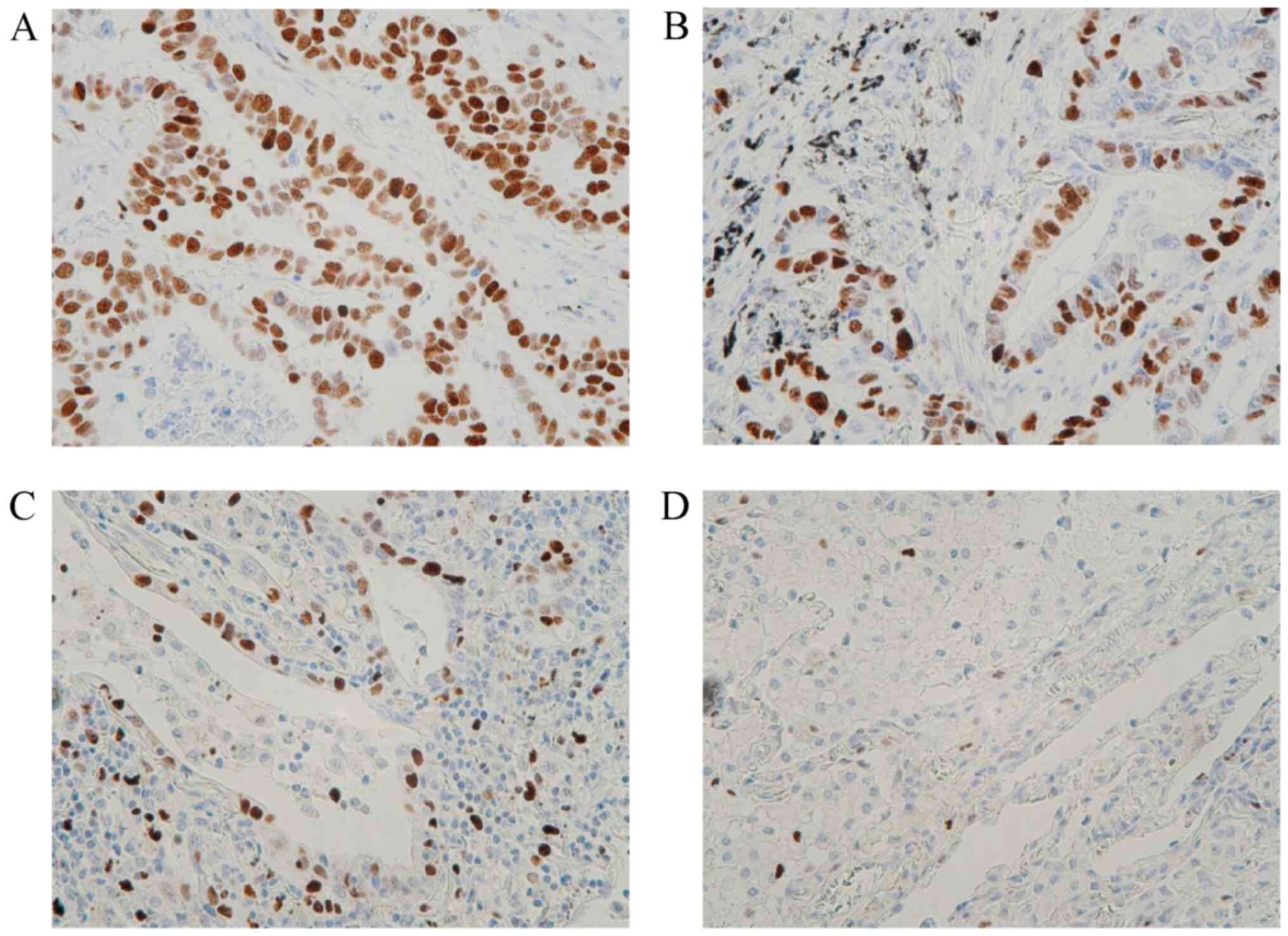
According to the IHC assessment of MCM2 expression in lung
adenocarcinoma tissues (Fig. 1),
35 (48.0%) of the 73 patients exhibited high MCM2 expression
levels. High MCM2 expression was statistically significantly
associated with lung adenocarcinoma subtypes (P=0.0051), N factor
(P=0.0117) and distant recurrence (P=0.0186). MCM2 is
differentially expressed among different subtypes (lepidic-,
acinar- and papillary-predominant) of lung adenocarcinoma, with the
lowest expression levels observed in lepidic-predominant lung
adenocarcinoma (Fig. 1D).
 | Table IBaseline characteristics of patients
(n=73) and association between MCM2 expression and
clinicopathological factors. |
Table I
Baseline characteristics of patients
(n=73) and association between MCM2 expression and
clinicopathological factors.
| | MCM2
expressionb | |
|---|
| Variables | No. of patients | High (n) | Low (n) | P-value |
|---|
| Sex | | | | 0.557 |
|
Male | 36 | 20 | 16 | |
|
Female | 37 | 17 | 20 | |
| Age, years (mean, 70;
range, 33-84) | | | | 0.727 |
|
≥70 | 37 | 20 | 17 | |
|
<70 | 36 | 17 | 19 | |
| Smoking status | | | | 0.564 |
|
Current or
former | 40 | 22 | 18 | |
|
Never | 33 | 15 | 18 | |
| Adenocarcinoma
subtypes | | | | 0.017a |
|
In
situ | 2 | 0 | 2 | |
|
Minimally
invasive | 2 | 0 | 2 | |
|
Lepidic-predominant | 8 | 0 | 8 | |
|
Papillary-predominant | 19 | 10 | 9 | |
|
Acinar-predominant | 29 | 17 | 12 | |
|
Solid-predominant | 9 | 7 | 2 | |
|
Micropapillary-predominant | 3 | 2 | 1 | |
| Pathological
stage | | | | 0.078 |
|
pIAB | 53 | 23 | 30 | |
|
pII-III | 20 | 14 | 6 | |
| T factor (mean
diameter, 23 mm; range, 10-74 mm) | | | | 0.362 |
|
T1 | 45 | 21 | 24 | |
|
T2 | 23 | 12 | 11 | |
|
T3-4 | 5 | 4 | 1 | |
| N factor | | | | 0.020a |
|
N0 | 56 | 25 | 31 | |
|
N1 | 4 | 1 | 3 | |
|
N2 | 13 | 11 | 2 | |
| EGFR status | | | | 0.397 |
|
Positive | 33 | 16 | 17 | |
|
Negative | 35 | 17 | 18 | |
|
Unknown | 5 | 4 | 1 | |
| Distant
recurrence | | | | 0.024a |
|
Yes | 15 | 12 | 3 | |
|
No | 58 | 25 | 33 | |
Survival analysis and prognostic
factors
The median follow-up time was 69 months (range,
6-120 months). To investigate the prognostic impact of MCM2
expression in patients with resected lung adenocarcinoma, patients
were grouped according to MCM2 expression (%) as follows: Groups 1
(<15 vs. ≥15), 2 (<20 vs. ≥20), 3 (<25 vs. ≥25), 4 (<30
vs. ≥30), 5 (<35 vs. ≥35) and 6 (<40 vs. ≥40). Group 4
exhibited the most significant difference in MCM2 expression in
terms of the OS. Therefore, ≥30% MCM2 expression in patients with
resected lung adenocarcinoma served as the cutoff value in survival
analysis. The results of the comparisons among groups are
summarized in Table II.
 | Table IIAssociation between OS or RFS and
MCM2 expression. |
Table II
Association between OS or RFS and
MCM2 expression.
| MCM2
expression | OS | RFS |
|---|
| Positive (%) | Positive (n) | Negative (n) | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| 15 | 60 | 13 | 0.237 | 2.399 | 0.562-10.240 | 0.677 | 1.254 | 0.431-3.644 |
| 20 | 50 | 23 | 0.040a | 3.563 | 1.059-11.990 | 0.035a | 3.143 | 1.082-9.130 |
| 25 | 46 | 27 | 0.012a | 4.702 | 1.397-15.830 | 0.008a | 4.204 | 1.447-12.220 |
| 30 | 37 | 36 | 0.001a | 5.697 | 1.936-16.760 | 0.003a | 3.745 | 1.568-8.944 |
| 35 | 31 | 42 | 0.001a | 4.672 | 1.840-11.870 | 0.009a | 2.873 | 1.299-6.356 |
| 40 | 26 | 47 | 0.002a | 4.008 | 1.697-9.465 | 0.010a | 2.784 | 1.283-6.043 |
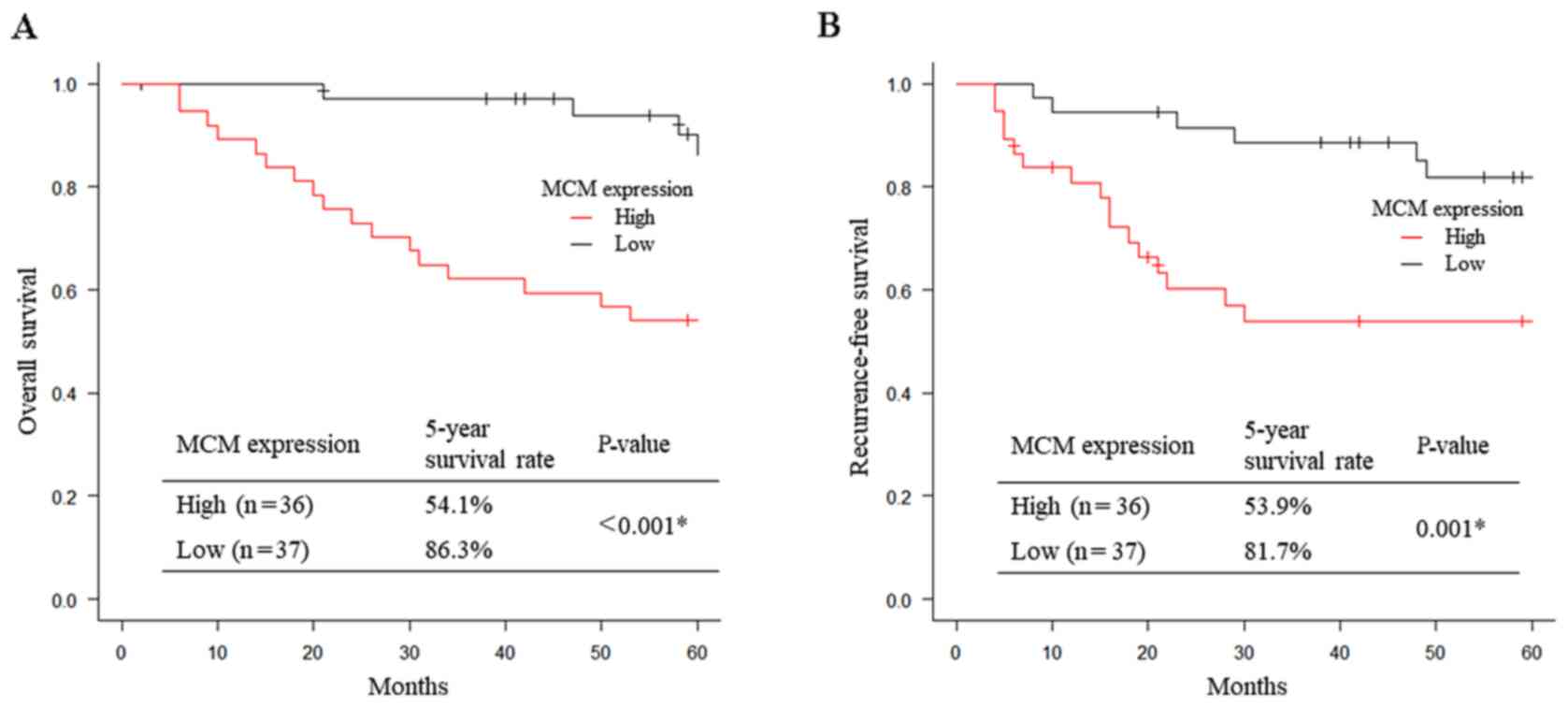
Patients with high MCM2 expression had significantly
worse 5-year OS and 5-year RFS compared with those with low MCM2
expression (OS: 54.1 vs. 86.3%, respectively, P<0.001, Fig. 2A; RFS: 53.9 vs. 81.7%,
respectively, P=0.001, Fig. 2B).
Of the 11 patients with stage IB lung adenocarcinoma, 2 (18.2%)
received postoperative adjuvant therapy with oral uracil-tegafur.
Furthermore, 10 (50.0%) of 20 patients with stage Ⅱ-Ⅲ lung
adenocarcinoma received intravenous platinum doublet-based
chemotherapy. However, 27 patients relapsed, of whom 17, 7 and 1
patients received standard anticancer therapy (according to the
guidelines published by the Japan Lung Cancer Society in 2016;
https://www.haigan.gr.jp/), EGFR-tyrosine kinase
inhibitors and immune checkpoint inhibitors, respectively. In
addition, 16 (59.3%), 8 (29.6%) and 4 (14.8%) patients received
first-, second- and third-line therapies, respectively, but 6
(22.2%) did not receive treatment upon disease recurrence.
As mentioned above, univariate and multivariate
survival analyses were performed to identify the potential
prognostic factors for OS and RFS (Table III). Univariate analysis revealed
that the significant prognostic factors for the OS of lung
adenocarcinoma included pathological stage and MCM2 expression
(P<0.001 and P<0.002, respectively), whereas those for the
RFS included pathological stage, EGFR mutation status and MCM2
expression (P<0.001, P<0.034 and P<0.003, respectively).
On multivariate survival analysis, high MCM2 expression
(pathological stage II-III) was found to be a strong prognostic
factor and was independent of and superior to the EGFR mutation
status (OS: HR=5.084, 95% CI: 1.715-15.080, P=0.003; RFS: HR=2.761,
95% CI: 1.090-6.998, P=0.032).
 | Table IIIUnivariate and multivariate survival
analyses in patients with lung adenocarcinoma. |
Table III
Univariate and multivariate survival
analyses in patients with lung adenocarcinoma.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | P-value | HR | 95% CI | P-value | Risk ratio | 95% CI |
|---|
| OS | | | | | | |
|
Age ≥70
years (vs. <70) | 0.089 | 2.114 | 0.893-5.002 | - | - | - |
|
Male sex
(vs. female) | 0.247 | 1.641 | 0.710-3.793 | - | - | - |
|
Smoking (vs.
no) | 0.909 | 0.960 | 0.480-1.923 | | | |
|
pStage I
(vs. II-III) |
<0.001a | 7.249 | 3.054-17.210 |
<0.001a | 6.688 | 2.783-16.070 |
|
EGFR
mutation-positive (vs. negative) | 0.8791 | 1.07 | 0.445-2.574 | - | - | - |
|
MCM2 high
(vs. low)b | 0.002a | 5.697 | 1.936-16.760 | 0.003a | 5.084 | 1.715-15.080 |
| RFS | | | | | | |
|
Age ≥70
years (vs. <70) | 0.300 | 1.514 | 0.691-3.318 | - | - | - |
|
Male sex
(vs. female) | 0.992 | 1.004 | 0.472-2.137 | - | - | - |
|
Smoking (vs.
no) | 0.550 | 0.824 | 0.436-1.556 | | | |
|
pStage I
(vs. II-III) |
<0.001a | 6.728 | 3.017-15.000 | 0.001a | 4.115 | 1.731-9.778 |
|
EGFR
mutation-positive (vs. negative) | 0.034a | 2.491 | 1.074-5.778 | 0.055 | 2.304 | 0.981-5.409 |
|
MCM2 high
(vs. low)b | 0.003a | 3.745 | 1.568-8.944 | 0.032a | 2.761 | 1.090-6.998 |
Discussion
MCM proteins serve as biological markers of
dysplasia and malignancy (11).
Although MCM proteins promote cancer cell proliferation, MCM
expression is a poor prognostic marker in several types of cancer,
such as breast cancer, renal cell carcinoma, prostate cancer,
colorectal cancer, lung cancer, glioma and cervical cancer
(4-6,12-15).
According to the protein-protein interaction network analysis of
201 proteins as previously reported (1), SCLC is functionally characterized by
molecular pathway activation for spliceosome, RNA transport, and
DNA replication and the cell cycle. In particular, the following 11
proteins have been identified as SCLC-specific proteins: MCM2,
MCM4, MCM6, MCM7 and MSH2 for tumor proliferation; regulator of
chromosome condensation 2, coronin-1C, chromodomain helicase
DNA-binding protein 4 and importin 9 for metastasis; and
phosphoglycerate dehydrogenase and thymidine phosphorylase for
cancer metabolism. For both SCLC and NSCLC, MCM2 is a potentially
poor prognostic factor according to an online Kaplan-Meier survival
analysis (1). Therefore, the
present study was focused on MCM2 expression in resected NSCLC
tissues and performed further survival analysis.
In our analysis of the correlation between MCM2
expression and clinicopathological characteristics of patients with
resected lung adenocarcinoma, those with a history of smoking, male
sex, or late-stage cancer exhibited significantly high MCM2
expression levels. Similarly, MCM2 has been found to be
significantly associated with tumor stage in patients with lung
cancer (16), and high MCM2
expression in NSCLC is more frequently observed among elderly
patients and patients with late-stage disease (17). Overall, high MCM2 expression is
linked to aggressive progression in the majority of cancer
types.
Moreover, multivariate analysis of the OS showed
that MCM2 expression may serve a biomarker and an independent
factor of unfavorable prognosis, consistent with previous studies
(17,18). As regards RSF, high MCM2 expression
in lung adenocarcinoma was shown to be associated with early
metastasis recurrence (Fig. 2B),
particularly distant metastasis (Table
I). These results provide useful information for thoracic
surgeons and medical oncologists and for postoperative follow-up
management.
MCM proteins, which are highly conserved helicases
and key regulatory components of eukaryotic DNA replication, are
overexpressed in several cancer types, and are therefore considered
as important targets of chemotherapy. The potential of MCMs as a
therapeutic target has been previously reported. Suppression of MCM
complexes was found to sensitize pancreatic cancer cells to
cytotoxic chemotherapy agents (gemcitabine and 5-FU) and enhance
treatment efficacy (19). MCM7 was
found to play an important role in cell proliferation by promoting
quiescence-reactivated proliferation and paclitaxel resistance
(20). In addition, MCM2 may serve
as a functional target for long non-coding RNAs and microRNAs in
regulating tumor cell proliferation, migration and apoptosis
(21,22). Moreover, the anti-hyperlipidemic
agent lovastatin, as an inhibitor of 3-hydroxy-3-methylglutaryl
coenzyme A has demonstrated antitumor activity by silencing MCM2,
triggering G1/S arrest in NSCLC cells (23). However, further research into
targeting MCM is required.
There were certain limitations to the present study.
First, this study was retrospective, rather than prospective or
multicenter; therefore, bias might exist. Second, the sample size
was small and possibly inadequate for obtaining reliable results.
Third, the postoperative observation period was relatively brief.
Further studies with a larger cohort are necessary to confirm our
results. Finally, no data on CD56 positivity in the tumor cells
were assessed in this study to evaluate neuroendocrine tumor
characteristics in association with high MCM2 expression.
In conclusion, MCM2 overexpression in patients with
lung adenocarcinoma appears to be an independent factor for an
unfavorable prognosis in terms of OS and RFS. Therefore, MCM2 may
represent a potential biomarker and therapeutic target for such
patients.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a Grant-in-Aid for
Scientific Research, Japan Society for the Promotion of Science
(grant no. 24592104), Ministry of Education, Culture, Sports,
Science and Technology, Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HSakai, first author, and HSaji, corresponding
author, served as the principal authors, performed data acquisition
and had full access to all the data in the study, and are
responsible for confirming the integrity and authenticity of the
data and the accuracy of the data analysis. HSakai and HSaji
contributed to study conception and design, coordination of the
study and revised the article for important intellectual content.
HK, TM, HM, NF, KK and KO enrolled patients and contributed to
preparing the manuscript. MC, JK, KF, TN and HN assessed the cells
for immunoreactivity and contributed to preparing the manuscript.
All the authors have read and approved the final version of the
manuscript to be published.
Ethics approval and consent to
participate
The Institutional Review Board of the St. Marianna
Medical University in Kanagawa, Japan approved this study
(accession no. 1461). Prior to the study, written informed consent
was obtained from all participants. All patient data remained
confidential throughout this research.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Fujii K, Miyata Y, Takahashi I, Koizumi H,
Saji H, Hoshikawa M, Takagi M, Nishimura T and Nakamura H:
Differential Proteomic Analysis between Small Cell Lung Carcinoma
(SCLC) and Pulmonary Carcinoid Tumors Reveals Molecular Signatures
for Malignancy in Lung Cancer. Proteomics Clin Appl.
12(e1800015)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bell SP and Dutta A: DNA replication in
eukaryotic cells. Annu Rev Biochem. 71:333–374. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Neves H and Kwok HF: In sickness and in
health: The many roles of the minichromosome maintenance proteins.
Biochim Biophys Acta Rev Cancer. 1868:295–308. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu YZ, Jiang YY, Hao JJ, Lu SS, Zhang TT,
Shang L, Cao J, Song X, Wang BS, Cai Y, et al: Prognostic
significance of MCM7 expression in the bronchial brushings of
patients with non-small cell lung cancer (NSCLC). Lung Cancer.
77:176–182. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gonzalez MA, Pinder SE, Callagy G, Vowler
SL, Morris LS, Bird K, Bell JA, Laskey RA and Coleman N:
Minichromosome maintenance protein 2 is a strong independent
prognostic marker in breast cancer. J Clin Oncol. 21:4306–4313.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kaur G, Balasubramaniam SD, Lee YJ,
Balakrishnan V and Oon CE: Minichromosome Maintenance Complex (MCM)
Genes Profiling and MCM2 Protein Expression in Cervical Cancer
Development. Asian Pac J Cancer Prev. 20:3043–3049. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nasri Nasrabadi P, Nayeri Z, Gharib E,
Salmanipour R, Masoomi F, Mahjoubi F and Zomorodipour A:
Establishment of a CALU, AURKA, and MCM2 gene panel for
discrimination of metastasis from primary colon and lung cancers.
PLoS One. 15(e0233717)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Goldstraw P and Groome P: Lung and pleural
tumours. In: TNM Classification of Malignant Tumours. 7th edition.
Sobin, LH, Gospodarowicz MK and Wittekind C (eds). Wiley-Blackwell,
Chichester, West Sussex, pp 136-150, 2009.
|
|
9
|
Travis W, Brambilla E and Müller-Hermelink
HK (eds): World Health Organization Classification of Tumours:
Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and
Heart. IARC Press, Lyon, 2004.
|
|
10
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Freeman A, Morris LS, Mills AD, Stoeber K,
Laskey RA, Williams GH and Coleman N: Minichromosome maintenance
proteins as biological markers of dysplasia and malignancy. Clin
Cancer Res. 5:2121–2132. 1999.PubMed/NCBI
|
|
12
|
Dudderidge TJ, Stoeber K, Loddo M,
Atkinson G, Fanshawe T, Griffiths DF and Williams GH: Mcm2,
Geminin, and KI67 define proliferative state and are prognostic
markers in renal cell carcinoma. Clin Cancer Res. 11:2510–2517.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dudderidge TJ, McCracken SR, Loddo M,
Fanshawe TR, Kelly JD, Neal DE, Leung HY, Williams GH and Stoeber
K: Mitogenic growth signalling, DNA replication licensing, and
survival are linked in prostate cancer. Br J Cancer. 96:1384–1393.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nishihara K, Shomori K, Fujioka S,
Tokuyasu N, Inaba A, Osaki M, Ogawa T and Ito H: Minichromosome
maintenance protein 7 in colorectal cancer: Implication of
prognostic significance. Int J Oncol. 33:245–251. 2008.PubMed/NCBI
|
|
15
|
Hua C, Zhao G, Li Y and Bie L:
Minichromosome Maintenance (MCM) Family as potential diagnostic and
prognostic tumor markers for human gliomas. BMC Cancer.
14(526)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Veena VS, George PS, Rajan K, Chandramohan
K, Jayasree K and Sujathan K: Immunocytochemistry on Sputum Samples
Predicts Prognosis of Lung Cancer. J Cytol. 36:38–43.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu YZ, Wang BS, Jiang YY, Cao J, Hao JJ,
Zhang Y, Xu X, Cai Y and Wang MR: MCMs expression in lung cancer:
Implication of prognostic significance. J Cancer. 8:3641–3647.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ramnath N, Hernandez FJ, Tan DF, Huberman
JA, Natarajan N, Beck AF, Hyland A, Todorov IT, Brooks JS and
Bepler G: MCM2 is an independent predictor of survival in patients
with non-small-cell lung cancer. J Clin Oncol. 19:4259–4266.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bryant VL, Elias RM, McCarthy SM, Yeatman
TJ and Alexandrow MG: Suppression of Reserve MCM Complexes
Chemosensitizes to Gemcitabine and 5-Fluorouracil. Mol Cancer Res.
13:1296–1305. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jia M, Zheng D, Wang X, Zhang Y, Chen S,
Cai X, Mo L, Hu Z, Li H, Zhou Z, et al: Cancer Cell enters
reversible quiescence through Intracellular Acidification to resist
Paclitaxel Cytotoxicity. Int J Med Sci. 17:1652–1664.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jin Y, Xiong A, Zhang Z, Li S, Huang H, Yu
TT, Cao X and Cheng SY: MicroRNA-31 suppresses medulloblastoma cell
growth by inhibiting DNA replication through minichromosome
maintenance 2. Oncotarget. 5:4821–4833. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu F, Yuan JH, Huang JF, Yang F, Wang TT,
Ma JZ, Zhang L, Zhou CC, Wang F, Yu J, et al: Long noncoding RNA
FTX inhibits hepatocellular carcinoma proliferation and metastasis
by binding MCM2 and miR-374a. Oncogene. 35:5422–5434.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang X, Teng Y, Yang F, Wang M, Hong X,
Ye LG, Gao YN and Chen GY: MCM2 is a therapeutic target of
lovastatin in human non-small cell lung carcinomas. Oncol Rep.
33:2599–2605. 2015.PubMed/NCBI View Article : Google Scholar
|