Introduction
Helicobacter pylori is known to be a major
cause of gastric cancer development and may contribute to
extra-gastric organ disease. Previous large-scale prospective
cohort studies have shown that H. pylori is a definite risk
factor for gastric cancer, and it is widely recognized that
atrophic gastritis (AG) associated with progression of H.
pylori-related gastritis significantly increases the risk of
cancer (1-4).
In recent years, it has also been shown that decreased gastric acid
secretion evaluated by pepsinogen (PG) is associated with
colorectal carcinogenesis (5,6) and
arteriosclerosis-related diseases such as diabetes mellitus
(7). The prevalence of H.
pylori has been reported to vary by race and country (8, 9) and
the incidence has decreased markedly in the developed world over
recent decades (10). The
prevalence of H. pylori in Japan is lower than in developing
countries and higher than in other developed countries (11). There were several studies reporting
the prevalence of H. pylori in rural and urban areas of
Japan (12-16).
Clarifying epidemiological indicators, such as the prevalence and
secular trend, of the disease is the first step in disease
prevention. H. pylori infections and AG are often
characterized by few symptoms, chronic progression, and a long
course. Therefore, estimation of these epidemiological indicators
requires population-based cohort screening and long-term
observation. However, only a few studies have investigated the
prevalence of H. pylori in a large population-based subjects
in Japan (16). As for the
prevalence of AG, there are very few reports on the natural history
of AG in a cohort of local residents and no reports on long-term
prognosis. The definitive diagnosis of H. pylori-related
gastritis and resulting gastric atrophy is based on histopathology
of the gastric mucosa. However, it is difficult to accurately
diagnose the severity and progression of H. pylori-related
AG by histopathology of several endoscopically collected specimens
since AG develops multifocally, in addition histopathology-based
diagnosis of AG involves subjective assessment without a gold
standard (17). There is general
agreement that the serum H. pylori antibody titer is
believed to be related to the activity of inflammation in H.
pylori-related gastritis (18,19)
and serum PG levels show dynamics that correlate with
histopathological changes and exocrine function in the gastric
mucosa corresponded to the extent of gastric atrophy (20-23).
In other words, serum PG are regarded as markers reflecting the
progression of chronic AG. Therefore, the present study used these
serum markers, which are more objective parameters, free of
discomfort, easy to accept and relatively inexpensive for mass
population. Measuring serum PG is the only viable method for
estimating the prevalence of functional AG in the large general
population (24). Song et
al reported a statistically significant decreasing trend in the
prevalence of functional atrophic corpus gastritis defined by serum
PG I measurements in the age group of 55 to 64 years from 1990
through 2009 based on a population-based study in Sweden (25). In Japan, the prevalence of AG is
expected to increase due to further aging of the population, but no
report has examined the prevalence of AG in a large-scale,
population-based samples.
The Research on Osteoarthritis/Osteoporosis Against
Disability (ROAD) study started in 2005 for the purpose of
prevention of musculoskeletal diseases and identification of risk
factors. This is based on an ongoing prospective survey in the
general population being conducted in Wakayama Prefecture, located
in the southwestern part of the main island of Japan. The first
baseline survey was conducted in 2005-2006. Participants in the
ROAD study were informed to undergo follow-up surveys at 3, 7, and
10 years after enrollment, when the baseline examinations were
repeated. The fourth survey was the 10-year follow-up and was
conducted in 2015-2016. Participants in this study were similar to
the general Japanese population in terms of physique, alcohol
consumption and other lifestyle habits (26). Wakayama Prefecture is one of the
high-risk areas for gastric and colorectal cancer mortality in
Japan; in 2005, gastric and colorectal cancer mortality ranked
within the top five among 47 prefectures in Japan, respectively
(27). Thus, the prevalence of AG
and H. pylori infection using this large-scale
population-based results could be generalizable to the Japanese
population in a high-risk area for gastric and colorectal
cancers.
The purpose of this study was to estimate and
compare the latest prevalence and secular trends of functional AG
and H. pylori infection determined by serological results
for H. pylori antibody and PG test with a 10-year interval
using the baseline and fourth survey data of the ROAD study.
Patients and methods
Study subjects
The present study involved the ROAD study cohorts
established in 2005. The ROAD study is a national, prospective
study of musculoskeletal diseases in Japan, consisting of a
population-based cohorts. Profiles of this longitudinal cohorts
were detailed in previous reports (28,29,30).
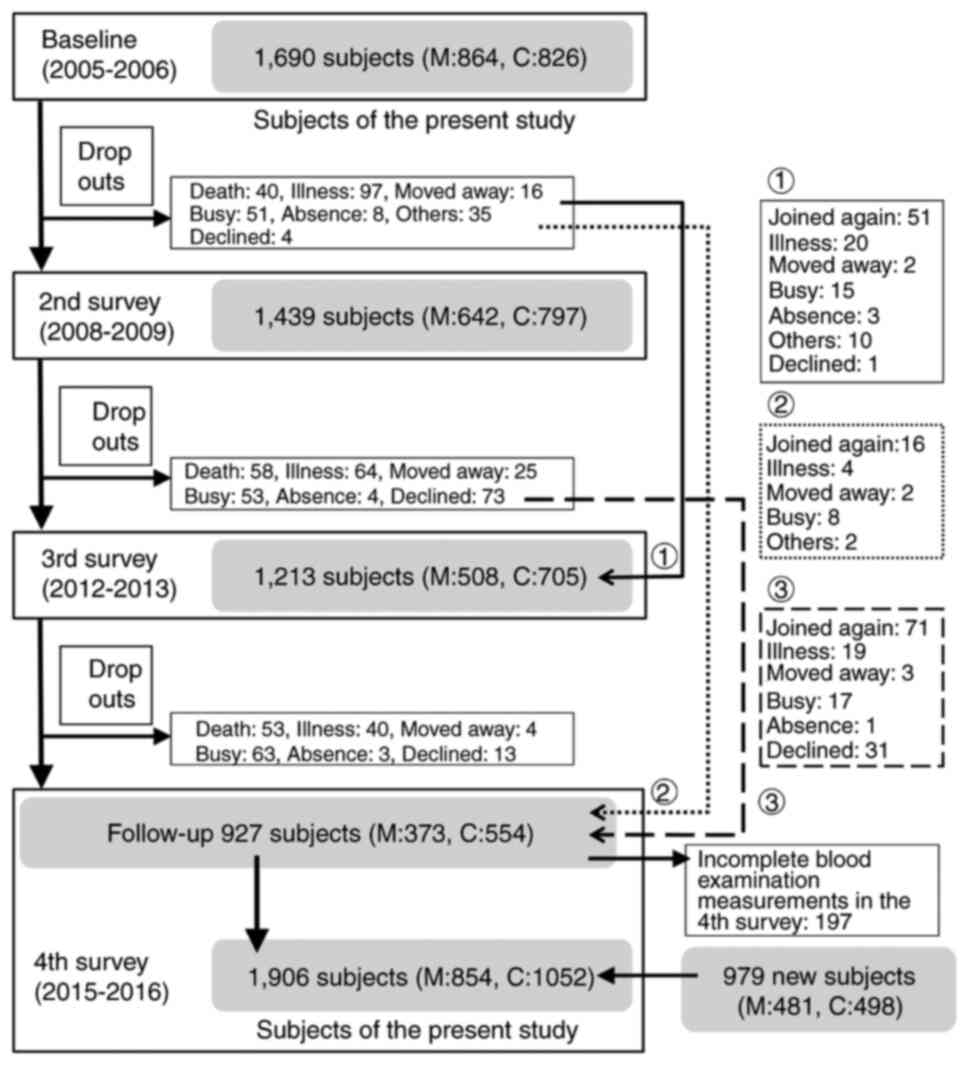
Briefly, a baseline database consists of the clinical and genetic
information of 1,690 residents surveyed between 2005 and 2006. The
subjects were 1,690 participants reported in the ROAD study,
recruited with reference to the lists of resident registrations in
two communities: 864 participants from a mountainous area in
Hidakagawa, Wakayama Prefecture and 826 participants from a coastal
area in Taiji, Wakayama Prefecture. Since the fourth survey is both
10-year follow-up to the baseline study and a new baseline database
for the subsequent 10-year period (the parent ROAD of this study is
an ongoing cohort study), in the fourth survey, new participants
recruited from the resident registration records of the two
communities using the same method as the baseline survey were
included. Therefore, the fourth survey included 979 new cohort
participants in addition to 927 cohort followers. In this
population-based cross-sectional study with a 10-year interval
survey, a total of 1,690 subjects in the baseline survey and 1,906
in the fourth survey who underwent blood examination were initially
included. Fig. 1 shows schematic
flow of subject's recruitment and survey of this study.
Participants were essentially asymptomatic for gastrointestinal
symptoms requiring prompt medical care and could be regarded as
representative of healthy middle-aged and older persons in the
general population. They answered an interviewer-administered
questionnaire consisting of lifestyle information such as drinking
habits, family history and medical history. Height and weight were
measured, from which the body mass index (BMI) [weight (kg)/height
(m)2] was calculated. According to the questionnaire at
the time of the fourth survey, 131/1906 (6.9%) of the subjects had
a history of H. pylori eradication therapy and we excluded
these H. pylori eradicated subjects from the analysis. At
the time of the health check, blood samples were obtained from the
participants. Serum samples were isolated from blood taken as
routine laboratory tests for the general health examinations and
stored below -20˚C until measurement of serum H. pylori
immunoglobulin (Ig) G antibody titers and serum PG levels. In this
study, blood samples at the baseline survey and the fourth survey
were used. However, two subjects in the baseline survey and two
subjects in the fourth survey with an insufficient amount of stored
serum were excluded from PG testing and serological testing for
H. pylori infections. Finally, a total of 1,557 subjects in
the baseline survey and 1,773 subjects in the fourth survey were
used for analysis in this study. Written informed consent was
provided by all participants prior to inclusion. This study was
performed in accordance with the Declaration of Helsinki and was
approved by the institutional ethics committees of the University
of Tokyo (approval nos. 1264 and 1326), Wakayama Medical University
(approval no. 373) and Tokyo University of Marine Science and
Technology (approval no. 187).
Serological analysis
Serum H. pylori antibody titers were measured
using an enzyme immunoassay (EIA kit; SRL, Tokyo, Japan) (31). Antibody titers above 10 U/ml were
classified as positive for H. pylori infection. Serum PG I
and PG II levels were measured using a modification (RIA-Beads Kit;
Dainabot, Tokyo, Japan) of radioimmunoassay (32). The gold standard for detecting
gastric atrophy is still histopathology (biopsy) of the gastric
mucosa, which was not selected in this study. However, there is
general consensus that serologic testing with pepsinogen may help
identify patients with AG (33). A
recent meta-analysis of 31 studies involving a total of 2,265 AG
patients showed that the summary sensitivity and specificity of AG
screening with serum pepsinogen were 0.69 (95% CI: 0.55-0.80) and
0.88 (95% CI: 0.77-0.94) respectively, indicating that serum
pepsinogen may be useful for noninvasive diagnosis of AG (34). Participants with AG were determined
based on PG test-positive criteria of PG I ≤70 ng/ml and PG I/II
ratio ≤3.0(23). These criteria
offer sensitivity of 70.5% and specificity of 97% for the diagnosis
of AG (23). The criterion for the
PG test used in the present study is the one most widely used for
the detection of AG in Japan and considered a reliable non-invasive
screening tool for AG.
Statistical analysis
Data for continuous variables are presented as means
± standard deviation (SD), and the differences were tested for
significance using unpaired t-tests (Student's t-test and Welch's
t-test) for comparisons of two groups. Differences in proportions
were compared using the χ2 test. The odds ratios (ORs)
were estimated by a logistic regression model. ORs and 95%
confidence intervals (CIs) were calculated using logistic
regression analysis. P<0.05 was considered to indicate a
statistically significant difference. Data analyses were performed
using SPSS version 27.0 software (SPSS, Chicago, IL) and STATA
(STATA Corp., College Station, TX).
Results
Background of study population
Table I shows the
characteristics of this study subjects used for analysis. There
were 1,557 participants at baseline and 1,773 in the fourth survey,
for a total of 3,330 participants. There were no significant
differences by gender between the two groups. The mean age was
65.70 (SD 12.17) years at baseline and 64.59 (SD 12.91) years in
the fourth survey, with significant difference between the two.
Current smokers accounted for 12.6% at baseline and 9.0% in the
fourth survey, and inhabitants of coastal regions accounted for
47.5 and 54.5%, respectively. At baseline, smoking tended to be
more frequent, and the percentage of subjects living in a coastal
community was lower (P<0.01). The percentage of non-drinkers and
the mean BMI tended to be higher in participants at baseline
(P<0.1). Therefore, smoking habits, alcohol use, BMI, and
community were also included in the model to control for
confounding effects in the subsequent analyses. The serum PG I or
II level was significantly higher at baseline than in the fourth
survey, whereas the PG I/II ratio was significantly lower at
baseline.
 | Table IComparison of background
characteristics of the participants in the baseline survey
(2005-2006) with those in the fourth survey (2015-2016). |
Table I
Comparison of background
characteristics of the participants in the baseline survey
(2005-2006) with those in the fourth survey (2015-2016).
| Characteristic | Baseline
survey | Fourth survey |
P-valuea |
|---|
| Total number of
subjects | 1,557 | 1,773 | |
| Age,
yearsb | 65.70 (12.17) | 64.59 (12.91) | <0.05 |
| BMI,
kg/m2b | 23.03 (3.42) | 22.83 (3.53) | 0.09 |
| H. pylori
antibody, U/mlb | 19.78 (23.04) | 14.91 (19.66) | <0.01 |
| PG I,
ng/mlb | 58.47 (39.96) | 55.14 (51.99) | <0.05 |
| PG II,
ng/mlb | 20.87 (13.88) | 15.30 (13.49) | <0.01 |
| PG
I/IIb | 3.19 (1.78) | 4.03 (1.86) | <0.01 |
| Sex,
Men/Womenc | 531/1,026
(34.1/65.9) | 572/1,201
(32.2/67.8) | 0.27 |
| Community,
Mountain/Coastalc | 817/740
(52.5/47.5) | 807/966
(45.5/54.5) | <0.01 |
| Current smoking
habit, -/+c | 1,326/191
(87.4/12.6) | 1,611/160
(91.0/9.0) | <0.01 |
| Current alcohol
use, -/+c | 946/603
(61.1/38.9) | 1,013/758
(57.2/42.8) | <0.05 |
| H. pylori
infection, -/+c | 745/812
(47.8/52.2) | 1,143/630
(64.5/35.5) | <0.01 |
| Atrophic gastritis,
-/+c | 933/624
(59.9/40.1) | 1,315/458
(74.2/25.8) | <0.01 |
Prevalence of AG and H. pylori
infection with a 10-year interval survey
Table II shows a
comparison of the prevalence of AG and H. pylori infection
at baseline and in the fourth survey. The prevalence of subjects
with AG diagnosed by the PG test was significantly lower in the
fourth survey (25.8%) compared to the baseline survey (40.1%), with
a crude OR of 0.52 (95% CI: 0.45-0.60), and the significance of the
difference did not change after adjustment. The percentage of H.
pylori infection was 35.5% in the fourth survey and 52.2% at
baseline, with a crude OR of 0.51 (95% CI: 0.44-0.58), and the
significance of the difference did not change after adjustment
(Table II). No significant gender
differences were observed in the prevalence of AG and H.
pylori infection (Table
III). Of a total of 1690 patients in the baseline survey (864
mountain, 826 coastal), 927 (373 mountain, 554 coastal) also
participated in the fourth survey, therefore the follow-up rate for
the same patients was 54.9% (43.2% in mountainous areas, 67.1% in
coastal areas). The prevalence of AG and H. pylori infection
in these follow-up participants was 33.5% (mountain 39.1%, coastal
29.8%) and 52.5% (mountain 56.0%, coastal 50.1%), respectively, at
baseline and in the fourth survey, they were 27.9% (34.9% in
mountain, 23.3% in coastal) and 36.4% (40.5% in mountain, 33.6% in
coastal), respectively. From the above results, the follow-up cases
(total, mountainous area, coastal area) that participated in both
the baseline and fourth survey showed almost the same tendency as
the results of overall participants. Subjects in mountainous areas
had a significantly higher mean age than subjects in coastal areas,
and the prevalence of AG and H. pylori infection in
mountainous areas tended to be higher than in coastal areas
(Table SI).
 | Table IIComparison of the prevalence of
Helicobacter pylori infection or atrophic gastritis between
the baseline survey (2005-2006) and the fourth survey
(2015-2016). |
Table II
Comparison of the prevalence of
Helicobacter pylori infection or atrophic gastritis between
the baseline survey (2005-2006) and the fourth survey
(2015-2016).
| Variable | Baseline survey, n
(%) | Fourth survey, n
(%) | ORa (95%CI) | ORb (95%CI) |
|---|
| H. pylori
infection | | | | |
|
(-) | 745 (47.8) | 1143 (64.5) | 1 (Ref) | 1 (Ref) |
|
(+) | 812 (52.2) | 630 (35.5) | 0.51
(0.44-0.58) | 0.51
(0.44-0.59) |
| Atrophic
gastritis | | | | |
|
(-) | 933 (59.9) | 1315 (74.3) | 1 (Ref) | 1 (Ref) |
|
(+) | 624 (40.1) | 458 (25.8) | 0.52
(0.45-0.60) | 0.53
(0.45-0.61) |
 | Table IIIComparison of the prevalence of
Helicobacter pylori infection or atrophic gastritis between
the baseline survey (2005-2006) and the fourth survey
(2015-2016). |
Table III
Comparison of the prevalence of
Helicobacter pylori infection or atrophic gastritis between
the baseline survey (2005-2006) and the fourth survey
(2015-2016).
| A, Men |
|---|
| Variable | Baseline survey, n
(%) | Fourth survey, n
(%) | ORa (95%CI) | ORb (95%CI) |
|---|
| H. pylori
infection | | | | |
|
(-) | 240 (45.2) | 372 (65.0) | 1 (Ref) | 1 (Ref) |
|
(+) | 291 (54.8) | 200 (35.0) | 0.44
(0.35-0.57) | 0.45
(0.35-0.58) |
| Atrophic
gastritis | | | | |
|
(-) | 297 (55.9) | 427 (74.7) | 1 (Ref) | 1 (Ref) |
|
(+) | 234 (44.1) | 145 (25.3) | 0.43
(0.33-0.56) | 0.46
(0.35-0.60) |
| B, Women |
| Variable | Baseline survey, n
(%) | Fourth survey, n
(%) | ORa (95%CI) | ORb (95%CI) |
| H. pylori
infection | | | | |
|
(-) | 505 (49.2) | 771 (64.2) | 1 (Ref) | 1 (Ref) |
|
(+) | 521 (50.8) | 430 (35.8) | 0.54
(0.46-0.64) | 0.53
(0.45-0.64) |
| Atrophic
gastritis | | | | |
|
(-) | 636 (62.0) | 888 (73.9) | 1 (Ref) | 1 (Ref) |
|
(+) | 390 (38.0) | 313 (26.1) | 0.58
(0.48-0.69) | 0.57
(0.47-0.69) |
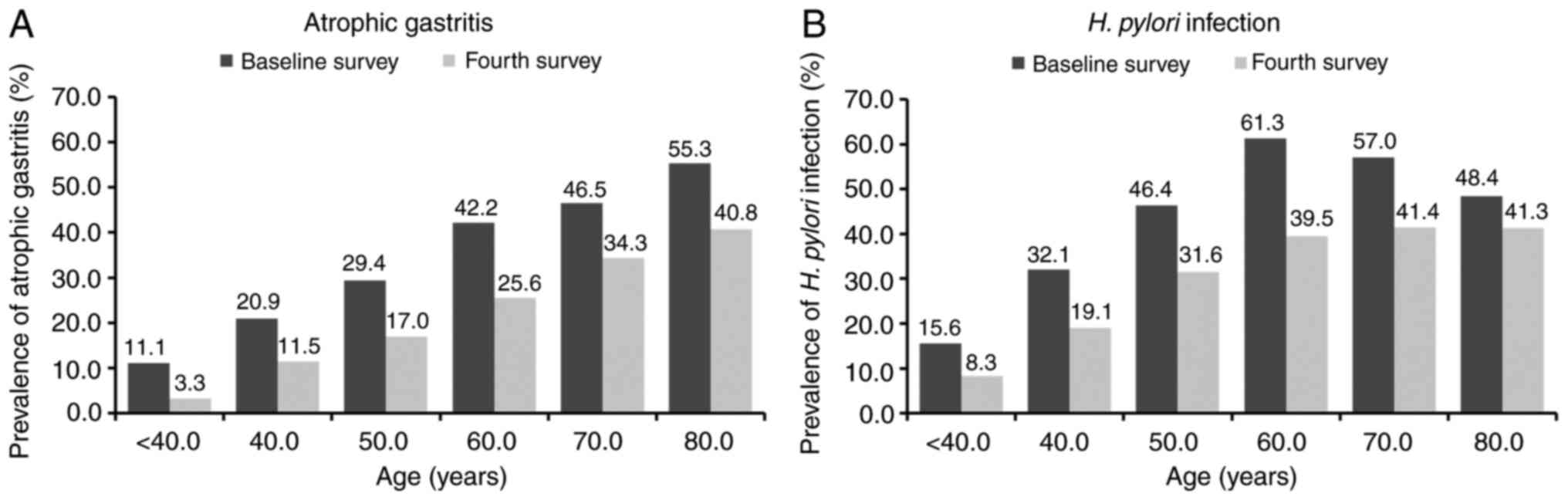
Fig. 2A shows the
age-specific prevalence of AG across the two surveys spanning 10
years. The prevalence of AG by the PG test was significantly higher
with age at baseline than in the fourth survey (P<0.01), and
there was no significant difference between men and women (data not
shown). Stratified by age, there were 105 subjects (baseline
survey: fourth survey=45:60) <40 years old, 317 (134:183) in
their 40s, 594 (265:329) in their 50s, 979 (429:550) in their 60s,
953 (525:428) in their 70s, and 382 (159:223) ≥80 years old. The
prevalence of AG-positive increased with age in both groups at
baseline and in the fourth survey. The prevalence rates of
AG-positive by age groups of <40, 40-49, 50-59, 60-69, 70-79,
and ≥80 years were 11.1, 20.9, 29.4, 42.2, 46.5, and 55.3%,
respectively, at baseline and 3.3, 11.5, 17.0, 25.6, 34.3, and
40.8%, respectively, in the fourth survey. AG-positive rates were
significantly lower in the 10-year follow-up group than in the
baseline group, in each age grade among subjects aged 40 years or
older, with crude ORs according to age groups of <40, 40-49,
50-59, 60-69, 70-79, and ≥80 years of 0.28, 0.49, 0.49, 0.47, 0.60,
and 0.56, respectively.
Fig. 2B shows the
age-specific prevalence of H. pylori infection in two
surveys at 10-year intervals. The prevalence of H. pylori
infection indicated an increasing trend with age, except for the
elderly group over 60 years old, in both the baseline and fourth
surveys. The prevalence rates of H. pylori infection
according to age groups of <40, 40-49, 50-59, 60-69, 70-79, and
≥80 years were 15.6, 32.1, 46.4, 61.3, 57.0, and 48.4%,
respectively, at baseline and 8.3, 19.1, 31.6, 39.5, 41.4, and
41.3%, respectively, in the fourth survey. H. pylori
seropositive rates were significantly lower in the fourth survey
than at baseline, in each age group among subjects aged 40 years or
older except for the oldest group, with a crude OR according to age
groups of <40, 40-49, 50-59, 60-69, 70-79, and ≥80 years of
0.49, 0.50, 0.53, 0.41, 0.53, and 0.75, respectively. Next, to
clarify whether the prevalence of H. pylori infection in the
elderly over 60 years old did not increase with age as shown in
Fig. 2B because of the effect of
natural eradication, subjects aged 60 years and older were divided
into age groups 60-69, 70-79, and 80 years and older. Then each age
group were classified into subgroups based on the presence or
absence of AG and H. pylori infection status, and for each
survey, we then compared the prevalence of subgroups within each
age group (Table IV). In the
baseline survey, the prevalence of HP (+) AG (+) or
HP (-) AG (-) did not differ significantly among age groups
60-69, 70-79, and 80 years and older. On the other hand, the
prevalence of HP (-) AG (+) showed a remarkably increasing
trend with age, whereas the prevalence of HP (+) AG (-)
showed a decreasing trend with age. From the above results, the
prevalence of HP (+) decreased, and the prevalence of
HP (-) AG (+) increased significantly with age in the
elderly aged 60 years and over. So, prevalence was thought to
exhibit an inverted U-shaped relationship (as shown in Fig. 2B). At the fourth survey, the
prevalence of HP (-) AG (-) or HP (+) AG (-)
decreased with age, whereas HP (+) AG (+) or HP (-)
AG (+) showed an increasing trend with age. As a result, as shown
in Fig. 2B, it was thought that
the prevalence of H. pylori infection was not significantly
different between the age groups of the elderly. In both the
baseline and the fourth surveys, the prevalence of HP (-) AG
(+) tended to increase with age in the elderly and the HP
(-) AG (+) subgroup proportions were significantly higher in the
70-79 and ≥80 age groups compared with the 60-69 age group.
[Baseline survey: Crude OR 2.04 (1.26-3.29) for age group 70-79
years vs age group 60-69 years, crude OR 2.93 (1.63-5.29) for age
group ≥80 years] [Fourth survey: Crude OR 2.51 (1.48-4.28) for age
group 70-79 years vs. age group 60-69 years, crude OR 3.50
(1.94-6.31) for age group ≥80 years].
 | Table IVComparison of the prevalence of
subgroups classified by Helicobacter pylori infection and
atrophic gastritis between age groups 60-69 years and 70-79 years
or ≥80 years in each survey. |
Table IV
Comparison of the prevalence of
subgroups classified by Helicobacter pylori infection and
atrophic gastritis between age groups 60-69 years and 70-79 years
or ≥80 years in each survey.
| A, Baseline
survey |
|---|
| | Age, years | Age, years |
|---|
| HP | AG | 60-69, n (%) | 70-79, n (%) | ≥80, n (%) | 70-79, OR
(95%CI) | ≥80, OR (95%
CI) |
|---|
| (-) | (-) | 135 (31.5) | 154 (29.3) | 49 (30.8) | 1 (Ref) | 1 (Ref) |
| (+) | (-) | 113 (26.3) | 127 (24.2) | 22 (13.8) | 0.99
(0.70-1.39) | 0.54
(0.31-0.94) |
| (+) | (+) | 150 (35.0) | 172 (32.8) | 55 (34.6) | 1.01
(0.73-1.38) | 1.01
(0.64-1.58) |
| (-) | (+) | 31 (7.2) | 72 (13.7) | 33 (20.8) | 2.04
(1.26-3.29) | 2.93
(1.63-5.29) |
| B, 4th survey |
| | Age, years | Age, years |
| HP | AG | 60-69, n (%) | 70-79, n (%) | ≥80, n (%) | 70-79, OR
(95%CI) | ≥80, OR (95%
CI) |
| (-) | (-) | 309 (56.2) | 210 (49.1) | 103 (46.2) | 1 (Ref) | 1 (Ref) |
| (+) | (-) | 100 (18.2) | 71 (16.6) | 29 (13.0) | 1.05
(0.74-1.48) | 0.87
(0.54-1.39) |
| (+) | (+) | 117 (21.3) | 106 (24.8) | 63 (28.3) | 1.33
(0.97-1.83) | 1.62
(1.11-2.36) |
| (-) | (+) | 24 (4.4) | 41 (9.6) | 28 (12.6) | 2.51
(1.48-4.28) | 3.50
(1.94-6.31) |
Discussion
This is the first to estimate the prevalence and
secular trends of AG with a 10-year interval in a large-scale
cross-sectional study using data from population-based cohort in
Japanese men and women. In this study, the prevalence of AG and
H. pylori infection were significantly lower in the fourth
survey than in the baseline survey. The prevalence of AG increased
with age, whereas the prevalence of H. pylori infection
increased with age except for the elderly group that showed an
inverted U-shaped association. Stratified for age, the prevalence
of AG and H. pylori infection were significantly lower in
the fourth survey than at baseline, and the curve of the fourth
survey shifted further to the right of the curve at baseline. Of a
total of 1690 patients in the baseline study (864 mountain, 826
coastal), 927 (373 mountain, 554 coastal) also participated in the
fourth study, therefore the follow-up rate for the same patients
was 54.9% (43.2% in mountainous areas, 67.1% in coastal areas). The
results in these follow-up participants also showed a similar trend
to the analysis results for the entire cohort (Table SI).
AG is generally recognized as a precursor of gastric
cancer (1), but there are few
epidemiological studies using large population-based data on the
prevalence of AG (35). As for the
prevalence and secular trends of AG using serological test results
based on population-based cohort data, there has been only one
report by Song et al in Sweden using only PG I as an
indicator of AG (25). As far as
we know, this is the first report of the prevalence of AG and
secular trends determined by PG test (PG I and I/II ratio) using
population-based cohort data in Japan. Since serum PG tests can be
measured easily, rapidly, at low cost, and with minimal
invasiveness, this approach could be used for evaluating functional
AG in a large population. PG I and the I/II ratio show different
changes during the natural history of AG. PG I is known to increase
from normal mucosa to non-atrophic H. pylori-related
gastritis and then decreases as AG extends, so if only PG I is used
as an indicator of gastric atrophy, low PG I would include not only
those with AG but also those with normal gastric mucosa. The
pepsinogen I/II ratio shows a continuous decrease during the
process from normal gastric mucosa through non-atrophic gastritis
to AG (36). Therefore, the PG
test was used as an indicator of gastric atrophy to estimate the
prevalence of AG in this population-based screening survey.
It is generally believed that differences in hygiene
levels or opportunities for oral infection during infancy affect
the prevalence of H. pylori infection (37). In developed countries, infection
rates are reported to be about 10-20% and increase with age
(8). The prevalence of H.
pylori infection in Japan has been showed in a few large
epidemiological studies. However, previous reports indicated the
prevalence in the Japanese population at a time when eradication
therapy for H. pylori-related gastritis was not generally
undergone. In 2008-2010, the prevalence of H. pylori
infection detected in urban inhabitants of Japan with a relatively
high proportion of young participants was estimated and shown to
increase with age (15). Moreover,
the graphs showing the prevalence of H. pylori infection by
age groups were clearly shifted to the right compared to previous
studies (38,39). In the present study as well, the
prevalence of H. pylori infection was significantly lower in
the fourth survey than at baseline; thus, the curve of the fourth
survey shifted further to the right of the curve of the baseline
survey. H. pylori is believed to disappear in the gastric
mucosa with extensive atrophy (4).
The general trend is that as gastric atrophy develops extensively
due to the persistence of H. pylori-related gastritis, the
number of H. pylori bacteria in the epithelium decreases
gradually and finally the bacteria are completely expelled from the
stomach, leading to the state of spontaneous eradication resulting
in the disappearance of bacterium-specific serum antibodies
(40,41). In addition, in elderly persons, it
is unavoidable that the antibody titer decreases with aging, and
contamination of eradicated cases is inevitable. In this study, the
HP (-) AG (+) subgroup proportions were significantly higher
in the 70-79 or ≥80 age groups compared with the 60-69 age group,
at both baseline and fourth survey. Thus, the prevalence of
HP (-) AG (+) tended to increase significantly with age in
the elderly. However, in follow-up cases analysis, when HP
(+) AG (+) in the baseline survey (n=140) were analysis target and
the outcome was HP (-) AG (+) in the fourth survey, the
estimated incidence compared with those aged 60-69 years was 0.82
(0.36 to 1.87) for age group 70-79 years and 0 for age group ≥80
years, respectively. Consequently, the trend towards a
significantly lower prevalence of HP infection with age in
the elderly did not appear to reflect spontaneous eradication of
the bacteria as an end result of the progression of chronic
atrophic gastritis, in the present analysis. The potential for
increased natural eradication of bacteria with aging requires
further analysis. As for autoimmune gastritis, the prevalence is
low (0.49%) in Japan (42).
Therefore, the possibility of autoimmune gastritis among the
examined AG cases with H. pylori-negative in this study was
considered negligible.
One of the limitations of the present study is that
the diagnosis of H. pylori infection and AG was based on
serological tests. However, if H. pylori-positive or
AG-positive misclassification by the serological test occurs
equally in all subjects, the risk of exposure misclassification was
underestimated. Second, although the participant population of the
present study consisted of a large number, these participants were
recruited from only two regions, the mountainous and coastal
regions, and thus may not be representative of the general
population. In this study, we selected two regions (mountain and
coast) located in the central and southern part of Wakayama
Prefecture, which has a low population movement according to the
Japanese census and is one of the regions with a high mortality
risk of gastric cancer and colorectal cancer in Japan (27). The values of anthropometric factors
(mean BMI values) of the participants in this study were not
significantly different from those of the general Japanese
population of the same age group. It is likely that the subject
selection in this study did not cause significant differences from
the general Japanese population of the same age group. Thus, the
results of this study may be generalizable to Japanese populations
in areas with high gastric and colorectal cancer risk. On the other
hand, the proportions of current smokers and drinking habits in
this study were lower than those of the general Japanese
population, suggesting that the study subjects may have had
healthier lifestyles. This selection bias should be considered when
generalizing the results of this study. Third, this study may have
a healthy user bias. Unhealthy individuals drop out and healthy
individuals remain for follow-up. Therefore, the possibility of
this bias should be considered when generalizing the results.
However, in this study, the results in the follow-up participants
also showed a similar trend to the analysis results for the entire
data. The fourth limitation is that the progressive spread of H.
pylori eradication therapy between 2005 and 2016 in Japan might
have affected the results to some extent. According to the
questionnaire at the time of the fourth survey, 131/1906 (6.9%) of
the subjects had a history of H. pylori eradication therapy
in the present study and we excluded these H. pylori
eradicated subjects from the analysis. Therefore, H. pylori
eradication therapy was likely to have had a limited impact on the
present findings.
In conclusion, this population-based cross-sectional
study with a 10-year interval survey using a large population
clarified that the prevalence of AG and H. pylori infection
decreased significantly. This change will probably contribute to
decreasing the future trends in the prevalence of H.
pylori-related diseases, including extra-gastric target
organs.
Supplementary Material
Comparison of the prevalence of
Helicobacter pylori infection or atrophic gastritis between
the baseline survey (2005-2006) and the fourth survey (2015-2016)
in follow-up subjects surveyed at 10-year intervals.
Acknowledgements
The authors would like to express their gratitude to
Dr Naoki Hirabayashi of Kawakami Clinic, Hidakagawa Town; Mrs.
Tomoko Takijiri, Mrs. Rie Takiguchi, Mrs. Kyoko Maeda, Ms. Ikuyo
Ueyama, Mrs. Michiko Mori, Mrs. Hisayo Sugimoto, and other members
of the public office in Hidakagawa Town; and Mrs. Tamako Tsutsumi,
Mrs. Kanami Maeda, Mrs. Megumi Takino, Mrs. Shuko Okada, Mrs.
Kazuyo Setoh, Mrs. Chise Ryouno, Mrs. Miki Shimosaki, Mrs. Chika
Yamaguchi, Mrs. Yuki Shimoji, and other members of the public
office in Taiji Town for helping to locate and schedule the
participants for examinations. The authors would also like to thank
Mrs. Kyoko Hattori, Mrs. Toki Sakurai, Mrs. Saeko Sahara and Mr.
Noriyuki Oe (Department of Prevention Medicine for Locomotive Organ
Disorders, 22nd Century Medical and Research Center, The University
of Tokyo, Tokyo, Japan) for their assistance in preparing and
appropriately reducing the interview data of study
participants.
Funding
Funding: This work was supported in part by a Grant-in-Aid for
Scientific Research from the Japan Society for the Promotion of
Science (grant no. 18K10063 to Izumi Inoue).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
II, NY, TM and MI conceived and planned the present
study. II, NY, TM, KM and MI analyzed and interpreted data. II
drafted the manuscript. TI, CH, SM, HO, HK, TA, KN and ST made
substantial contributions to the study design and protocol, data
collection and screening, and revising the draft critically for
important intellectual content. NY and MI edited the final draft.
II and NY confirm the authenticity of all the raw data. All authors
read and approved the final version.
Ethics approval nand consent to
participate
The study was conducted with the approval of the
ethics committees of the University of Tokyo (approval nos. 1264
and 1326), Tokyo University of Marine Science and Technology
(approval no. 187), and the University of Wakayama Medical
University (approval no. 373). All participants provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Vries AC, van Grieken NC, Looman CW,
Casparie MK, de Vries E, Meijer GA and Kuipers EJ: Gastric cancer
risk in patients with premalignant gastric lesions: A nationwide
cohort study in the Netherlands. Gastroenterology. 134:945–952.
2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tatsuta M, Iishi H, Nakaizumi A, Okuda S,
Taniguchi H, Hiyama T, Tsukuma H and Oshima A: Fundal atrophic
gastritis as a risk factor for gastric cancer. Int J Cancer.
53:70–74. 1993.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Uemura N, Okamoto S, Yamamoto S, Matsumura
N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N and Schlemper RJ:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ohata H, Kitauchi S, Yoshimura N, Mugitani
K, Iwane M, Nakamura H, Yoshikawa A, Yanaoka K, Arii K, Tamai H, et
al: Progression of chronic atrophic gastritis associated with
Helicobacter pylori infection increases risk of gastric cancer. Int
J Cancer. 109:138–143. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kanno T, Matsuki T, Oka M, Utsunomiya H,
Inada K, Magari H, Inoue I, Maekita T, Ueda K, Enomoto S, et al:
Gastric acid reduction leads to an alteration in lower intestinal
microflora. Biochem Biophys Res Commun. 381:666–670.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Inoue I, Kato J, Yoshimura N, Maeda Y,
Moribata K, Shingaki N, Deguchi H, Enomoto S, Maekita T, Ueda K, et
al: Elevated risk of recurrent colorectal neoplasia with
Helicobacter pylori-associated chronic atrophic gastritis: A
follow-up study of patients with endoscopically resected colorectal
neoplasia. Mol Clin Oncol. 1:75–82. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yu TY, Wei JN, Kuo CH, Liou JM, Lin MS,
Shih SR, Hua CH, Hsein YC, Hsu YW, Chuang LM, et al: The impact of
gastric Atrophy on the incidence of diabetes. Sci Rep.
7(39777)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Graham DY, Malaty HM, Evans DG Jr, Klein
PD and Adam E: Epidemiology of Helicobacter pylori in an
asymptomatic population in the United States. Effect of age, race,
and socioeconomic status. Gastroenterology. 100:1495–1501.
1991.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mégraud F, Brassens-Rabbé MP, Denis F,
Belbouri A and Hoa DQ: Seroepidemiology of Campylobacter pylori
infection in various populations. J Clin Microbiol. 27:1871–1973.
1989.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Official Statistics of Sweden: Cancer
Incidence in Sweden 2011. The National Board of Health and Welfare,
Stockholm, 2013.
|
|
11
|
Graham DY, Adam E, Klein PD, Evans DJ Jr,
Evans DG, Hazell SL, Alpert LC, Michaletz PA and Yoshimura HH:
Epidemiology of Campylobacter pylori infection. Gastroenterol Clin
Biol. 3:84B–88B. 1989.PubMed/NCBI
|
|
12
|
Asaka M, Kudo M, Kato M, Sugiyama T and
Takeda H: Review article: Long-term Helicobacter pylori
infection-from gastritis to gastric cancer. Aliment Pharmacol Ther.
12 (Suppl 1):S9–S15. 1998.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yamagata H, Kiyohara Y, Aoyagi K, Kato I,
Iwamoto H, Nakayama K, Shimizu H, Tanizaki Y, Arima H, Shinohara N,
et al: Impact of Helicobacter pylori infection on gastric cancer
incidence in a general Japanese population: The Hisayama study.
Arch Intern Med. 13:1962–1968. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shikata K, Doi Y, Yonemoto K, Arima H,
Ninomiya T, Kubo M, Tanizaki Y, Matsumoto T, Iida M and Kiyohara Y:
Population-based prospective study of the combined influence of
cigarette smoking and Helicobacter pylori infection on gastric
cancer incidence: The Hisayama study. Am J Epidemiol.
168:1409–1415. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tamura T, Morita E, Kondo T, Ueyama J,
Tanaka T, Kida Y, Hori Y, Inoue S, Tomita K, Okada R, et al:
Prevalence of Helicobacter pylori infection measured with urinary
antibody in urban area of Japan, 2008-2010. Nagoya J Med Sci.
74:63–70. 2012.PubMed/NCBI
|
|
16
|
Hirayama Y, Kawai T, Otaki J, Kawakami K
and Harada Y: Prevalence of Helicobacter pylori infection with
healthy subjects in Japan. J Gastroenterol Hepatol. 29 (Suppl
4):S16–S19. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Guarner J, Herrera-Goepfert R, Mohar A,
Sanchez L, Halperin D, Ley C and Parsonnet J: Interobserver
variability in application of the revised Sydney classification for
gastritis. Hum Pathol. 30:1431–1434. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eaton KA and Krakowka S: Chronic active
gastritis due to Helicobacter pylori in immunized gnotobiotic
piglets. Gastroenterology. 103:1580–1586. 1992.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Loffeld RJ, Werdmuller BF, Kusters JG and
Kuipers EJ: IgG antibody titer against Helicobacter pylori
correlates with presence of cytotoxin associated gene A-positive H.
pylori strains. FEMS Immunol Med Microbiol. 28:139–141.
2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Miki K, Ichinose M, Shimizu A, Huang SC,
Oka H, Furihata C, Matsushima T and Takahashi K: Serum pepsinogens
as a screening test of extensive chronic gastritis. Gastroenterol
Jpn. 22:133–141. 1987.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hirshiowitz BI: Pepsinogen: Its origins,
secretion and excretion. Physiol Rev. 37:475–511. 1957.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Samloff IM, Varis K, Ihamaki T, Siurala M
and Rotter JI: Relationships among serum pepsinogen I, serum
pepsinogen II, and gastric mucosal histology. A study in relatives
of patients with pernicious anemia. Gastroenterology. 83:204–209.
1982.PubMed/NCBI
|
|
23
|
Ichinose M, Yahagi N, Oka M, Ikeda H, Miki
K and Omata M: Screeening for gastric cancer in Japan. In: Wu GY,
Aziz K, eds. Cancer screening for common malignancies. Totowa, New
Jersey: Humana Press 87-102, 2001.
|
|
24
|
Agréus L, Kuipers EJ, Kupcinskas L,
Malfertheiner P, Di Mario F, Leja M, Mahachai V, Yaron N, van Oijen
M, Perez GP, et al: Rationale in diagnosis and screening of
atrophic gastritis with stomach specific plasma biomarkers. Scand J
Gastroenterol. 47:136–147. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Song H, Held M, Sandin S, Rautelin H,
Eliasson M, Söderberg S, Hallmans G, Engstrand L, Nyrén O and Ye W:
Increase in the prevalence of atrophic gastritis among adults age
35 to 44 years old in Northern Sweden between 1990 and 2009. Clin
Gastroenterol Hepatol. 13:1592–1600. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Muraki S, Oka H, Akune T, Mabuchi A, En-yo
Y, Yoshida M, Saika A, Suzuki T, Yoshida H, Ishibashi H, et al:
Prevalence of radiographic knee osteoarthritis and its association
with knee pain in the elderly of Japanese population-based cohorts:
The ROAD study. Osteoarthr Cartil. 17:1137–1143. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cancer Statistics. Cancer Information
Service, National Cancer Center, Japan (Vital Statistics of Japan,
Ministry of Health, Labour and Welfare). https://ganjoho.jp/reg_stat/statistics/data/dl/index.html#pref_mortality.
Accessed August 5, 2022.
|
|
28
|
Yoshimura N, Muraki S, Oka H, Mabuchi A,
En-Yo Y, Yoshida M, Saika A, Yoshida H, Suzuki T, Yamamoto S, et
al: Prevalence of knee osteoarthritis, lumbar spondylosis and
osteoporosis in Japanese men and women: The research on
osteoarthritis/osteoporosis against disability study. J Bone Miner
Metab. 27:620–628. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yoshimura N, Muraki S, Oka H, Kawaguchi H,
Nakamura K and Akune T: Cohort profile: Research on
osteoarthritis/osteoporosis against disability (ROAD) study. Int J
Epidemiol. 39:988–995. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yoshimura N, Oka H, Muraki S, Akune T,
Hirabayashi N, Matsuda S, Nojiri T, Hatanaka K, Ishimoto Y, Nagata
K, et al: Reference values for hand grip strength, muscle mass,
walking time, and one-leg standing time as indices for locomotive
syndrome and associated disability: The second survey of the ROAD
study. J Orthop Sci. 16:768–777. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kawai S, Arai K, Lin Y, Nishiyama T,
Sasakabe T, Wang C, Miwa H and Kikuchi S: Comparison of the
detection of Helicobacter pylori infection by commercially
available serological testing kits and the 13C-urea
breath test. J Infect Chemother. 25:769–773. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ichinose M, Miki K, Furihata C, Kageyama
T, Hayashi R, Niwa H, Oka H, Matsushima T and Takahashi K:
Radioimmunoassay of serum group I and group II pepsinogens in
normal controls and patients with various disorders. Clin Chim
Acta. 126:183–191. 1982.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lahner E, Zagari RM, Zullo A, Di Sabatino
A, Meggio A, Cesaro P, Lenti MV, Annibale B and Corazza GR: Chronic
atrophic gastritis: Natural history, diagnosis and therapeutic
management. A position paper by the Italian society of hospital
gastroenterologists and digestive endoscopists [AIGO], the italian
society of digestive endoscopy [SIED], the Italian society of
gastroenterology [SIGE], and the Italian society of internal
medicine [SIMI]. Dig Liv Dis. 51:1621–1632. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Huang YK, Yu JC, Kang WM, Ma ZQ, Ye X,
Tian SB and Yan C: Significance of serum pepsinogens as a biomarker
for gastric cancer and atrophic gastritis screening: A systematic
review and meta-analysis. PLoS One. 10(e0142080)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Weck MN and Brenner H: Prevalence of
chronic atrophic gastritis in different parts of the world. Cancer
Epidemiol Biomarkers Prev. 15:1083–1094. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Karita M, Noriyasu A, Kosako E, Teramukai
S and Matsumoto S: Relationship between pepsinogen I&II and H.
pylori infection considered with grade of atrophy and
gastroduodenal diseases. Dig Dis Sci. 48:1839–1845. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Karita M, Teramukai S and Matsumoto S:
Risk of Helicobacter pylori transmission from drinking well water
is higher than that from infected intrafamilial members in Japan.
Dig Dis Sci. 48:1062–1067. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fujisawa T, Kumagai T, Akamatsu T,
Kiyosawa K and Matsunaga Y: Changes in seroepidemiological pattern
of Helicobacter pylori and hepatitis A virus over the last 20 years
in Japan. Am J Gastroenterol. 94:2094–2099. 1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kawai T, Yamamoto K, Fukuzawa M, Yamagishi
T, Yagi K, Fukuzawa M, Kataoka M, Kawakami K, Itoi T, Sakai Y, et
al: Helicobacter pylori infection and reflux esophagitis in young
and middle-aged Japanese subjects. J Gastroenterol Hepatol. 25
(Suppl 1):S80–S85. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Karnes WE Jr, Samloff IM, Siurala M, Kekki
M, Sipponen P, Kim SW and Walsh JH: Positive serum antibody and
negative tissue staining for Helicobacter pylori in subjects with
atrophic body gastritis. Gastroenterology. 101:167–174.
1991.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kokkola A, Kosunen TU, Puolakkainen P,
Sipponen P, Harkonen M, Laxen F, Virtamo J, Haapiainen R and
Rautelin H: Spontaneous disappearance of Helicobacter pylori
antibodies in patients with advanced atrophic corpus gastritis.
APMIS. 111:619–624. 2003.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Notsu T, Adachi K, Mishiro T, Fujihara H,
Toda T, Takaki S and Kinoshita Y: Prevalence of autoimmune
gastritis in individuals undergoing medical checkups in Japan.
Intern Med. 58:1817–1823. 2019.PubMed/NCBI View Article : Google Scholar
|