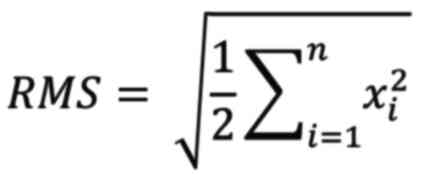Introduction
Oral squamous cell carcinoma (OSCC) stands as one of
the most prevalent malignant neoplasms globally (1). The invasion of OSCC can affect
adjacent mandibular bone, necessitating its removal as an integral
component of oncologic treatment (2).
Tumor resection is associated with loss of function,
such as chewing, swallowing, and speaking, facial aesthetics, and a
corresponding reduction in postoperative health-related quality of
life (HRQOL) (3). Vascularized
free tissue transfer, predominantly sourced from the fibula
(4) or scapula (5), is a commonly employed technique for
mandible reconstruction, aiming to achieve the optimal balance of
functionality and aesthetics in anatomical restoration. This
elaborate procedure heavily relies on surgical skill, and there is
no consistent standard for shaping the bone graft, rendering it
challenging to predict the impact of healing on the patient's
postoperative facial appearance (6).
The development of computer-aided virtual surgical
planning (VSP), combined with computer-aided design and
computer-aided manufacturing (CAD/CAM) for the production of
surgical guides for guided surgery, is now considered a standard
procedure for mandibular reconstruction and is commercially
available (7). These procedures
provide the potential for a more precise and customized surgical
approach, precise positioning of the vascularized free tissue
graft, reduced surgical and graft ischemia time, and improved
postoperative esthetic results (8,9).
Nonetheless, commercial manufacturing processes can be time
consuming, potentially causing delays in tumor therapy (8,10).
VSP and CAD/CAM techniques have become
comprehensible and feasible even for individuals not specialized in
bioinformatics. Software-based planning solutions such as the
Mimics Innovation Suite (Materialise, Leuven, Belgium) or the
open-source project Blender (Blender®; Blender
Foundation and Institute; Amsterdam, The Netherlands) enable the
integration of planning processes directly in clinical settings,
facilitating a more adaptable and personalized approach to
addressing each patient's requirements within a shorter
timeframe.
Three-dimensional (3D) printed models of the VSP can
be used to accurately model and pre-bend standardized
osteosynthesis plates preoperatively, leading to further time
savings during the procedure (7).
Moreover, as digital planning determines the position of the bone
segments, potential dental rehabilitation options can be discussed
with the patient at an early stage of therapy (11). However, it remains uncertain
whether in-house VSP and guided surgery can effectively contribute
to the restoration of the patient's preoperative facial appearance
during the postoperative healing process. The primary objective is
not to predict the patient's soft tissue reconstruction. The aim is
not to illustrate the planned bone reconstruction and the actual
surgery but to show their impact on the existing soft tissues. In
the present study, we performed in-house VSP and guided mandibular
reconstruction in 32 patients with OSCC using fibular or scapular
vascularized free tissue transfer. We evaluated the impact of the
outlined procedures on the accuracy of postoperative hard and soft
tissue reconstruction and identified risk factors associated with
poor outcomes.
Materials and methods
Patients
This retrospective analysis was performed on a
cohort of 32 patients diagnosed with locally invasive OSCC. The
sample size of 32 was determined using G*Power (v.3.1.9.2;
University of Duesseldorf, Duesseldorf, Germany) with a
significance level set at 0.05, a power of 0.95, and an estimated
large effect size of 0.6. All patients underwent segmental
mandibular resection and subsequent reconstruction. No cases
involved complete mandibular resection. Among the 32 patients, 26
received a fibular vascularized free tissue transfer. In the
remaining six patients, either the extent of soft tissue resection
was too extensive for a fibular graft, or vascular supply from the
lower leg was deemed unsuitable.
In these cases, a scapular vascularized free tissue
transfer from the right shoulder was employed instead. The patient
population consisted of 22 male and 10 female individuals, with
ages ranging from 47 to 82 years. The clinical characteristics of
all patients are detailed in Table
I. In assessing baseline clinical characteristics, tumor stages
T1 and T2 were grouped together, as well as T3 and T4, while AJCC
stages I and II were also grouped together, along with AJCC stages
III and IV.
 | Table IPatient clinical baseline
characteristics. |
Table I
Patient clinical baseline
characteristics.
| N | Age at surgery,
years | Sex | pT | pN | M | G | Stage | AT | Tx | Segments | Plate | OS | TBC, days |
|---|
| 1 | 75 | F | 3 | 2b | 0 | 3 | III | + | Fibula | 2 | Mini | + | 174 |
| 2 | 53 | M | 3 | 0 | 0 | 1 | III | + | Fibula | 1 | Mini | + | 162 |
| 3 | 65 | M | 4 | 0 | 0 | 1 | IVA | + | Fibula | 2 | Mini | + | 279 |
| 4 | 47 | F | 4 | 2b | 0 | 3 | IVA | + | Fibula | 3 | Mini | + | 244 |
| 5 | 58 | M | 1 | 0 | 0 | 2 | I | - | Fibula | 2 | Mini | - | 197 |
| 6 | 78 | M | 2 | 2a | 0 | 2 | IVA | + | Fibula | 2 | Reco | + | 230 |
| 7 | 51 | M | 1 | 0 | 0 | 2 | I | - | Fibula | 2 | Reco | - | 116 |
| 8 | 67 | M | 4 | 0 | 0 | 2 | IVA | + | Fibula | 2 | Reco | + | 188 |
| 9 | 66 | M | 3 | 0 | 0 | 2 | III | + | Fibula | 2 | Reco | - | 157 |
| 10 | 51 | M | 3 | 1 | 0 | 2 | III | + | Fibula | 3 | Reco | - | 225 |
| 11 | 81 | F | 4 | 0 | 0 | 2 | IVA | + | Fibula | 3 | Reco | + | 325 |
| 12 | 75 | F | 1 | 0 | 0 | 2 | I | - | Fibula | 2 | Reco | - | 49 |
| 13 | 68 | M | 2 | 0 | 0 | 2 | II | - | Fibula | 2 | Reco | - | 204 |
| 14 | 66 | M | 2 | 0 | 0 | 2 | II | - | Fibula | 3 | Reco | - | 287 |
| 15 | 65 | M | 4 | 2b | 0 | 2 | IVA | + | Fibula | 3 | Reco | + | 168 |
| 16 | 58 | M | 2 | 0 | 0 | 2 | II | - | Fibula | 2 | Reco | - | 336 |
| 17 | 66 | F | 2 | 1 | 0 | 2 | III | + | Fibula | 2 | Reco | - | 354 |
| 18 | 76 | M | 2 | 0 | 0 | 2 | II | - | Fibula | 1 | Reco | - | 290 |
| 19 | 65 | F | 4 | 0 | 0 | 2 | IVA | + | Fibula | 3 | Reco | + | 157 |
| 20 | 58 | M | 3 | 2b | 0 | 3 | IVA | + | Fibula | 2 | Reco | - | 43 |
| 21 | 73 | M | 2 | 2b | 0 | 2 | IVA | + | Fibula | 2 | Reco | + | 251 |
| 22 | 54 | F | 4 | 2b | 0 | 2 | IVA | + | Fibula | 2 | Reco | + | 200 |
| 23 | 70 | M | 1 | 0 | 0 | 1 | I | - | Fibula | 1 | Reco | - | 188 |
| 24 | 56 | M | 4 | 2c | 0 | 2 | IVA | + | Fibula | 3 | Reco | - | 268 |
| 25 | 76 | F | 2 | 0 | 0 | 2 | II | - | Fibula | 2 | Reco | + | 189 |
| 26 | 53 | M | 4 | 0 | 0 | 2 | IVA | + | Fibula | 3 | Reco | + | 197 |
| 27 | 63 | F | 4 | 3b | 0 | 2 | IVB | + | Scapula | 2 | Reco | - | 45 |
| 28 | 62 | F | 4 | 2c | 0 | 2 | IVA | + | Scapula | 2 | Reco | - | 168 |
| 29 | 62 | F | 4 | 3b | 0 | 3 | IVB | + | Scapula | 1 | Reco | - | 248 |
| 30 | 77 | M | 4 | 3b | 0 | 2 | IVB | + | Scapula | 2 | Reco | - | Mis |
| 31 | 60 | M | 4 | 3b | 0 | 3 | IVB | + | Scapula | 2 | Reco | - | Mis |
| 32 | 82 | M | 3 | 2b | 0 | 3 | IVA | + | Scapula | 1 | Reco | - | 884 |
For analysis of nodal status, patients were
categorized into two groups: those with lymph involvement
(positive) and those without (negative). Other factors evaluated
included postoperative adjuvant therapy (radiation and/or drug
therapy), the number of graft segments employed for reconstruction,
the type of osteosynthesis used (2.0 mm mini-plate osteosynthesis
vs. 2.7 mm reconstruction plate osteosynthesis), the duration
between preoperative and postoperative control imaging, and the
evaluation of postoperative preservation in occlusal support
zones.
All participants provided written informed consent
for their inclusion in the study, which adhered to principles
outlined in the Declaration of Helsinki. Additionally, the study
received review and approval from the local ethics committee of the
University Medical Center Goettingen (Goettingen, Germany; approval
no. 14/7/19).
In-house VSP and CAD/CAM-based
fabrication of surgical guides
Computed tomography (CT) scans of the
head-neck-thorax region, performed for initial tumor staging, and
angio-CT to evaluate vascular supply of the lower extremity (each
with a 0.6 mm slice thickness), were used for VSP. All CT
examinations were performed at the Department of Diagnostic and
Interventional Radiology, University Medical Center Goettingen,
Germany. CTA and CT scans were performed using a third-generation
dual-energy CT scanner (SOMATOM Definition AS & Force and
SOMATOM Definition AS Edge, Siemens Healthineers, Forchheim,
Germany). Virtual planning for all cases was performed by PB or NM,
and case analysis was performed by GH. All analyses were performed
twice; with the second round of analyses performed at a minimum
interval of 5 to 14 days later. The surgical procedure was
performed as a collaborative effort by the staff of the Department
of Oral and Maxillofacial Surgery at the University of Goettingen,
enduring a high professional standard.
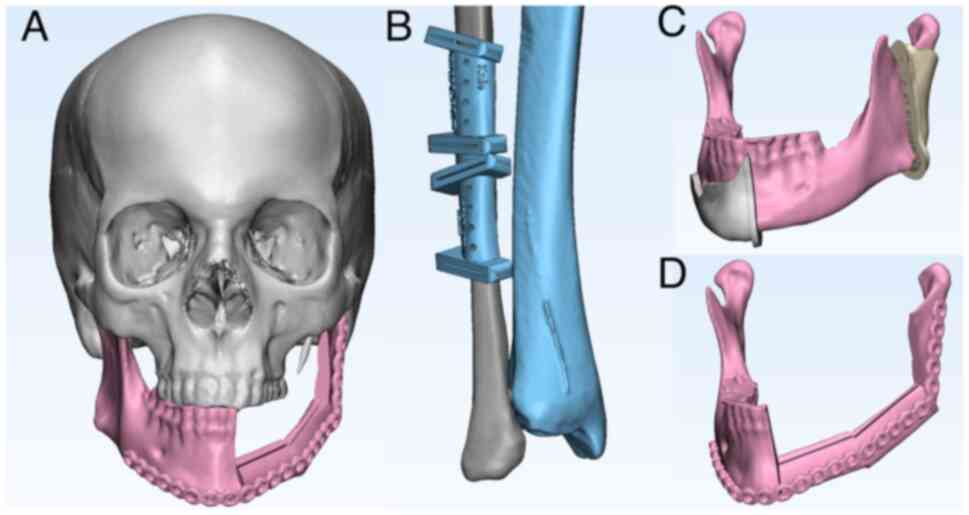
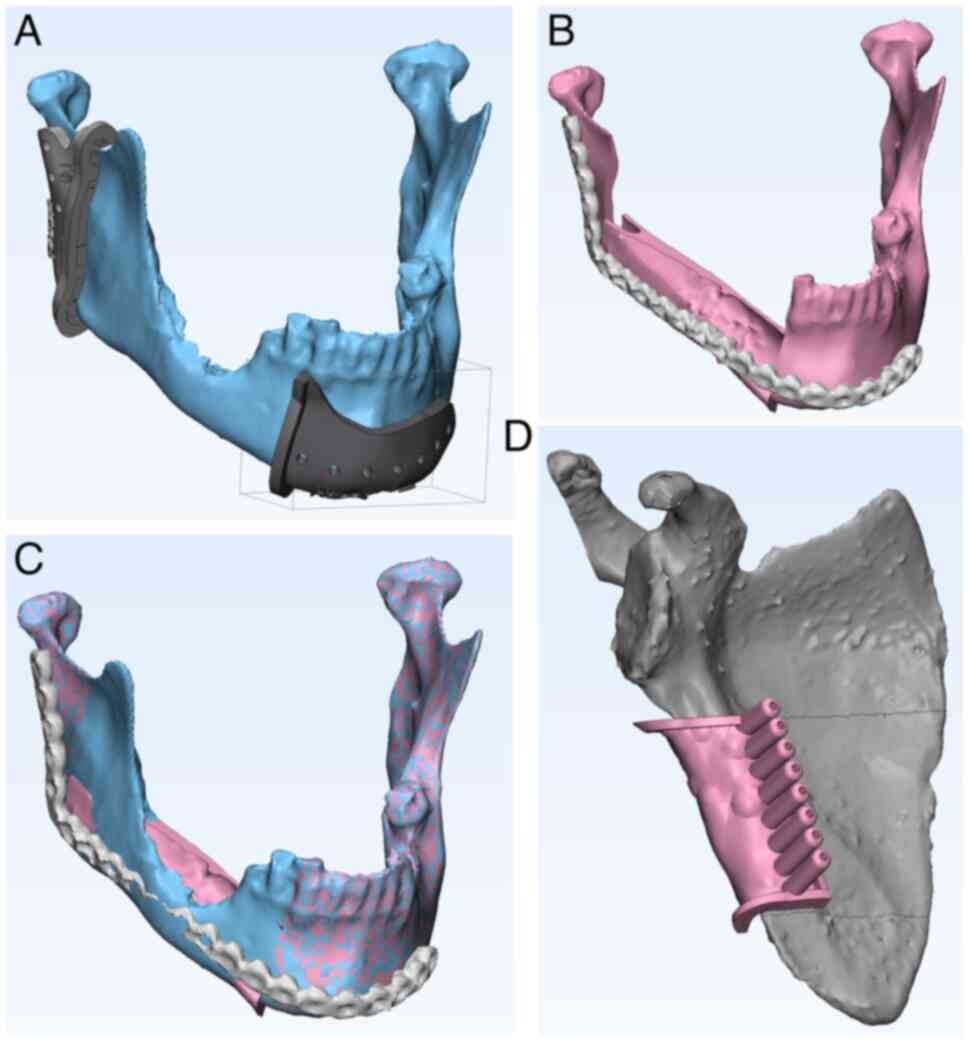
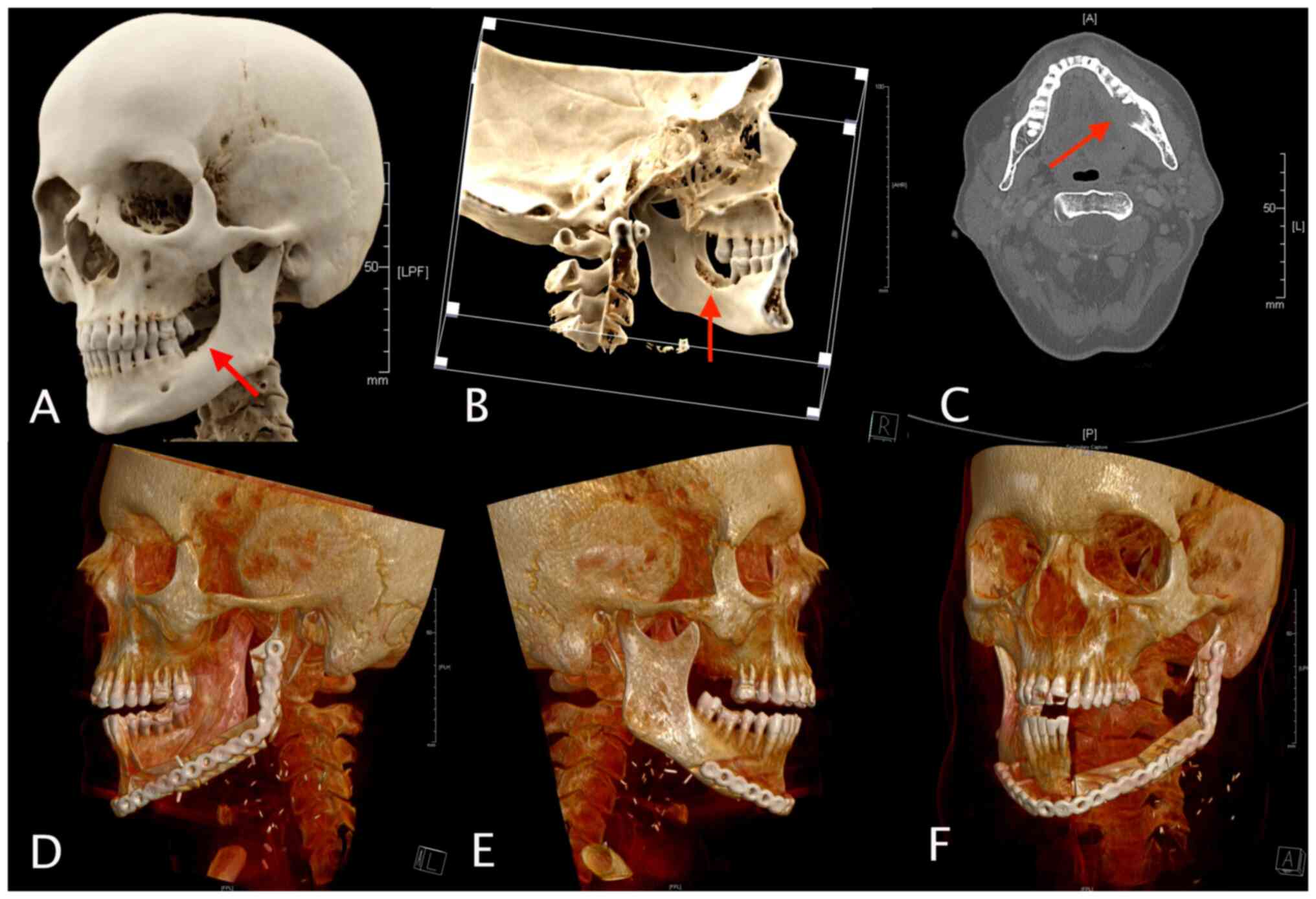
The visible mandibular bone defect (resulting from
tumor invasion) was virtually resected with a safety margin of 1 cm
both anteriorly and posteriorly, as illustrated in Figs. 1C and 2A). Subsequently, the 3D model of the
fibula or scapula was inserted into the bone defect to properly
reconstruct the external contour of the original jaw segment
(Figs. 1A, 1D, 2B
and 2C). The individual fibula and
scapula segments were virtually osteotomized (Figs. 1B and 2D). A 3D model of the entire
reconstructed neomandible was printed using a Monoprice Inventor
IIIP 3D printer (Monoprice, California, USA) with PLA material
(Polymaker, Houston, USA). For 27 cases, a customized 2.7 mm
reconstruction osteosynthesis plate (KLS Martin, Tuttlingen,
Germany) was employed, tailored to fit the segments and fixed to
the printed model using osteosynthesis screws (KLS Martin,
Tuttlingen, Germany). In five cases, 2.0 mm mini osteosynthesis
plates from the same manufacturer were utilized as an alternative
(KLS Martin, Tuttlingen, Germany).
The osteosynthesis plates were digitally segmented
from the CBCT DICOM data set, and the resulting 3D model was
inserted into the VSP (Fig. 1D).
Subsequently, two surgical guides for the mandibular osteotomies
(one for the anterior and one for the posterior) and a graft guide
for the fibula/scapular osteotomies were fabricated using an
in-house CAD/CAM-based procedure (Figs. 1B, 1C, 2A
and 2D). All VSP steps were
performed using the Mimics In-novation Suite software (Materialise,
Leuven, Belgium). The final surgical guides were 3D printed from
surgical guide resin (FormLabs, Massachusetts, USA) using the
FormLabs Form 3B+ 3D printer (FormLabs, Massachusetts, USA) and
sterilized. The pre-bent osteosynthesis plates were sterilized and
prepared for surgery.
Guided mandibular reconstruction
Following the initial neck dissection (selective
level 1-3, with more if needed) and cervical vascular preparation
for vascularized free tissue transfer, mandibular resection was
performed during intraoral tumor resection. The affected mandibular
segment was exposed, and the surgical guides (anterior and
posterior resection planes) were positioned and fixed to the
mandible. The mandible was then osteotomized along the
predetermined planes using a jigsaw. Screw holes for the pre-bent
osteosynthesis plate were predrilled using shafts modeled in
surgical guides. The entire primary OSCC (soft tissue with attached
mandibular bone) was sent for pathology, and marginal sections were
taken and submitted for frozen section analysis. Defect
reconstruction was performed only in the case of R0 resection.
In parallel, the fibula harvest was performed using
a two-team approach. When the bony fibula was visualized and the
vascular pedicle (A./V. fibularis) was identified, the fibula was
pre-osteotomized using the jigsaw, and the graft guide was
positioned over the vascular perforator with the attached skin
island. The guide was secured with osteosynthesis screws, and the
individual segments were osteotomized using a jigsaw while
protecting the vascular pedicle in predetermined planes.
Subsequently, the individual fibular segments were screwed to the
pre-bent osteosynthesis plates.
The 3D-arranged bone graft with an attached vascular
pedicle was transferred to the head and secured in place through
the predrilled holes in the fibular segments. The skin island was
sutured into the soft tissue defect, and the vascular pedicle was
directed cervically for microvascular reanastomosis, performed with
the aid of an operating microscope (Carl Zeiss Meditec, Oberkochen,
Germany).
In cases involving scapular harvesting, following
the necessary intraoperative repositioning and visualization of the
bony scapula and vascular pedicle, the scapula was pre-osteotomized
from the margo lateralis, inverted, and the cutting guide was
securely attached to the scapular segment from below using
osteosynthesis screws, as illustrated in Fig. 2D. The predetermined segments were
subsequently osteotomized using the jigsaw, and the segment was
fixed to the pre-bent osteosynthesis plate in accordance with the
fibula procedure.
Morphometric evaluation
Postoperative control CT scans (0.6 mm slice
thickness) were imported into the Mimics suite software
(Materialise, Leuven, Belgium), and virtual 3D models were
generated through semi-automatic thresholding and tessellation. A
fixed threshold range of -700 to +2,200 was applied for soft
tissues, while a threshold range of +300 to the highest was used
for bony structures.
The 3D models were imported into the 3-Matic
software (Materialise, Leuven, Belgium), where the trimming
function was used to remove existing artifacts (e.g., caused by
dental crowns). The models were aligned using the classical
Iterative Closest Point (ICP) algorithm and overlapping regions
were evaluated using the 3-Matic Part Comparison Analysis function.
This function calculates the distance between closed points among
the surface triangles of 3D surface mesh and automatically aligns
and calculates the deviations between the corresponding point
pairs. The surface deviations are visualized in a heatmap. The
analysis provides values for the mean deviation of the same mesh
surface points (MSD=Mean Surface Distance), along with the
associated standard deviation (SD) and root mean square (RMS)
values. The exact RMS value was calculated using the following
equation:
If point Y in the postoperative 3D surface mesh has
the closest point Y' in the preoperative 3D surface mesh, then Xn
is the distance between Y and Y', where n denotes the total number
of point pairs in both 3D surface meshes. The RMS value represents
the sum of the averaged 3D deviations and functions as an indicator
of the extent to which the deviations between the two individual
datasets deviate from zero (12).
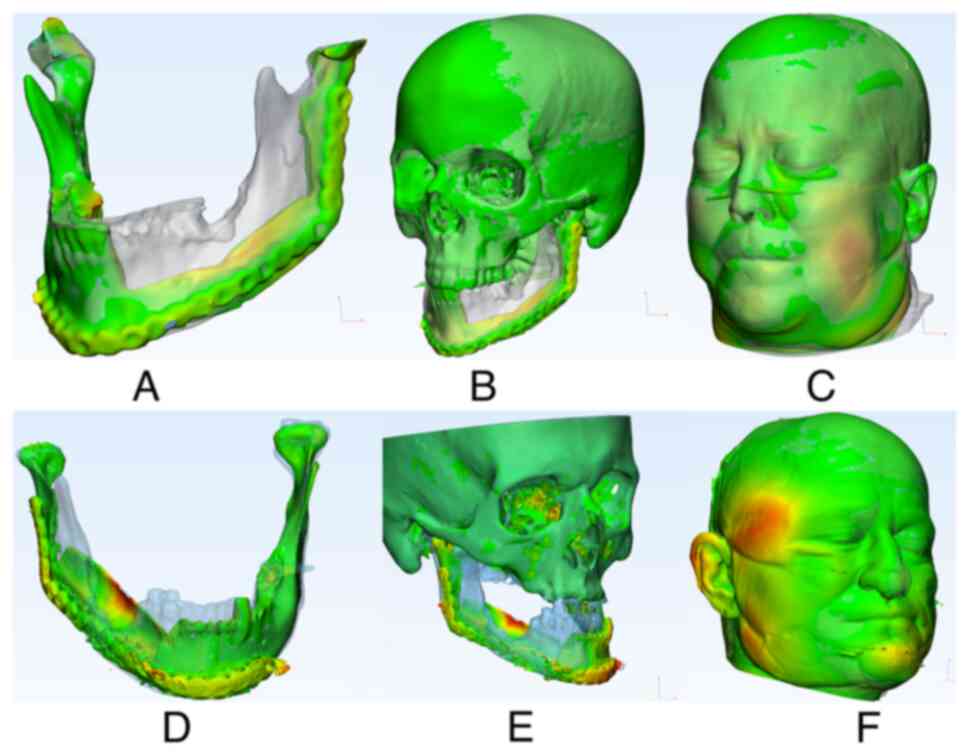
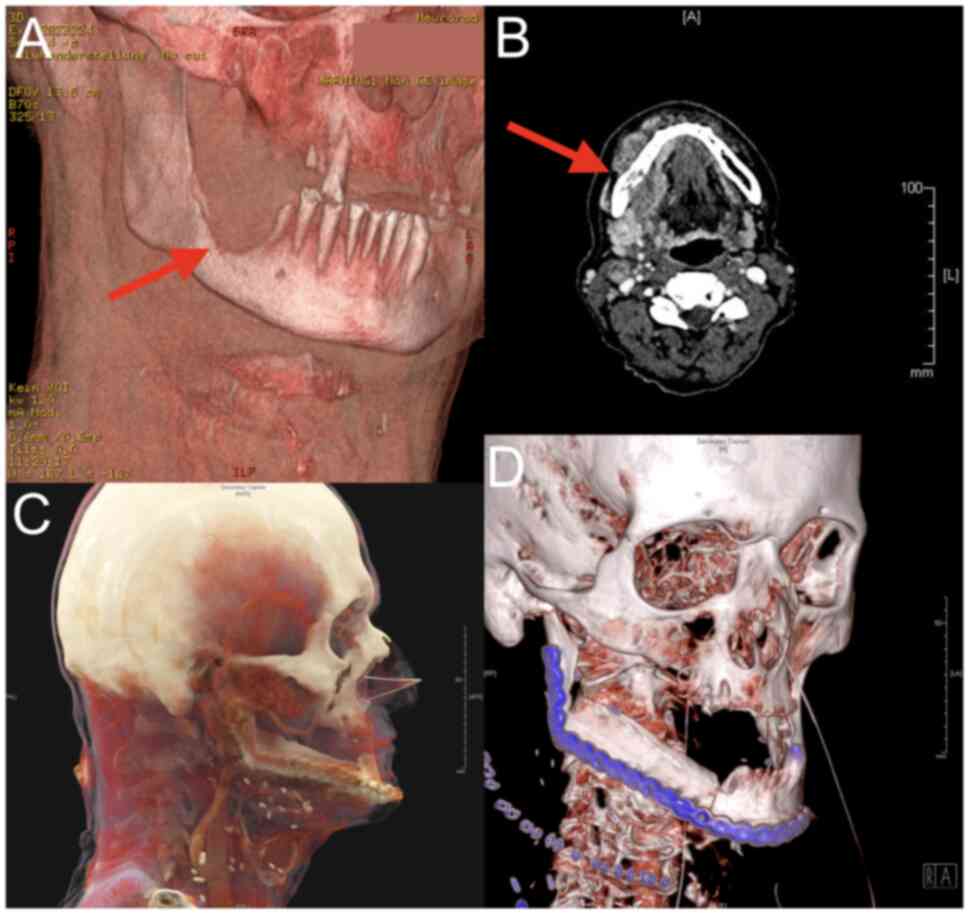
An example of the generated heatmaps is depicted in Fig. 3A-F for further illustration.
Statistical analysis
All variables, including clinical data and 3D model
analysis, were summarized as absolute and relative frequencies or
mean ± SD and median (minimum; maximum), as appropriate. The impact
of the type of osteosynthesis (reconstruction plate osteosynthesis
vs. mini-plate osteosynthesis) on reconstruction accuracy was
assessed by employing separate linear models for all reconstruction
measures, with the type of osteosynthesis serving as the predictor.
Model improvement was assessed through likelihood ratio tests on
models where osteosynthesis type was not considered as a predictor.
The resulting p-values were corrected for multiple testing using
Holm's procedure.
Similar examinations were conducted for all other
potential risk factors affecting reconstruction quality. To account
for the influence of osteosynthesis type, osteosynthesis type was
included as an additional predictor in the linear models. Alongside
the results of the likelihood ratio tests, the coefficients from
the resulting model fits are presented with 95% confidence
intervals and their associated p-values.
Due to the screening nature of this study,
unadjusted p-values are reported. A significance level of alpha=5%
was set for all statistical tests, and all analyses were performed
using the statistical software R (version 4.1.2; R Core Team 2021).
The R package ordinal [version 2019.12.10; (13)] was utilized for the ordinal
regression model, while the R package logistf [version 1.24.1;
(14)] was employed for the Firth
correction in the logistic regression models.
Results
3D performance analysis
Descriptive data for the part comparison analysis,
aimed at assessing the reconstruction's accuracy in comparison to
the preoperative baseline, are shown in Table II. Regarding the 3D morphometric
evaluation, data were collected from all 32 cases, and they are
presented separately for the 26 fibula cases and all 6 scapula
cases.
 | Table IIDescriptive data of the part
comparison analysis. |
Table II
Descriptive data of the part
comparison analysis.
| Parameter | All cases
(n=32) | Fibula cases
(n=26) | Scapula cases
(n=6) |
|---|
| MSD skull | | | |
|
Mean ±
SD | 0.76±0.31 | 0.68±0.24 | 1.14±0.3 |
|
Median (min;
max) | 0.68 (0.33;
1.5) | 0.61 (0.33;
1.26) | 1.1 (0.74;
1.51) |
| MSD mandible | | | |
|
Mean ±
SD | 1.5±0.5 | 1.31±0.34 | 2.15±0.55 |
|
Median (min;
max) | 1.4 (0.85;
3.2) | 1.14 (0.34;
0.85) | 2.06 (1.68;
3.23) |
| MSD soft
tissue | | | |
|
Mean ±
SD | 3.2±2.0 | 2.87±1.83) | 5.52±1.48 |
|
Median (min;
max) | 2.3 (1.3; 9.5) | 2.26 (1.27;
9.46) | 5.21 (4.07;
7.56) |
|
Missing | 2 | 0 | 2 |
| RMS skull | | | |
|
Mean ±
SD | 1.8±0.8 | 1.64±0.64 | 2.44±1.07 |
|
Median (min;
max) | 1.6 (0.91;
4.5) | 1.43 (0.91;
3.21) | 2.02 (1.57;
4.48) |
| RMS mandible | | | |
|
Mean ±
SD | 2.3±0.8 | 2.08±0.62 | 3.06±0.94 |
|
Median (min;
max) | 2.2 (1.2; 4.9) | 1.88 (1.18;
3.23) | 2.75 (2.39;
4.94) |
| RMS soft
tissue | | | |
|
Mean ±
SD | 5.3±2.9 | 4.92±2.77 | 7.83±3.04 |
|
Median (min;
max) | 4.1 (2.1; 14) | 4.04 (2.12;
13.87) | 8.47 (3.62;
10.74) |
|
Missing | 2 | 0 | 2 |
The analysis revealed deviations between
preoperative and postoperative data. The smallest differences in
MSD were observed in the whole skull comparison. In this
comparison, the majority of the bone tissue remained unaffected by
the surgery, and thus maintaining consistency postoperatively. In
the mandible analysis, a mean deviation of 1.5 mm was observed,
which exceeded the data for the entire skull. The largest mean
deviation in the MSD was seen in the soft tissue comparison, where
a mean of 3.2 mm was measured.
Two scapula cases could not be evaluated for soft
tissue appearance due to the patients' absence at the 6-month
control CT scan. Consequently, the postoperative CT scan at two
weeks was utilized for the analysis of the skull and mandible.
Factors influencing reconstruction
precision
Potential risk factors, such as sex, age at surgery,
adjuvant therapy performed, occlusal support zone after surgery,
number of segments transplanted, AJCC stage, nodal status, T stage,
graft type, time between preoperative and first postoperative
follow-up CT scan, and type of osteosynthesis, were examined to
identify possible associations with one of three reconstruction
accuracy measures (RMS cranial, RMS mandible, RMS soft tissue).
Linear models were employed to fit the reconstruction accuracy
measure, with the risk factor serving as a predictor. Table III shows the combinations of
reconstruction and risk factors where the risk factor significantly
improved the model's predictive ability. Table IV provides details on the model
fits that underlie these results.
 | Table IIICombinations of risk factors and
reconstruction accuracy measures where the inclusion of the risk
factor significantly improved the model prediction of the
reconstruction accuracy in linear models. |
Table III
Combinations of risk factors and
reconstruction accuracy measures where the inclusion of the risk
factor significantly improved the model prediction of the
reconstruction accuracy in linear models.
| Risk factor | Reconstruction | P-value |
|---|
| Type of
transplant | RMS skull | 0.033a |
| Type of
transplant | RMS mandible | 0.006b |
| Adjuvant
therapy | RMS soft
tissue | 0.036a |
| AJCC stage | RMS soft
tissue | 0.036a |
| Nodal status | RMS soft
tissue | 0.033a |
 | Table IVSelected model coefficient; CI and
P-values from the multivariate analysis model (likelihood ratio
tests). |
Table IV
Selected model coefficient; CI and
P-values from the multivariate analysis model (likelihood ratio
tests).
| Reconstruction | Risk factor | Term | Modeled value
estimation | CI | P-value |
|---|
| RMS skull | Transplant | Reference
value | 1.473 | [0.80; 2.15] |
<0.001a |
| | | Type of transplant
(scapula) | 0.7675 | [0.07; 1.47] | 0.033b |
| | | Type of transplant
(scapula) | 0.2005 | [-0.55; 0.95] | 0.59 |
| RMS mandible | Transplant | Reference
value | 1.995 | [1.36; 2.63] |
<0.001a |
| | | Type of transplant
(scapula) | 0.9619 | [0.30; 1.62] | 0.006c |
| | | Type of
osteosynthesis (reconstruction osteosynthesis plate) | 0.09915 | [-0.61; 0.81] | 0.777 |
| RMS soft
tissue | Adjuvant
therapy | Reference
value | 5.925 | [3.3; 8.53] |
<0.001a |
| | | No adjuvant
therapy | -2.463 | [-4.8; -0.17] | 0.036b |
| | | Type of
osteosynthesis (reconstruction osteosynthesis plate) | 0.1446 | [-2.7; 2.97] | 0.917 |
| RMS soft
tissue | AJCC stage | Reference
value | 3.461 | [0.31; 6.60] | 0.033b |
| | | AJCC stage
III/IV | 2.463 | [0.17; 4.80] | 0.036b |
| | | Type of
osteosynthesis (reconstruction osteosynthesis plate) | 0.1446 | [-2.68; 3.00] | 0.917 |
| RMS soft
tissue | Nodal status | Reference
value | 4.515 | [1.8; 7.20] | 0.002c |
| | | Positive nodal
status | 2.292 | [0.2; 4.40] | 0.033b |
| | | Type of
osteosynthesis (reconstruction osteosynthesis plate) | -0.426 | [-3.2; 2.40] | 0.758 |
The findings from this screening analysis indicate
that the type of bone graft used for neo-mandible formation (fibula
vs. scapula) is a potential risk factor affecting reconstruction
accuracy. Patients who underwent scapular grafts had significantly
higher deviations between preoperative baseline data and
postoperative reconstruction results. Moreover, high AJCC stage
(III and IV), positive nodal status (N+), and the use of adjuvant
therapy are likely risk factors associated with poorer
postoperative soft tissue reconstruction outcomes. The different
osteosynthesis plate systems used for osteosynthetic neomandible
fixation do not affect the accuracy of fit. This is despite a 0.7
mm difference in plate diameter.
Discussion
The destruction of the bony mandible resulting from
the progression of OSCC can have a significant effect on patients'
abilities related to speech, chewing, swallowing, facial
appearance, and their overall health-related quality of life
(HRQOL) (15). In addition to
oncologic considerations, achieving functional and esthetic
restoration and facilitating social reintegration are essential
objectives of treatment.
Mandibular reconstruction involving vascularized
free tissue transfer is a surgically challenging and time-consuming
procedure. Therefore, the use of VSP and guided surgery should
contribute to increased efficiency, as well as enhance
predictability and precision in the outcomes (16).
In the present study, we evaluated the effect of
in-house VSP and guided mandibular reconstruction on reconstruction
accuracy, specifically examining deviations in the patient's
preoperative and postoperative hard and soft tissue appearance.
Case study examples of the procedures conducted are depicted in
Figs. 4 and 5, showcasing both fibular and scapular
bone graft cases, respectively. The patient population under
investigation comprised 22 males and 10 females, with a mean age of
65 years (ranging from 47 to 82 years). This demographic profile is
in line with other OSCC patient collectives described in the
literature (2,6), making it a representative sample.
The fibular free flap was primarily employed for
neomandibular reconstruction (81%), followed by the scapular free
flap (19%), aligning with findings reported by others (17). Mandibular reconstruction is most
commonly required in patients with advanced T4 tumor stage,
accounting for 44% of all patients. This can be readily attributed
to the tendency of T4 tumors to infiltrate adjacent structures,
necessitating mandibular bone replacement more frequently (18,19).
While malignant bone infiltration by tumor cells is rare in
early-stage tumors, small tumors near the mandible may require
resection and reconstruction to achieve a tumor-free margin as part
of the oncologic treatment approach.
We identified potential confounding factors that
could have an impact on both reconstruction accuracy and
postoperative appearance of facial soft tissue. Higher tumor stages
(AJCC stages III and IV) are associated with poorer preoperative
and postoperative soft tissue appearance. This association can be
partly attributed to the fact that patients at these stages
experience considerably greater tissue deficits after resection,
necessitating more extensive and complicated reconstruction
procedures. Ritschl et al reported a poorer level of
concordance between VSP data and postoperative reconstruction for
higher grade defects in contrast to smaller reconstruction defects
(8).
Our findings revealed an association between
positive nodal status (N+) and poorer postoperative facial soft
tissue appearance, aligning with the results reported by Lee et
al (20). This association may
be attributed to the relationship between positive nodal status,
higher tumor stage, and concomitant adjuvant therapy.
Our findings revealed that adjuvant therapy also has
a negative effect on postoperative facial appearance. It should be
noted that patients with an oncologic indication for adjuvant
therapy usually have more advanced tumor disease, necessitating
more extensive mandibular reconstruction and, consequently,
carrying a higher potential for error. Furthermore, it is worth
noting that radiotherapy may affect the processes of soft tissue
remodeling, with cases of scarring and complex inflammatory
processes documented in the literature (21). These effects could potentially
affect the postoperative facial appearance. This side effect is
also visible in Fig. 3F, where
there is a deviation in the right temporal region. This deviation
was not initially associated with the surgery but was still
affected by swelling that persisted six months after surgery.
Our data suggest that the type of bone graft used
for neomandible formation significantly impacts the quality of
postoperative reconstruction. Significantly higher deviations in
hard tissue data between preoperative and postoperative states were
observed in patients who underwent mandibular reconstruction with a
scapular graft compared to those who received a fibular graft. To
the best of our knowledge, there are no comparable studies in the
literature. This could potentially be attributed to the greater
complexity involved in the guided shaping of the scapular flap
compared to a fibular flap, primarily due to the adherent
musculature and the associated potential for error in positioning
the graft guide on the lateral margo. Furthermore, a critical
consideration for opting for the scapular flap is the presence of a
substantial soft tissue defect, which is a significant factor to
bear in mind when assessing the quality of the postoperative
reconstruction. Regarding the analysis of the number of bone
segments utilized for neo-mandible reconstruction, our findings
revealed that the majority of mandibular defects can be replaced
with two bone segments.
There is currently no available literature
addressing the impact of the osteosynthesis type (one
reconstruction plate vs. multiple mini plates) on the postoperative
reconstruction accuracy. In our study, we did not observe a
significant influence of the osteosynthesis type on the quality of
postoperative reconstruction. What has been thoroughly studied in
the literature, however, is the impact of the osteosynthesis type
on postoperative complications, such as plate infection, plate
fracture, pseudoarthrosis, loosened osteosynthesis screws, or
exposed osteosynthesis material (22).
López-Arcas et al (23) and van Gemert et al (24) did not observe any differences in
the aforementioned complications. However, Al-Bustani et al
proposed an increased complication rate when employing mini-plate
osteosynthesis for neomandible reconstruction (25). In contrast, patient-specific
implants (PSIs) have demonstrated higher reconstruction accuracy
and better fit compared to manually shaped plates (26).
The VSP and guided surgery evaluated in this study
are based on an in-house developed and established procedure. A
disadvantage of existing commercially available reconstruction
procedures using PSIs is that the processing time can extend to
several weeks in certain cases. However, due to tumor progression,
rapid surgery becomes imperative. Utilizing the in-house procedure
outlined here allows for expedited tumor surgery, followed by
precise virtual planned mandibular reconstruction. Besides the
time-saving aspect mentioned earlier, it's essential not to
overlook the cost-benefit ratio. This aspect has been thoroughly
explored by Rommel et al (27) and Tarsitano et al (28), although it was not the primary
focus of this study.
This study aims to introduce a new digital algorithm
designed to render the analysis of 3D reconstruction more
objective. Barr et al clearly describe the problem of
defining the reconstruction accuracy (29). Various studies have endeavored to
analyze the reconstruction accuracy through metric analyses
involving distance and angles (30,31).
Unfortunately, this type of evaluation overlooks the complexity of
3D factors and fails to acknowledge the potential for magnified
errors. A meta-analysis conducted by Serrano et al
underscores the necessity for a unified analysis of guided
reconstructions to increase comparability (9). In the present collective, the mean
MSD of the mandible was 1.5 mm (± 0.5 mm SD; range 0.85-3.2 mm).
The MSD for the cranial data was 0.76 mm (± 0.31 mm SD; range
0.33-1.5 mm), and the RMS values were 2.3 for the mandibular data
(± 0.8 SD; range 1.2-4.9) and 1.8 for the cranial data (± 0.8 SD;
range 0.91-4.5).
Comparative data in the literature are still
limited. Studies conducted by Ritschl et al (8) and Moe et al (32) employed similar assessment
approaches (8,32). In Moe et al's study, which
involved a cohort of 26 cases (24 fibulas and two scapulas), the
average MSD values for the mandible were 1.9 mm, accompanied by an
associated RMS value of 3.72. Ritschl et al reported MSD
values of 0.5 mm (range-0.6-6.1) and RMS values of 2.2 (range
1.5-11.1) when comparing mandibles from preoperative 3D models with
postoperative 3D models. The data from both studies align closely
with the present analysis. When comparing the pre- and
postoperative status of the patients regarding the soft tissue
relevant to the facial appearance, the points exhibited an average
of 3.2 mm (± 2.0 mm SD; range 1.3-9.5 mm). The corresponding the
RMS value was 5.3 (± 2.9 SD; range 2.1-14).
As elucidated earlier, achieving consistent analysis
of VSP and guided mandibular reconstruction is difficult. It is
imperative to assess the accuracy of the virtual planning, surgical
guide fabrication, and postprocessing protocols at each step. This
diligence ensures that accuracy is maintained throughout the
process and that potential errors within this multi-step protocol
do not accumulate and introduce bias into the study results. Hence,
all materials used in this study were produced in accordance with
strict manufacturer specifications, encompassing the manufacturing
process, assembly, and sterilization of the guides. However, it's
worth noting that this study has other limitations, primarily
stemming from the unequal distribution of group sizes.
It's important to emphasize that 3D models were
generated using CT data with a slice thickness of 0.6 mm. In most
cases, only positive data in favor of VSP and guided surgery are
published, while negative results are often interpreted as an
absence of correlation (9).
Therefore, there is a need for large, randomized studies to gain a
deeper understanding of the complexity involved in a 3D anatomical
reconstruction approach.
In conclusion, high tumor stage, positive nodal
status, and the use of adjuvant therapy contribute to more
substantial deviations in the preoperative and postoperative facial
soft tissue appearance among OSCC patients. Differences between
preoperative hard tissue data and postoperative reconstruction
outcomes are greater in patients who underwent a scapular free flap
for neomandible formation compared to those who received a fibular
flap. In-house VSP and guided mandibular reconstruction can deliver
satisfactory clinical results and can be performed quickly,
enabling patients with advanced OSCC to benefit from this
technology.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GH and PB designed the present study. GH, NM, BS and
BW collected all the data. GH, AL and PB analyzed and interpreted
all data and confirmed the authenticity of all the raw data. GH, TK
and PB were instrumental in drafting the manuscript. AL and HS made
substantial contributions to the analysis and interpretation of the
data. TK and HS made substantial contributions to the conception
and design of the study and revised the manuscript. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
tenets of the Declaration of Helsinki and reviewed and approved by
the local ethics committee of the University Medical Center
Goettingen (Goettingen, Germany; approval no. 14/7/19). Written
informed consent was obtained from all subjects involved in the
study.
Patient consent for publication
All patients gave written informed consent for
publication.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Verhelst P-J, Dons F, Van Bever PJ,
Schoenaers J, Nanhekhan L and Politis C: Fibula free flap in head
and neck reconstruction: Identifying risk factors for flap failure
and analysis of postoperative complications in a low volume
setting. Craniomaxillofac Trauma Reconstr. 12:183–192.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
van Beek FE, Jansen F, Mak L,
Lissenberg-Witte BI, Buter J, Vergeer MR, Voortman J, Cuijpers P,
Leemans CR and Verdonck-de Leeuw IM: The course of symptoms of
anxiety and depression from time of diagnosis up to 2 years
follow-up in head and neck cancer patients treated with primary
(chemo)radiation. Oral Oncol. 102(104576)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hidalgo DA: Fibula free flap: A new method
of mandible reconstruction. Plast Reconstr Surg. 84:71–79.
1989.PubMed/NCBI
|
|
5
|
Swartz WM, Banis JC, Newton ED, Ramasastry
SS, Jones NF and Acland R: The osteocutaneous scapular flap for
mandibular and maxillary reconstruction. Plast Reconstr Surg.
77:530–545. 1986.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Petrovic I, Panchal H, Franca PDDS,
Hernandez M, McCarthy CC and Shah J: A Systematic review of
validated tools assessing functional and aesthetic outcomes
following fibula free flap reconstruction of the mandible. Head
Neck. 41(248)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ghai S, Sharma Y, Jain N, Satpathy M and
Pillai AK: Use of 3-D printing technologies in craniomaxillofacial
surgery: A review. Oral Maxillofac Surg. 22:249–259.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ritschl LM, Kilbertus P, Grill FD, Schwarz
M, Weitz J, Nieberler M, Wolff KD and Fichter AM: In-house,
open-source 3D-software-based, CAD/CAM-planned mandibular
reconstructions in 20 consecutive free fibula flap cases: An
explorative cross-sectional study with three-dimensional
performance analysis. Front Oncol. 11(731336)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Serrano C, van den Brink H, Pineau J,
Prognon P and Martelli N: Benefits of 3D printing applications in
jaw reconstruction: A systematic review and meta-analysis. J
Craniomaxillofac Surg. 47:1387–1397. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nyirjesy SC, Heller M, von Windheim N,
Gingras A, Kang SY, Ozer E, Agrawal A, Old MO, Seim NB, Carrau RL,
et al: The role of computer aided design/computer assisted
manufacturing (CAD/CAM) and 3-dimensional printing in head and neck
oncologic surgery: A review and future directions. Oral Oncol.
132(105976)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Berrone M, Crosetti E, Tos PL, Pentenero M
and Succo G: Fibular osteofasciocutaneous flap in computer-assisted
mandibular reconstruction: Technical aspects in oral malignancies.
Acta Otorhinolaryngol Ital. 36:469–478. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sharma N, Aghlmandi S, Cao S, Kunz C,
Honigmann P and Thieringer FM: Quality characteristics and clinical
relevance of in-house 3D-printed customized polyetheretherketone
(PEEK) implants for craniofacial reconstruction. J Clin Med.
9(2818)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Christensen R: ‘Ordinal-regression models
for ordinal data.’. R Package Version. 12–10. 2019.
|
|
14
|
Heinze G and Schemper M: A solution to the
problem of separation in logistic regression. Stat Med.
21:2409–2419. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Hoene G, Gruber RM, Leonhard JJ, Wiechens
B, Schminke B, Kauffmann P, Schliephake H and Brockmeyer P:
Combined quality of life and posttraumatic growth evaluation during
follow-up care of patients suffering from oral squamous cell
carcinoma. Mol Clin Oncol. 15(189)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Iglesias-Martín F, Oliveros-López LG,
Fernández-Olavarría A, Serrera-Figallo MA, Gutiérrez-Corrales A,
Torres-Lagares D and Gutiérrez-Pérez JL: Advantages of surgical
simulation in the surgical reconstruction of oncological patients.
Med Oral Patol Oral Cir Bucal. 23:e596–e601. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Brown JS, Lowe D, Kanatas A and Schache A:
Mandibular reconstruction with vascularised bone flaps: A
systematic review over 25 years. Br J Oral Maxillofac Surg.
55:113–126. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hollows P, McAndrew PG and Perini MG:
Delays in the referral and treatment of oral squamous cell
carcinoma. Br Dent J. 188:262–265. 2000.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang SH, Hahn E, Chiosea SI, Xu ZY, Li
JS, Shen L and O'Sullivan B: The role of adjuvant
(chemo-)radiotherapy in oral cancers in the contemporary era. Oral
Oncol. 102(104563)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee H, Roh JL, Cho KJ, Choi SH, Nam SY and
Kim SY: Number of positive lymph nodes better predicts survival for
oral cavity cancer. J Surg Oncol. 119:675–682. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kim J, Shin ES, Kim JE, Yoon SP and Kim
YS: Neck muscle atrophy and soft-tissue fibrosis after neck
dissection and postoperative radiotherapy for oral cancer. Radiat
Oncol J. 33:344–349. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dean A, Alamillos F, Heredero S,
Redondo-Camacho A, Guler I and Sanjuan A: Fibula free flap in
maxillomandibular reconstruction. Factors related to osteosynthesis
plates' complications. J Craniomaxillofac Surg. 48:994–1003.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
López-Arcas JM, Arias J, Del Castillo JL,
Burgueño M, Navarro I, Morán MJ, Chamorro M and Martorell V: The
fibula osteomyocutaneous flap for mandible reconstruction: A
15-year experience. J Craniomaxillofac Surg. 68:2377–2384.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
van Gemert JTM, Abbink JH, van Es RJJ,
Rosenberg AJWP, Koole R and Van Cann EM: Early and late
complications in the reconstructed mandible with free fibula flaps.
J Surg Oncol. 117:773–780. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Al-Bustani S, Austin GK, Ambrose EC,
Miller J, Hackman TG and Halvorson EG: Miniplates versus
reconstruction bars for oncologic free fibula flap mandible
reconstruction. Ann Plast Surg. 77:314–317. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wurm MC, Hagen J, Nkenke E, Neukam FW and
Schlittenbauer T: The fitting accuracy of pre-bend reconstruction
plates and their impact on the temporomandibular joint. J
Craniomaxillofac Surg. 47:53–59. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rommel N, Kesting MR, Rohleder NH, Bauer
FMJ, Wolff KD and Weitz J: Mandible reconstruction with free fibula
flaps: Outcome of a cost-effective individual planning concept
compared with virtual surgical planning. J Craniomaxillofac Surg.
45:1246–1250. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tarsitano A, Battaglia S, Crimi S, Ciocca
L, Scotti R and Marchetti C: Is a computer-assisted design and
computer-assisted manufacturing method for mandibular
reconstruction economically viable? J Craniomaxillofac Surg.
44:795–799. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Barr ML, Haveles CS, Rezzadeh KS, Nolan
IT, Castro R, Lee JC, Steinbacher D and Pfaff MJ: Virtual surgical
planning for mandibular reconstruction with the fibula free flap: A
systematic review and meta-analysis. Ann Plast Surg. 84:117–122.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Geusens J, Sun Y, Luebbers HT, Bila M,
Darche V and Politis C: Accuracy of Computer-aided
design/computer-aided manufacturing-assisted mandibular
reconstruction with a fibula free flap. J Craniofac Surg.
30:2319–2323. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schepers RH, Raghoebar GM, Vissink A,
Stenekes MW, Kraeima J, Roodenburg JL, Reintsema H and Witjes MJ:
Accuracy of fibula reconstruction using patient-specific CAD/CAM
reconstruction plates and dental implants: A new modality for
functional reconstruction of mandibular defects. J Craniomaxillofac
Surg. 43:649–657. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Moe J, Foss J, Herster R, Powell C, Helman
J, Ward BB and VanKoevering K: An in-house computer-aided design
and computer-aided manufacturing workflow for maxillofacial free
flap reconstruction is associated with a low cost and high
accuracy. J Craniomaxillofac Surg. 79:227–236. 2021.PubMed/NCBI View Article : Google Scholar
|