1. Introduction
Multiple myeloma (MM) has become the second-most
common hematological malignancy, accounting for 10% of all
hematological malignancies (1). MM
can be identified by serum immunofixation electrophoresis and the
features of MM are the buildup of clonal proliferative malignant
plasma cells in the bone marrow as well as the release of
monoclonal immunoglobulin (M protein). Anemia, hypercalcemia,
osteolytic lesions and renal insufficiency are some of its clinical
manifestations (which are commonly referred to as ‘CRAB’ symptoms,
denoting hypercalcemia, renal failure, anemia, and bone
destruction) (2). In the past two
decades, a notable development has been made in the treatment of
MM. MM treatment has been linked to extremely positive outcomes
owing to the rapid uptake of autologous hematopoietic stem cell
transplantation, the advent of immunomodulatory medications
(IMids), and proteasome inhibitors (PIs), with small molecule
antitumor medications, such as dexamethasone and other
glucocorticoids (3,4). However, MM is considered a fatal
illness (5). The most important
concerns include improving patient prognoses, decreasing adverse
drug reactions, extending the lives of patients, and enhancing
their quality of life. Myeloma cells may die directly or indirectly
with the use of an immunomodulator (6), and MM cells may be indirectly
affected by changes to the bone marrow microenvironment.
Thalidomide was the first novel medication to be authorized by the
US Food and Drug Administration (FDA) for the management of MM in
1999. It has been reported to inhibit tumor necrosis factor
production, MM cell growth, and anti-angiogenesis, albeit its
therapeutic efficacy is significantly hampered by the neurological
side effects of thalidomide, which include drowsiness and
peripheral neuropathy (7).
Lenalidomide and pomalidomide are two new IMiDs that were created
and used as the result of preclinical studies and subsequent
clinical trials. Both could be utilized in MM therapy regimens.
Lenalidomide was the first thalidomide analog to be marketed, and
it was more potent than its parent drug despite only two
differences at the molecular level: The addition of an amino group
at position four of the phthaloyl ring and the removal of a
carbonyl group from the phthaloyl ring (8). Lenalidomide was developed by Celgene
Corporation (now part of Bristol Myers Squibb) in the U.S. and was
approved by the FDA on December 17, 2005 for fast-track marketing
for the treatment of anemia caused by myelodysplastic syndromes
associated with deletions of chromosome 5q, and has since been
approved for a variety of indications, including MM (9). Lenalidomide, which is a structural
and functional analog of thalidomide, can enhance the
immunomodulatory, anticancer, and tolerability properties of
thalidomide (10). Lenalidomide
has been a global bestseller for numerous years since its
introduction to the market, but with the entry of generics into the
market, there has been a significant decrease in the price of
lenalidomide, rendering it available to a wider range of
patients.
2. Pharmacological mechanism
Lenalidomide, a second-generation imine medication,
is relatively less toxic and with a higher potency when compared
with thalidomide (11).
Lenalidomide has been demonstrated to exhibit an array of effects
and mechanisms of action that can contribute to its antitumor
properties (12) (Fig. 1).
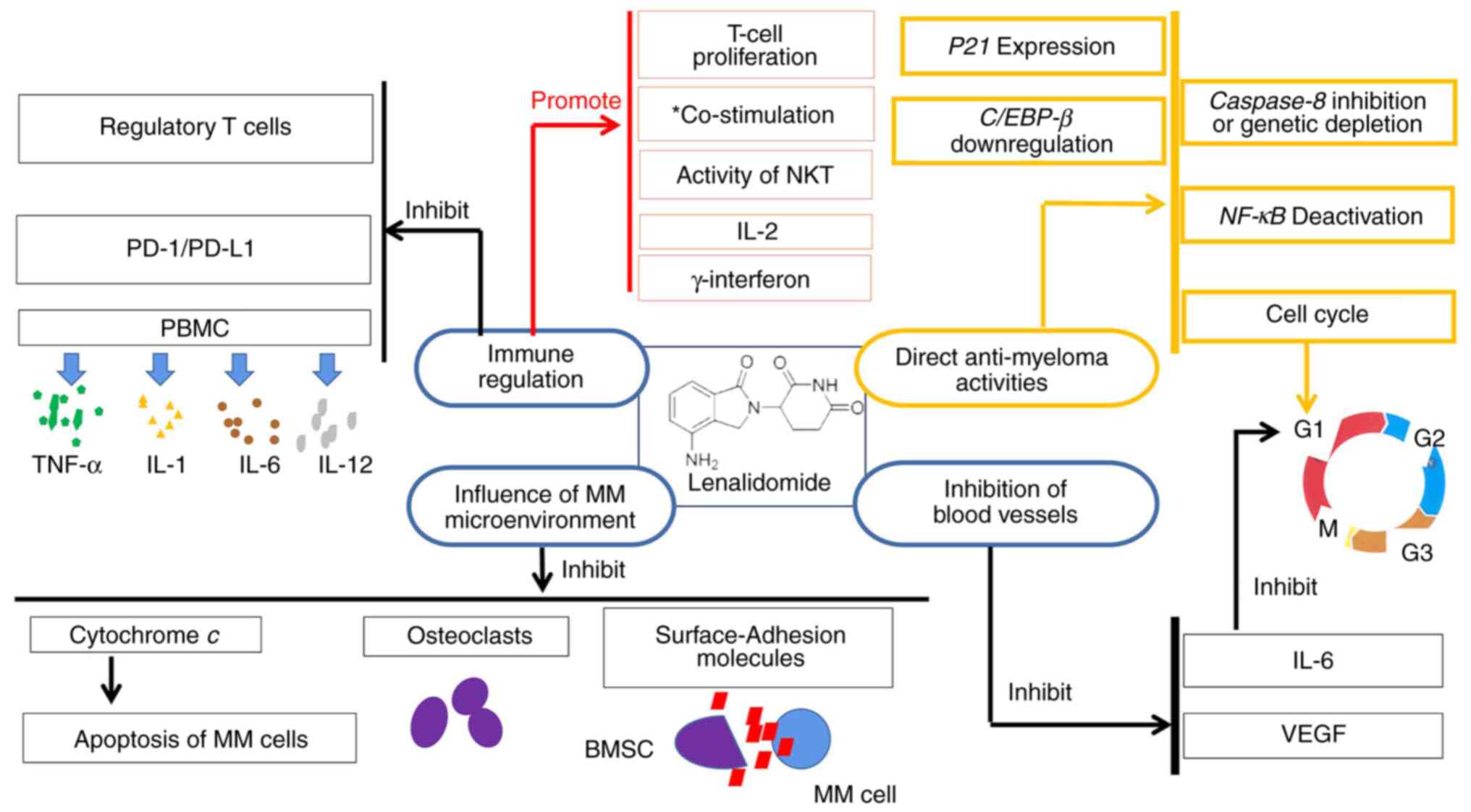 | Figure 1Pharmacological mechanism of
lenalidomide. *Co-stimulation of CD4+ and
CD8+ T cells. MM, multiple myeloma; VEGF, vascular
endothelial growth factor; IL, interleukin; NKT, natural killer
cells and T lymphocytes; C/EBP-β CCAAT/, enhancer binding
protein-β; NF-κB, nuclear factor-κB; BMSC, bone marrow stromal
cell; PBMC, human peripheral blood mononuclear cell; TNF-α, tumor
necrosis factor-Α; PD-1 programmed death-1; PD-L1, programmed death
ligand-1. |
Non-immune regulation
Vascular endothelial growth factor (VEGF) is
inhibited by lenalidomide, which makes it challenging for tumor
cells to form blood vessels. It blocks VEGF and can prevent the
production of interleukin-6 (IL-6) (13). As per a previous study, IL-6 is a
cytokine with a wide range of inflammatory and immune regulatory
properties (14). In addition,
IL-6 can promote the progression of MM. Lenalidomide has also been
linked to the growth arrest of myeloma cells in the G1 phase
(15,16), and this direct cytotoxicity is
associated with a decrease in IL-6 production. However, the precise
mechanism underlying this effect is unknown.
A well-known mitochondrial protein, cytochrome
c, can maintain life by transporting electrons to the
respiratory chain and allowing continued ATP production. Cell
survival and apoptosis significantly depend on cytochrome c
(17). By influencing the release
of cytochrome c, lenalidomide can impact the apoptosis of MM
cells (18). In addition, by
altering caspase-8, lenalidomide can also affect the apoptosis of
MM cells (19,20).
Lenalidomide can directly induce MM cell apoptosis
and cell cycle arrest. Previous studies have demonstrated several
downstream changes after lenalidomide treatment, which may be
associated with the direct anti-myeloma activities of the drug, in
addition to the previously mentioned mechanism. These changes
include the upregulation of P21 expression (21), nuclear factor-κB (NF-κB)
deactivation (22), CCAAT/enhancer
binding protein-β (C/EBP-β) downregulation (23), and caspase-8 inhibition or genetic
depletion (20).
By suppressing the production of surface-adhesion
molecules on both MM cells and bone marrow stromal cells (BMSCs),
lenalidomide prevents contact between them (24). Lenalidomide can prevent MM-related
bone damage by either directly preventing osteoporosis development
or by indirectly decreasing the tumor load. The effect of
lenalidomide on osteoclasts can slow the development of MM, as
osteoclasts have been demonstrated to increase MM growth and
medication resistance (25).
Immune regulation
In contrast to other anti-MM medications,
lenalidomide possesses immunoregulatory effects. First, it can
improve the co-stimulation of CD4+ and CD8+ T
cells (26). When compared to the
first-generation IMiD thalidomide, lenalidomide increases T-cell
proliferation and the production of IL-2 and γ-interferon (27). Lenalidomide can also inhibit
regulatory T cells, which are a subset of immunosuppressive T
cells, that are important for self-tolerance and the reaction of
the the immune system to tumor cells (28). With the natural killer (NK)
cell-surface markers, lenalidomide increases the activity of NK and
T lymphocytes (NKT) (29). In
patients with MM who have received suitable treatment, NK cell
proliferation is promoted, an important pharmacological effect of
lenalidomide. It has also been demonstrated that the ability of
human peripheral blood mononuclear cells (PBMC) to produce the
pro-inflammatory cytokines tumor necrosis factor-α (TNF-α), IL-1,
IL-6, and IL-12 is inhibited by the effect of lenalidomide
(30). Lenalidomide reduces the
immune checkpoint inhibitor programmed death-1 (PD-1) expression on
both T and NK cells in patients with MM; it also reduces the
expression of PD-1 and programmed death ligand-1 (PD-L1) on MM
cells (31-33).
3. Pharmacokinetics
Oral lenalidomide is promptly and effectively
absorbed (>90% of the dose) under fasting conditions as per the
results of a control study conducted on healthy volunteers
(34). Drug oral absorption
effectiveness can be affected by food type. The maximum
concentration (Cmax) and the area under the
concentration-time curve (AUC) both decreased by 20-50%,
respectively, when combined with a high-fat diet. The increase in
AUC and Cmax was dose-dependent, with minimal to
moderate inter-individual variability in plasma exposure. After 24
h, ~80% (34,35) of the oral dose was eliminated in
the urine. Lenalidomide has a very brief (3-4 h) half-life and it
does not build up in plasma when administered repeatedly.
Renal function is the only significant factor
affecting lenalidomide plasma exposure as per a study that assessed
the dose range of patients with MM (36). These researchers also confirmed
that plasma AUC and Cmax were proportional to the dose
used in the treatment of patients with MM, without exhibiting any
differences when compared with the healthy volunteers.
4. Clinical research and application
The application of lenalidomide for the treatment of
MM was approved by the FDA in March 2006. Since then, clinical
trials and real-world studies (37-61)
on the single-drug (R) treatment of lenalidomide and the
combination of lenalidomide and dexamethasone (RD) as well as
various treatment schemes based on the combination of RD have been
widely conducted. These studies aimed to confirm the therapeutic
efficacy of lenalidomide in patients with newly diagnosed MM (NDMM)
or relapsed refractory MM (RRMM) (Table I).
 | Table IUse of lenalidomide in clinical
trials and real-world studies in MM. |
Table I
Use of lenalidomide in clinical
trials and real-world studies in MM.
| Lenalidomide-based
treatment scheme | Patients | Key trials or
studies | Efficacy | Notable adverse
effects | (Refs.) |
|---|
| RD-R | NDMM | Clinical study | EFS, 10.4
months | Neutropenia,
21% | (37) |
| | | (NCT02215980) | Median PFS, 20.2
months | Infection, 10% | |
| | | | OS rate in 3 years,
74% | Dermatosis, 7% | |
| RD | | | EFS, 6.9
months | Neutropenia,
18% | |
| | | | Median PFS, 18.3
months | Infection, 12% | |
| | | | OS rate in 3 years,
63% | Dermatosis, 3% | |
| Not responded | RRMM | Randomized | CR, 3% | Data not
available | (38) |
| to first-line
RD | | controlled trial
by | VGPR, 18% | | |
| | | Lund et
ala | PR, 39% | | |
| | | (NCT01430546) | | | |
| | | (NCT01450215) | | | |
| RD-R vs. RD | | | CR, 11% | Neutropenia
and thrombocytopenia were more frequent in RD-R | |
| | | | VGPR, 31% | | |
| | | | PR, 38% | | |
| | | | TTP (24.9 months
vs. not reached) | | |
| VRD | NDMM | Phase 3 study | Median PFS, 43
months | ≥ Grade 3 AEs,
82% | (39) |
| | | (S0777) | Median OS, 75
months | | |
| | | | CR, 16% | | |
| | | | ≥PR, 82% | | |
| RD | NDMM | | Median PFS, 30
months | ≥ Grade 3 AEs,
75% | |
| | | | Median OS, 64
months | | |
| | | | CR, 8% | | |
| | | | ≥PR, 72% | | |
| VRD | NDMM | Real world study
by | Median PFS, 26.5
months | Data not
available | (66) |
| | | Medhekar et
al | | | |
| Lenalidomide- | NDMM | Meta-analysis | PFS was increased
compared with |
Gastrointestinal | (41) |
| based
treatment | | | conventional
therapy alone | problems (RR
2.36) | |
| | | | | Thromboembolic
events | |
| | | | | (RR 2.55) | |
| | | | | Second primary
cancers | |
| | | | | (RR 2.61) | |
| VRD + ASCT | NDMM | Joseph et al
and | Median PFS, 63
months | Data not
available | (42,43) |
| | | McCaughan et
al | Median OS, 123.4
months | | |
| CD38 | NDMM | Part 1 of a
phase | MRD negativity,
50% | Neutropenia,
23% | (44) |
| monoclonal | | 3 trial | | Infections,
12% | |
| antibody, | | (NCT03617731) | | | |
| isatuximab and | | | | | |
| VRD | | | | | |
| Dara-VRD | NDMM | Griffin trial
by | sCR, 62.6% | Grade 3/4
hematologic | (45) |
| VRD | | Voorhees et
al | MRD negativity,
51.0% | AEs and infections
were | |
| | |
(NCT02874742)b | sCR, 45.4% | more common
with | |
| | | | MRD negativity,
20.4% | Dara-VRD, but | |
| | | | | infection rates
were similar | |
| VRD | NDMM | Attal et
al | Median PFS, 36
months | Neutropenia,
92% | (46) |
| | | (NCT01191060) | CR, 48% |
Gastrointestinal | |
| | | | | disorders, 28% | |
| | | | | Infections,
20% | |
| VRD + ASCT + | | | Median PFS, 50
months | Neutropenia,
47% | |
| VRD | | | CR, 59% |
Gastrointestinal | |
| | | | | disorders, 7% | |
| | | | | Infections, 9% | |
| VRD + ASCT | | | ORR, 100% | | (46) |
| | | | CR + sCR,
73.3% | | |
| | | | VGPR, 95.6% | | |
| | | | PFS rate in 2
years, 84.5% | | |
| | | | OS rate in 2 years,
100% | | |
| VRD | RRMM | Phase 2 study | ORR, 86% | Peripheral
neuropathy, 62% | (47) |
| | | | VGPR, 66% | ≥ Grade 3 AEs,
1.9% | |
| | | | Median PFS, 35.1
months | | |
| VRD | RRMM | Phase 2 study | PFS rate in 1 year,
45.5% | Thrombocytopenia,
54.5% | (48) |
| | | (JCOG0904) | OS rate in 3 years,
70.0% | Sensory
peripheral | |
| | | | | neuropathy,
22.7% | |
| Weekly IRD | NDMM | Integrated | Median PFS, 25.8
months | Data not
available | (49) |
| | | analysis of
four | OS rate in 3 years,
96% | | |
| | | phase I/II
studiesc | | | |
| IRD | NDMM | Kumar et
al | ORR, 80% | ≥ Grade 3 AEs,
68% | (50) |
| | | | CR, 32% | | |
| | | | VGPR, 63% | | |
| | | | Median PFS, 29.4
months | | |
| IRD + ASCT | NDMM | Clinical trial | ORR, 100% | ≥ Grade 3 AEs,
86% | (51) |
| | | (NCT01850524) | CR, 44% | | |
| | | | VGPR, 76% | | |
| | | | Median PFS, 29.4
months | | |
| IRD | | | CR, 26% | ≥3 Grade TEAEs
88% | |
| | | | VGPR, 63% | | |
| | | | Median PFS, 35.3
months | Severe TEAEs,
66% | |
| | | | | Mortality, 8% | |
| Ixazomib and | NDMMd | Patel et
al | Median PFS, 73
months | ≥3 Grade
hematologic AEs | (52) |
| lenalidomide | | | CR/sCR, 43% | Neutropenia,
46.88% | |
| | | | | Leukopenia,
20.31% | |
| | | | | Thrombocytopenia,
15.63% | |
| Ixazomib and | | | Median PFS, 73
months | ≥3 Grade
nonhematologic | |
| lenalidomide | | | CR/sCR, 43% | AEs | |
| | | | | Lung infections,
26.6% | |
| | | | | Diarrhe, a
12.5% | |
| | | | | Rash
(maculopapular), 12.5% | |
| IRD | RRMM | Phase 3 clinical
trial | Median OS, 53.6
months | ≥3 Grade AEs,
80.1% | (53) |
| | | (NCT01564537) | | Serious AEs,
56.8% | |
| IRD | RRMM | Multicenter
real- | Median PFS, 11.9
months | Data not
available | (54) |
| | | world
studye | (IgG type 19.3
months) | | |
| | | | Median OS, not
attained | | |
| Dara-KRD | NDMM | Open phase 1b | ORR, 95% | Diarrhea: | (55) |
| | | research | CR, 67% | • Any grades AEs,
68% | |
| | | | PR, 86% | • ≥3 Grade AEs,
18% | |
| | | | | Lymphopenia: | |
| | | | | • Any grades AEs,
64% | |
| | | | | • ≥3 Grade AEs,
59% | |
| | | | | Cough: | |
| | | | | • Any grades AEs,
59% | |
| | | | | • ≥3 Grade AEs,
5% | |
| | | | | Upper respiratory
tract | |
| | | | | infection: | |
| | | | | • Any grades AEs,
59% | |
| | | | | • ≥3 Grade AEs,
5% | |
| VRD | NDMM | Phase 3 clinical
trial | Median PFS, 34.4
months | Fatigue, 6% | (56) |
| | | (NCT01863550) | | Hyperglycemia,
4% | |
| | | | | Peripheral
neuropathy, 8% | |
| | | | | Dyspnea, 2% | |
| | | | | Diarrhoea, 5% | |
| | | | | Thrombotic events,
2% | |
| | | | | Serious AEs,
22% | |
| KRD | | | Median PFS, 34.6
months | Fatigue, 6% | |
| | | | | Hyperglycemia,
6% | |
| | | | | Peripheral
neuropathy, <1% | |
| | | | | Dyspnea, 7% | |
| | | | | Diarrhoea, 3% | |
| | | | | Thrombotic events,
5% | |
| | | | | Serious AEs,
45% | |
| KRD + ASCT + | NDMM | Phase 2
multicenter | sCR, 76% | Neutropenia,
34% | (57) |
| KRD | | investigation | PFS rate in 5
years, 72% | Lymphocytopenia,
32% | |
| | | (NCT01816971) | OS rate in 5 years,
84% | Infection, 22% | |
| | | | | Cardiac events,
3% | |
| KRD + | NDMM | Phase 2 study | Median PFS, 56.4
months | Hematogenous,
74% | (58) |
| ASCT + R | | (NCT02405364) | CR, 64.3% | Infectious,
22% | |
| | | | sCR, 61.9% | | |
| KRD | RRMM | Randomized | Median OS, 48.3
months | ≥3 Grade AEs,
87% | (59) |
| RD | | controlled
study | Median OS, 40.4
months | ≥3 Grade AEs,
83.3% | |
| | | (NCT01080391) | | | |
| KRD | RRMM | Multicenter
real- | ORR, 68.2% | ≥3 Grade AEs,
48% | (60) |
| | | world
investigation | Median PFS, 8.8
months | | |
| | | | Median OS, 29
months | | |
| KRD | RRMM | Study on the use
of | VGPR, 57% | Data not
available | (61) |
| | | KRD
reinduction | | | |
| KRD + | | | VGPR, 77% | | |
| HDCT/ASCT | | | Median PFS, 23.3
months | | |
Application of lenalidomide alone and
with dexamethasone in MM
The conventional course of treatment for young
patients with NDMM is autologous-hematopoietic stem cell
transplantation (ASCT) after effective induction therapy (62). Lenalidomide was authorized by the
FDA in 2017 for use as a maintenance therapy for patients with MM
following ASCT (63).
A clinical study (NCT02215980) (37) showed the effectiveness and
viability of continuing 10 mg lenalidomide monotherapy (RD-R) daily
in comparison with continuous RD after receiving
dose/schedule-adjusted RD in elderly, moderately healthy
individuals with NDMM who did not undergo ASCT. Additionally, the
data revealed that low-dose lenalidomide may be used without
dexamethasone after nine cycles of RD, and the outcomes were
comparable with those of continuous RD.
According to a randomized controlled trial by Lund
et al (38), a single
medication, lenalidomide, may be useful for the prolonged treatment
of RRMM once patients exhibit preliminary responsiveness to the
induction of the RD regimen. In a subsequent phase 2 clinical trial
(NCT01450215), patients with RRMM who responded to first-line RD in
an observational study (NCT01430546) received up to 24 cycles of R
or RD as extended treatment. The median reaction time in the
observational study was 1.7 months, with a range of 0.6-9.6 months.
In these two investigations, 11% of the patients experienced a
complete response (CR) to all treatments received. Very good
partial response (VGPR) and partial response (PR) were observed in
31 and 38% of the patients, respectively. In the subset of patients
who were not enrolled in the second phase of the experiment, the
equivalent remission rates were 3, 18, and 39%, respectively. RD
did not develop within the median time to progress (TTP) during a
median follow-up of 36 months for the surviving patients; RD-R was
24.9 months (95% CI, 12.5-not calculable; P<0.001).
Application of lenalidomide combined
with bortezomib and dexamethasone in MM
The first PI to receive FDA approval was bortezomib
(V) (64). It can connect to the
amino acid residues of the 26S proteasome and block the
ubiquitin-proteasome system pathway, thereby preventing the
breakdown of protein products involved in fighting tumors (65). Bortezomib is indispensable in the
management of MM. In a phase 3 study and real world research data
(39,66), the combination of bortezomib and RD
(VRD) was established to be associated with an excellent response
rate, manageable toxicity, and therapeutic advantages.
A phase 3 study (S0777) (39) revealed that when VRD was used
instead of RD alone, progression-free survival (PFS) and overall
survival (OS) were significantly improved in newly diagnosed
patients without immediate ASCT. Additionally, a study by Medhekar
et al (66) which analyzed
patient characteristics and treatment outcomes, revealed a median
PFS of 26.5 months that was shorter than the pivotal median PFS of
43 months achieved in the SWOG S0777 study (39). According to a meta-analysis
(41), PFS was continually
increased with lenalidomide treatment compared with conventional
therapy alone.
In previous research (42,43),
sequential ASCT was administered to 751 of the 1,000 consecutive
patients receiving VRD induction therapy. The median PFS and OS for
this population were 63 and 123.4 months, respectively. The most
recent data from the Griffin trial and German-Speaking Myeloma
Multicenter Group (GMMG-HD7) trial (44,45,67)
investigations indicated that in patients deemed eligible for ASCT,
the reaction rate of adding CD38 monoclonal antibody, isatuximab to
VRD, and the negative rate of measurable residual disease (MRD)
were both improved. Although the Griffin trial recently showed that
the combination of these four medications offers considerable
benefits in terms of PFS (45),
neither research included data on PFS or OS. Therefore, the CD38
monoclonal antibody and VRD quadruple induction procedure may
become the norm for patients with MM who are deemed ASCT
candidates. The pursuit of negative MRD may also be advantageous
for patients who have reached VGPR. Specifically, an analysis of
patients who participated in the PETHEMA/GEM 2012 trial (68) established that negative MRD can
improve the prognosis of high-risk patients with cytogenetics.
However, the use of the VRD scheme for consolidation treatment
after ASCT is still debatable. The published ASCT investigations on
consolidation therapy with the VRD scheme (46), however, were performed throughout
two cycles, which revealed improved VGPR and CR after
consolidation.
The median age of the study participants was 73
years (range 65-91 years) in a phase 2 study of lenalidomide
combined with bortezomib and dexamethasone for treating
transplant-ineligible patients with MM (47). The total effective rate was 86 and
66% of the patients achieved VGPR or improved remission. The median
OS was not attained, the mean PFS was 35.1 months (95% CI, 30.9-not
reached), and the mean follow-up period was 30 months.
Additionally, a phase 2 study (JCOG0904) (48) revealed that patients with RRMM
undergoing VRD treatment exhibited satisfactory 1-year PFS (45.5%)
and 3-year OS (70%) outcomes.
Application of lenalidomide combined
with ixazomib and dexamethasone in MM
A reversible PI called ixazomib (I) with oral
bioavailability was produced by Millenium Pharmaceuticals, Inc.
(now Takeda Oncology) (69). The
drug functions by binding to and inhibiting the subunits of the 20S
proteasome. The FDA approved its use in combination with
lenalidomide and dexamethasone (IRD) in November 2015 for treating
patients with MM who have already undergone at least a single
therapy. Globally, however, clinical trials involving ixazomib for
NDMM and real-world research applications are still ongoing.
In a previous study, a total of 25 patients with
NDMM receiving weekly IRD, as well as 18 other patients receiving
twice-weekly IRD, then received ≥1 dose of ixazomib maintenance
(49). The median PFS for the
weekly IRD group was 25.8 months (95% CI, 9.2-34.8), and for
twice-weekly IRD group, it was 26.3 months (95% CI, 5.7-not
reached). Patients in the two groups showed a 3-year OS of 96 and
77%, respectively. A study by Kumar et al (50) treated patients with NDMM using
ixazomib for maintenance after examining the long-term
effectiveness and safety of the weekly complete oral combination of
IRD. In the study, induction was halted in 23 patients and ASCT was
performed. The remaining 42 patients showed an overall response
rate (ORR) of 80%, of which 63% had VGPR and 32% had CR. This
finding reveals that the IRD procedure can be administered for a
considerable amount of time without any signs of cumulative
toxicity. Furthermore, a double-blind, placebo-controlled
TOURMALINE-MM2 clinical trial (NCT01850524) (51) was conducted, which involved
patients with NDMM who were not candidates for or were unable to
undergo ASCT. The median PFS of the IRD group was 35.3 months and
the median follow-up duration was 53.3 months; 63% had VGPR and 26%
had CR.
The response rates of the patients following ASCT
increased over time, which was partly due to the advantages of
lenalidomide maintenance that were noted in the previous trial.
This finding led the researchers to add ixazomib to 64 patients
with NDMM after ASCT, to compare the effects of R and IR (52). The median survival time was not
attained, the CR/strict CR (sCR) was 43%, and the median follow-up
period was 62 months (25-82 months). The median PFS of the patients
was 73 months. The addition of lenalidomide to the maintenance of
the drug resulted in a superior PFS than anticipated, and it was
safe and was well tolerated when compared with the historical usage
of lenalidomide alone.
A double-blind, placebo-controlled TOURMALINE- MM1
phase 3 clinical trial (53) for
treating patients with relapsed and refractory MM revealed that
there were no new or worse safety concerns. Moreover, among
patients with RRMM, the OS rate of those taking IRD was
statistically improved compared with those taking placebo-RD.
According to a multicenter real-world study conducted by Japanese
researchers using the Kansai Myeloma Forum database (https://myeloma.jp/), IRD treatment exhibited stronger
efficacy than other types of treatments in patients with IgG-type
RRMM in actual clinical practice (54), but no additional clinical trials
and studies are available to support these findings.
Application of lenalidomide combined
with carfilzomib, dexamethasone and CD38 monoclonal antibody,
daratumumab (Dara), in MM
In 2012, the FDA authorized carfilzomib (K) for the
treatment of MM. The drug, which is a tetrapeptide epoxy ketone,
specifically targets and permanently inhibits the proteasome
(70). The FDA originally
authorized the single medication therapy for patients with MM who
had received at least two treatments in 2012. Later, the FDA
approved the use of lenalidomide with dexamethasone or carfilzomib
in conjunction with dexamethasone to treat RRMM (71). Furthermore, several clinical
studies on carfilzomib are concurrently being performed on patients
with NDMM.
The effectiveness of Dara in combination with
carfilzomib, lenalidomide, and dexamethasone (Dara-KRD) in treating
patients with NDMM was explored in an open phase 1b research
(55). Regardless of fulfilling
the transplantation requirements, 22 patients received Dara-KRD
treatment for up to 13 cycles that lasted 28 days or until ASCT. An
ORR of 95% was achieved, of which 86% was PR and 67% was CR. Hence,
Dara-KRD appears to be well tolerated. In another multicenter,
open-label, phase 3 randomized controlled trial (56), patients with NDMM who did not
immediately receive ASCT were compared in terms of VRD and KRD
data. The median PFS was 34.4 months [95% CI, 30.1-not estimable
(NE)] for VRD and 34.6 months (95% CI, 28.8-37.8) for KRD; KRD did
not increase the PFS in patients with NDMM in this randomized phase
3 study.
Patients with NDMM who were eligible for
transplantation underwent four cycles of KRD induction, ASCT, four
cycles of KRD consolidation, and ten cycles of KRD maintenance in a
phase 2 multicenter investigation that evaluated the use of KRD and
ASCT in the treatment of NDMM (57); sCR was the major endpoint after
eight cycles of KRD. In total, 76 patients were enrolled in the
study, their median age ranged from 40 to 76 years. Furthermore,
the sCR rate after eight cycles was 60%. The sCR rate was 76% in
the intention to treat (ITT) population. The 5-year PFS and OS
rates of ITT were 72 and 84% after a median follow-up of 56 months.
In patients with NDMM treated with KRD and ASCT, there was a
significant incidence of negative sCR and MRD at the end of the
consolidation of KRD. PFS and OS may be extended by prolonging the
consolidation treatment for KRD, and safety and tolerance can be
effectively managed. Another phase 2 study on KRD and ASCT
(58) involved eight KRD
treatments, ASCT for all patients, and a year-long course of
lenalidomide, with the primary objective of sCR. Poor cytogenetics
affected 21% of the 46 individuals. Of the 42 patients assessed
following consolidation, 26 patients (61.9%) and 27 patients
(64.3%) had sCR and CR, respectively. In conclusion, eight cycles
of KRD resulted in a quick and favorable response in patients with
NDMM who qualified for transplantation, however, of note
cardiovascular side effects need to be constantly monitored.
Eligible patients were randomly assigned in a ratio
of 1:1 to receive KRD or RD treatment for 28 days in a randomized
controlled study on RRMM (59). In
patients who had previously received a single therapy, the median
OS was extended by 11.4 months for KRD compared with RD, and in
patients who had previously received two therapies, the median OS
was extended by 6.5 months for KRD compared with RD. Hence, it can
be surmised that in RRMM, KRD has a markedly lower risk of
mortality and a higher survival rate compared with RD. The
therapeutic benefit of KRD is, however, most apparent during the
initial recurrence. Similar findings were obtained by Japanese
researchers conducting a multicenter real-world investigation using
the Kansai Myeloma Forum database (60). They identified that 107 patients
had received KRD therapy. The ORR was 68.2% and the median PFS and
OS were 8.8 and 29 months, respectively. The results of 44 patients
who had salvage high-dose chemotherapy (HDCT) plus ASCT following
KRD reinduction were examined in a study on the use of KRD
reinduction and salvage ASCT after first-line transplantation for
RRMM (61). All patients had
first-line high-dose chemotherapy plus ASCT (HDCT/ASCT), with a
median progression time of 2.9 (1.2-13.5) years. After reinduction
and before the salvage transplantation, 25/44 patients (57%)
achieved VGPR; however, after salvage HDCT/ASCT, the percentage
increased to 34/44 (77%). Given that the median PFS following
rescue HDCT/ASCT was 23.3 months, KRD considerably prolonged PFS
following rescue HDCT/ASCT and was enhanced by maintenance
treatment.
5. Safety and tolerability
Neutropenia, thrombocytopenia and
anemia
The Myeloma XI experiment was conducted at 110
National Health Service hospitals in England, Wales, and Scotland.
It was an open-label, randomised, phase 3 adaptive design trial
with three selection steps (72).
Hematological adverse effects (AEs), such as neutropenia [362 (33%)
patients], thrombocytopenia [72 (7%) patients], and anemia [42 (4%)
patients], were the most frequent grade 3 or 4 AEs among
lenalidomide users. Compared with 150 (17%) of the 874 individuals
under observation, 494 (45%) of the 1,097 patients receiving
lenalidomide experienced serious AEs. Therefore, complete blood
counts, including white blood cells and their counts, platelet
counts, hemoglobin, and hematocrit, should be checked weekly at
baseline and during the first 8 weeks of lenalidomide treatment,
and monthly thereafter. If neutropenia is present, physicians
should consider treating the patient with growth factors (G-CSF).
Dose adjustments in the event of grade 3 or 4 thrombocytopenia or
neutropenia should be made by an experienced physician with
reference to the medication package insert. Following the
development of hematologic toxicity, if continued lenalidomide
therapy results in improved bone marrow function (no hematologic
toxicity for at least two consecutive cycles and an absolute
neutrophil count ≥1.5x109/l and platelet count
≥100x109/l at the start of a new cycle using the current
dose level), and the lenalidomide dose can be reinstated to the
original level.
Thyroid dysfunction
Cases of hypothyroidism and hyperthyroidism have
been reported in patients taking lenalidomide (73). Effective management of
comorbidities affecting thyroid function should be achieved prior
to treatment with lenalidomide. The authors recommend continuous
monitoring of thyroid function at baseline and during
treatment.
Peripheral neuropathy
One of the main reasons that has caused numerous
physicians to abandon the use of thalidomide is peripheral
neuropathy (74). No worsening of
peripheral neuropathy was observed in patients with NDMM treated
with long-term lenalidomide (37).
In a prospective clinical and neurophysiological study of long-term
neurotoxicity of lenalidomide applications, investigators confirmed
that the neuropathy induced by lenalidomide is usually subclinical
or mild. Neurotoxicity was independent of cumulative lenalidomide
dose and hematologic response (75).
Tumor lysis syndrome
Due to the antitumor activity of lenalidomide, the
complication of tumor lysis syndrome (TLS) may occur. However,
there have been rare reports of TLS in patients with MM treated
with lenalidomide. Nonetheless, caution should be exercised when
administering lenalidomide to patients with a high pre-treatment
tumor load, and these patients should be closely monitored, with
particular attention to the first cycle, and appropriate
precautions taken.
Severe skin reactions (including
allergic reactions)
Angioedema, hypersensitivity, and severe skin
reactions, including Stevens-Johnson syndrome (SJS), toxic
epidermal necrolysis (TEN), and drug reactions with eosinophilia
and systemic symptoms (DRESS), have been reported with the use of
lenalidomide (76-78).
DRESS may be associated with skin reactions (for example, rash or
epidermal exfoliative dermatitis), eosinophilia, fever, and/or
systemic complications of lymphadenopathy, such as hepatitis,
nephritis, pneumonia, myocarditis, and/or pericarditis (78). These events can have fatal
consequences. In addition, lenalidomide should be avoided in
patients who have experienced a grade 4 rash with prior thalidomide
use. If a grade 2-3 rash occurs, suspending or discontinuing the
drug should be considered. If angioedema, hypersensitivity, grade 4
rash, exfoliative or maculopapular rash, or suspected SJS, TEN, and
DRESS occur, the drug must be discontinued and must not be
restarted after these reactions have resolved.
Second primary tumor
In a retrospective pooled analysis of 11 clinical
trials of lenalidomide for RRMM, the overall incidence rate (IR,
events per 100 patient-years) of second primary malignancies (SPM)
was 3.62. The IR for aggressive SPM (hematologic and solid tumors)
was 2.08, which was in line with background cancer incidence.
Non-invasive second primary tumors include basal cell or squamous
cell skin cancers. In another analysis, pooled data from pivotal
phase 3 trials of relapsed or refractory MM (N=703) showed an IR of
3.98 (95% CI, 2.51-6.31) for SPM with lenalidomide/dexamethasone
and 1.38 (95% CI, 0.44-4.27) for placebo/dexamethasone (79). When considering treatment with
lenalidomide, the physician should weigh both the potential benefit
of lenalidomide and the risk of a second primary malignancy.
Hepatotoxicity
Liver failure, including death, has been reported in
patients treated with lenalidomide in combination with other drugs
(80,81). The mechanism of drug-induced severe
hepatotoxicity is not known, but in some cases, preexisting viral
liver disease, elevated baseline liver enzymes and treatment with
antibiotics may also be a risk factor. Commonly, abnormal liver
function test values were reported, which were generally
asymptomatic and reversible with suspension of dosing. Once
parameters return to baseline values, treatment at a lower dose may
be considered. Lenalidomide is excreted through the kidneys
(36). Dose adjustment in patients
with renal insufficiency is particularly important to avoid higher
hematological adverse effects or hepatotoxicity that may result
from elevated blood levels. Hepatic function monitoring may be
indicated by the clinician, particularly if there has been a
history of viral liver infection or concurrent viral liver
infection, or if lenalidomide is used in combination with
medications known to cause abnormalities in liver function.
Infections
Patients with MM are more likely to develop
infections, including pneumonia (72). For patients with NDMM, treatment
with lenalidomide in combination with dexamethasone was associated
with a higher incidence of infection in the former compared with
treatment with melphalan, prednisone, and thalidomide (MPT)
(82,83). For patients with NDMM previously
treated with ASCT, maintenance therapy with lenalidomide was
associated with a higher incidence of infection in the former
compared with placebo (84). All
patients should seek immediate medical attention at the first sign
of infection (for example, cough and fever) for empirical
anti-infective treatment by a hematologist. Cases of viral
reactivation, including severe cases of re-inactivation of herpes
zoster (85) or hepatitis B virus
(HBV) (86), have been reported in
patients treated with lenalidomide. Some cases of herpes zoster
turn into disseminated herpes zoster, herpes zoster meningitis, or
ocular herpes zoster, requiring suspension or permanent
discontinuation of lenalidomide therapy and adequate antiviral
therapy. Patients with prior HBV infection and treated with
lenalidomide have progressed to acute liver failure in some cases,
leading to discontinuation of lenalidomide and adequate antiviral
therapy. HBV status should be clarified prior to initiating
lenalidomide therapy. For patients who have tested positive for HBV
infection, it is recommended to consult a specialist experienced in
the treatment of hepatitis B. Lenalidomide should be used with
caution in patients with a history of HBV infection, including
those who are anti-HBc antibody-positive but HBsAg-negative. These
patients should be closely monitored for signs and symptoms of
active HBV infection throughout the course of treatment.
Venous and arterial
thromboembolism
Lenalidomide combined with dexamethasone for the
treatment of patients with MM increases the risk of venous
thrombosis (especially the risk of deep vein thrombosis and
pulmonary embolism) (87).
Clinicians routinely apply aspirin or rivaroxaban to prevent
thrombosis (88). Lenalidomide in
combination with dexamethasone for the treatment of patients with
MM increases the risk of arterial thrombosis (especially myocardial
infarction and cerebrovascular events), and the risk of arterial
thrombosis is lower when lenalidomide is combined with melphalan
and prednisone. Lenalidomide monotherapy is associated with a lower
risk of arterial thrombosis than lenalidomide in combination with
other drugs for the treatment of MM (89).
Teratogenicity
Thalidomide, the first-generation IMiD, was once
recommended as a sedative antiemetic for pregnant women to reduce
their pregnancy reactions before being used as a therapeutic agent
for MM (90), which was withdrawn
due to its teratogenicity (91).
Lenalidomide has been shown to be teratogenic, as its predecessor,
thalidomide (92). Hui et
al used pregnant cynomolgus monkeys to study the teratogenic
potential of lenalidomide (93).
All of the fetuses of the lenalidomide-treated group had
deformities upon external fetal inspection, including anomalies of
the upper and lower limbs. Therefore, lenalidomide is
contraindicated in pregnant women and women who are likely to
become pregnant if all contraceptive requirements have not been
met.
Tolerability
Cereblon (CRBN) is the central target molecule for
lenalidomide. It is suggested that the emergence of lenalidomide
resistance is influenced by low CRBN expression, CRBN mutations,
and genes encoding downstream proteins (94). In addition, in a prospective
multicenter, single-arm clinical trial, researchers combined
longitudinal single-cell RNA sequencing (scRNA-seq) to study the
molecular dynamics of MM resistance mechanisms. This study revealed
new MM molecular resistance pathways including hypoxia tolerance,
protein folding and mitochondrial respiration, and it was
identified that peptidyl prolyl isomerase A (PPIA), a central
enzyme in the protein folding reaction pathway, may be a new target
for drug-resistant MM. CRISPR-Cas9 deletion of PPIA or inhibition
of PPIA with a small-molecule inhibitor (cyclosporine) markedly
sensitized MM tumor cells to proteasome inhibitors (95). Hematological grade 4 or
nonhematological grade 3/4 AEs and drug resistance are the main
factors that lead to lenalidomide discontinuation in clinical
research and in clinical practice (37,96).
6. Dosage in special populations
Medications for patients with renal
insufficiency
No dose adjustment is required for patients with
creatinine clearance (CLcr) ≥60 ml/min. Dose adjustments should be
made for patients with CLcr <60 ml/min at the start of
treatment. Lenalidomide can be administered at a full dose of 25 mg
per day 21/28 (daily on days 1-21 of each repetitive 28-day cycle)
in patients with a CLcr >30 and can be given daily to patients
with a CLcr <30, even on dialysis, at a dose of at least 15 mg
per day (97).
Elderly patients
In a multicenter, open-label, phase 3 FIRST trial
(MM-020/IFM07-01), of the 1,623 patients receiving medication in
the present study, 94% (1,521/1,623) were 65 years or older and 35%
(561/1,623) were 75 years or older. The 1,623 were randomly
assigned to the following three groups: Rd in 28-day cycles until
disease progression (Rd pers), to the same combination for 18
cycles (Rd18), or to MPT for 72 weeks. The proportion of patients
>75 years of age was similar across study groups (Rd pers, 33%;
Rd18, 34%; MPT, 33%). The incidence of most adverse reaction
categories (for example, all adverse reactions, grade 3/4 AEs,
serious adverse reactions) was higher in older subjects (>75
years of age) than in younger subjects (≤75 years of age) in all
treatment groups. Grade 3/4 AEs were consistently reported at
higher rates for systemic disease and site-of-administration status
body systems in older subjects than in younger subjects in all
groups (difference of at least 5%). The incidence of grade 3 or 4
AEs for infections and contagions, cardiac diseases (including
heart failure and congestive heart failure), skin and subcutaneous
tissue diseases, and renal and urologic diseases (including renal
failure) was consistently slightly higher in older subjects than in
younger subjects in all groups (<5% difference). These trends
were not clear with respect to the incidence of grade 3/4 adverse
reactions in other body systems (for example, blood and lymphatic
system disorders, infections and infectious heart disease, and
vascular disease). The incidence of serious adverse reactions was
generally higher in older subjects than in younger subjects in all
groups (83). In another study,
the population pharmacokinetic analysis included patients of
advanced age, and the results of the analysis showed no effect of
age on the clearance (plasma exposure) of lenalidomide (98). Because elderly patients are more
likely to have decreased renal function, caution should be
exercised in dose selection and renal function should be
monitored.
Pregnant and lactating women
As already mentioned, lenalidomide should be
contraindicated during pregnancy. Women who are at risk of becoming
pregnant should use effective contraception. If a female patient
becomes pregnant while using lenalidomide, treatment must be
discontinued and she is asked to seek evaluation and advice from a
physician with expertise or experience in teratology. It is
uncertain whether lenalidomide is secreted through human milk;
therefore, it is recommended that breastfeeding women discontinue
breastfeeding during treatment with lenalidomide.
7. Conclusion
Lenalidomide, a second-generation IMiD, is highly
regarded in the treatment of patients with NDMM and RRMM owing to
its several advantages over thalidomide, which is a
first-generation immunosuppressant. Lenalidomide kills MM cells in
diverse ways, including through direct induction and immune
modulation. Lenalidomide is an oral medication that is quickly and
well absorbed; however, the renal function of the patient taking it
is affected in terms of the plasma exposure concentration.
Lenalidomide has a variety of uses in the treatment of MM,
including induction therapy and maintenance therapy. It can also be
used in combination with other medications such as dexamethasone,
PIs, and CD38 monoclonal antibodies. Although several clinical
trials have revealed positive outcomes with lenalidomide, there is
less real-world research evidence for NDMM relative to RRMM.
Despite the fact that the neurological side-effects of
lenalidomide, particularly those affecting the peripheral nerves,
are markedly reduced compared with those of thalidomide, the
clinical application of lenalidomide should be cautious, especially
in relation to its performance in the blood system, infections,
thrombosis, teratogenic potential and other unpleasant
responses.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the National Natural
Science Foundation (grant no. 81302044), the Natural Science
Foundations of Shandong Province (grant no. ZR2020MH124), the
Promotive Research Fund for Excellent Young and Middle-aged
Scientists of Shandong Province (grant no. BS2013YY009), and the
Projects of Medical and Health Technology Development Program of
Shandong Province (grant no. 2016WS0407).
Availability of data and materials
Not applicable.
Authors' contributions
CWZ was responsible for the conception and writing
of the article. YNW collected information and XLG approved the
articles and performed modifications with regard to language
editing. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bird SA and Boyd K: 9. Multiple myeloma:
An overview of management. Palliat Care Soc Pract.
13(1178224219868235)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nijhof IS, van de Donk NWCJ, Zweegman S
and Lokhorst HM: Current and new therapeutic strategies for
relapsed and refractory multiple myeloma: An update. Drugs.
78:19–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shah UA and Mailankody S: Emerging
immunotherapies in multiple myeloma. BMJ. 370(m3176)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pinto V, Bergantim R, Caires HR, Seca H,
Guimarães JE and Vasconcelos MH: Multiple Myeloma: Available
therapies and causes of drug resistance. Cancers (Basel).
12(407)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cowan AJ, Green DJ, Kwok M, Lee S, Coffey
DG, Holmberg LA, Tuazon S, Gopal AK and Libby EN: Diagnosis and
management of multiple myeloma: A review. JAMA. 327:464–477.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Delforge M, Vlayen S and Kint N:
Immunomodulators in newly diagnosed multiple myeloma: Current and
future concepts. Expert Rev Hematol. 14:365–376. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Burgess J, Ferdousi M, Gosal D, Boon C,
Matsumoto K, Marshall A, Mak T, Marshall A, Frank B, Malik RA and
Alam U: Chemotherapy-Induced peripheral neuropathy: Epidemiology,
pathomechanisms and treatment. Oncol Ther. 9:385–450.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tageja N: Lenalidomide-current
understanding of mechanistic properties. Anticancer Agents Med
Chem. 11:315–326. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cives M, Simone V, Brunetti O, Longo V and
Silvestris F: Novel lenalidomide-based combinations for treatment
of multiple myeloma. Crit Rev Oncol Hematol. 85:9–20.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Quach H, Kalff A and Spencer A:
Lenalidomide in multiple myeloma: Current status and future
potential. Am J Hematol. 87:1089–1095. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jabbour E, Thomas D, Kantarjian H, Zhou L,
Pierce S, Cortes J and Verstovsek S: Comparison of thalidomide and
lenalidomide as therapy for myelofibrosis. Blood. 118:899–902.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kotla V, Goel S, Nischal S, Heuck C, Vivek
K, Das B and Verma A: Mechanism of action of lenalidomide in
hematological malignancies. J Hematol Oncol. 2(36)2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tawara K, Scott H, Emathinger J, Ide A,
Fox R, Greiner D, LaJoie D, Hedeen D, Nandakumar M, Oler AJ, et al:
Co- Expression of VEGF and IL-6 Family Cytokines is Associated with
Decreased Survival in HER2 Negative Breast Cancer Patients:
Subtype-Specific IL-6 Family Cytokine-Mediated VEGF Secretion.
Transl Oncol. 12:245–255. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Matthes T, Manfroi B and Huard B:
Revisiting IL-6 antagonism in multiple myeloma. Crit Rev Oncol
Hematol. 105:1–4. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou J, Shen Q, Lin H, Hu L, Li G and
Zhang X: Decitabine shows potent anti-myeloma activity by depleting
monocytic myeloid-derived suppressor cells in the myeloma
microenvironment. J Cancer Res Clin Oncol. 145:329–336.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Díaz T, Rodríguez V, Lozano E, Mena MP,
Calderón M, Rosiñol L, Martínez A, Tovar N, Pérez-Galán P, Bladé J,
et al: The BET bromodomain inhibitor CPI203 improves lenalidomide
and dexamethasone activity in in vitro and in vivo models of
multiple myeloma by blockade of Ikaros and MYC signaling.
Haematologica. 102:1776–1784. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kulikov AV, Shilov ES, Mufazalov IA,
Gogvadze V, Nedospasov SA and Zhivotovsky B: Cytochrome c: The
Achilles' heel in apoptosis. Cell Mol Life Sci. 69:1787–1797.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li L, Hua Y, Dong M, Li Q, Smith DT, Yuan
M, Jones KR and Ren J: Short-term lenalidomide (Revlimid)
administration ameliorates cardiomyocyte contractile dysfunction in
ob/ob obese mice. Obesity (Silver Spring). 20:2174–2185.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhou L, Huang X, Niesvizky R, Pu Z and Xu
G: Caspase-8 regulates the antimyeloma activity of bortezomib and
lenalidomide. J Pharmacol Exp Ther. 379:303–309. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou L: Caspase-8: Friend or Foe in
bortezomib/lenalidomide-based therapy for myeloma. Front Oncol.
12(861709)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Felici C, Passarelli A, Cafforio P,
Racanelli V, Leone P and Tucci M: Lenalidomide arrests cell cycle
and modulates PD1-dependent downstream mTOR intracellular signals
in melanoma cells. Melanoma Res. 33:357–363. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wong AH, Shin EM, Tergaonkar V and Chng
WJ: Targeting NF-κB signaling for multiple myeloma. Cancers
(Basel). 12(2203)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li S, Pal R, Monaghan SA, Schafer P,
Ouyang H, Mapara M, Galson DL and Lentzsch S: IMiD immunomodulatory
compounds block C/EBP{beta} translation through eIF4E
down-regulation resulting in inhibition of MM. Blood.
117:5157–5165. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bou Zerdan M, Nasr L, Kassab J, Saba L,
Ghossein M, Yaghi M, Dominguez B and Chaulagain CP: Adhesion
molecules in multiple myeloma oncogenesis and targeted therapy. Int
J Hematol Oncol. 11(IJH39)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Qu X, Mei J, Yu Z, Zhai Z, Qiao H and Dai
K: Lenalidomide regulates osteocytes fate and related
osteoclastogenesis via IL-1β/NF-κB/RANKL signaling. Biochem Biophys
Res Commun. 501:547–555. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cho SF, Lin L, Xing L, Li Y, Wen K, Yu T,
Hsieh PA, Munshi N, Wahl J, Matthes K, et al: The immunomodulatory
drugs lenalidomide and pomalidomide enhance the potency of AMG 701
in multiple myeloma preclinical models. Blood Adv. 4:4195–4207.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Castelli R, Cassin R, Cannavò A and Cugno
M: Immunomodulatory drugs: new options for the treatment of
myelodysplastic syndromes. Clin Lymphoma Myeloma Leuk. 13:1–7.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Neuber B, Dai J, Waraich WA, Awwad MHS,
Engelhardt M, Schmitt M, Medenhoff S, Witzens-Harig M, Ho AD,
Goldschmidt H and Hundemer M: Lenalidomide overcomes the
immunosuppression of regulatory CD8(+)CD28(-) T-cells. Oncotarget.
8:98200–98214. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Richardson K, Keam SP, Zhu JJ, Meyran D,
D'Souza C, Macdonald S, Campbell K, Robbins M, Bezman NA, Todd K,
et al: The efficacy of combination treatment with elotuzumab and
lenalidomide is dependent on crosstalk between natural killer
cells, monocytes and myeloma cells. Haematologica. 108:83–97.
2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bodera P and Stankiewicz W:
Immunomodulatory properties of thalidomide analogs: Pomalidomide
and lenalidomide, experimental and therapeutic applications. Recent
Pat Endocr Metab Immune Drug Discov. 5:192–196. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Benson DM Jr, Bakan CE, Mishra A,
Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J,
Smith MK, et al: The PD-1/PD-L1 axis modulates the natural killer
cell versus multiple myeloma effect: a therapeutic target for
CT-011, a novel monoclonal anti-PD-1 antibody. Blood.
116:2286–2294. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hallett WH, Jing W, Drobyski WR and
Johnson BD: Immunosuppressive effects of multiple myeloma are
overcome by PD-L1 blockade. Biol Blood Marrow Transplant.
17:1133–1145. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tamura H, Ishibashi M, Yamashita T,
Tanosaki S, Okuyama N, Kondo A, Hyodo H, Shinya E, Takahashi H,
Dong H, et al: Marrow stromal cells induce B7-H1 expression on
myeloma cells, generating aggressive characteristics in multiple
myeloma. Leukemia. 27:464–472. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen N, Kasserra C, Reyes J, Liu L and Lau
H: Single-dose pharmacokinetics of lenalidomide in healthy
volunteers: Dose proportionality, food effect, and racial
sensitivity. Cancer Chemother Pharmacol. 70:717–725.
2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen N, Weiss D, Reyes J, Liu L, Kasserra
C, Wang X, Zhou S, Kumar G, Weiss L and Palmisano M: No clinically
significant drug interactions between lenalidomide and
P-glycoprotein substrates and inhibitors: results from controlled
phase I studies in healthy volunteers. Cancer Chemother Pharmacol.
73:1031–1039. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen N, Ye Y, Liu L, Reyes J, Assaf MS,
Kasserra C, Zhou S and Palmisano M: Lenalidomide at therapeutic and
supratherapeutic doses does not prolong QTc intervals in the
thorough QTc study conducted in healthy men. Basic Clin Pharmacol
Toxicol. 113:179–186. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Larocca A, Bonello F, Gaidano G,
D'Agostino M, Offidani M, Cascavilla N, Capra A, Benevolo G, Tosi
P, Galli M, et al: Dose/schedule-adjusted Rd-R vs continuous Rd for
elderly, intermediate-fit patients with newly diagnosed multiple
myeloma. Blood. 137:3027–3036. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lund J, Gruber A, Lauri B, Duru AD,
Blimark C, Swedin A, Hansson M, Forsberg K, Ahlberg L, Carlsson C,
et al: Lenalidomide versus lenalidomide + dexamethasone prolonged
treatment after second-line lenalidomide + dexamethasone induction
in multiple myeloma. Cancer Med. 7:2256–2268. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Durie BGM, Hoering A, Abidi MH, Rajkumar
SV, Epstein J, Kahanic SP, Thakuri M, Reu F, Reynolds CM, Sexton R,
et al: Bortezomib with lenalidomide and dexamethasone versus
lenalidomide and dexamethasone alone in patients with newly
diagnosed myeloma without intent for immediate autologous stem-cell
transplant (SWOG S0777): A randomised, open-label, phase 3 trial.
Lancet. 389:519–527. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mohammad NS, Nazli R, Zafar H and Fatima
S: Effects of lipid based Multiple Micronutrients Supplement on the
birth outcome of underweight pre-eclamptic women: A randomized
clinical trial. Pak J Med Sci. 38:219–226. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zou Y, Lin M, Sheng Z and Niu S:
Bortezomib and lenalidomide as front-line therapy for multiple
myeloma. Leuk Lymphoma. 55:2024–2031. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Joseph NS, Kaufman JL, Dhodapkar MV,
Hofmeister CC, Almaula DK, Heffner LT, Gupta VA, Boise LH, Lonial S
and Nooka AK: Long-Term follow-up results of lenalidomide,
bortezomib, and dexamethasone induction therapy and risk-adapted
maintenance approach in newly diagnosed multiple myeloma. J Clin
Oncol. 38:1928–1937. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
McCaughan GJ, Gandolfi S, Moore JJ and
Richardson PG: Lenalidomide, bortezomib and dexamethasone induction
therapy for the treatment of newly diagnosed multiple myeloma: A
practical review. Br J Haematol. 199:190–204. 2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Goldschmidt H, Mai EK, Bertsch U, Fenk R,
Nievergall E, Tichy D, Besemer B, Dürig J, Schroers R, von Metzler
I, et al: Addition of isatuximab to lenalidomide, bortezomib, and
dexamethasone as induction therapy for newly diagnosed,
transplantation-eligible patients with multiple myeloma (GMMG-HD7):
Part 1 of an open-label, multicentre, randomised,
active-controlled, phase 3 trial. Lancet Haematol. 9:e810–e821.
2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Voorhees PM, Kaufman JL, Laubach J, Sborov
DW, Reeves B, Rodriguez C, Chari A, Silbermann R, Costa LJ,
Anderson LD Jr, et al: Daratumumab, lenalidomide, bortezomib, and
dexamethasone for transplant-eligible newly diagnosed multiple
myeloma: the GRIFFIN trial. Blood. 136:936–945. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Attal M, Lauwers-Cances V, Hulin C, Leleu
X, Caillot D, Escoffre M, Arnulf B, Macro M, Belhadj K, Garderet L,
et al: Lenalidomide, bortezomib, and dexamethasone with
transplantation for myeloma. N Engl J Med. 376:1311–1320.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
O'Donnell EK, Laubach JP, Yee AJ, Chen T,
Huff CA, Basile FG, Wade PM, Paba-Prada CE, Ghobrial IM, Schlossman
RL, et al: A phase 2 study of modified lenalidomide, bortezomib and
dexamethasone in transplant-ineligible multiple myeloma. Br J
Haematol. 182:222–230. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Iida S, Wakabayashi M, Tsukasaki K,
Miyamoto K, Maruyama D, Yamamoto K, Takatsuka Y, Kusumoto S, Kuroda
J, Ando K, et al: Bortezomib plus dexamethasone vs thalidomide plus
dexamethasone for relapsed or refractory multiple myeloma. Cancer
Sci. 109:1552–1561. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Dimopoulos MA, Laubach JP, Echeveste
Gutierrez MA, Grzasko N, Hofmeister CC, San-Miguel JF, Kumar S,
Labotka R, Lu V, Berg D, et al: Ixazomib maintenance therapy in
newly diagnosed multiple myeloma: An integrated analysis of four
phase I/II studies. Eur J Haematol. 102:494–503. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kumar SK, Berdeja JG, Niesvizky R, Lonial
S, Laubach JP, Hamadani M, Stewart AK, Hari P, Roy V, Vescio R, et
al: Ixazomib, lenalidomide, and dexamethasone in patients with
newly diagnosed multiple myeloma: Long-term follow-up including
ixazomib maintenance. Leukemia. 33:1736–1746. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Facon T, Venner CP, Bahlis NJ, Offner F,
White DJ, Karlin L, Benboubker L, Rigaudeau S, Rodon P, Voog E, et
al: Oral ixazomib, lenalidomide, and dexamethasone for
transplant-ineligible patients with newly diagnosed multiple
myeloma. Blood. 137:3616–3628. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Patel KK, Shah JJ, Feng L, Lee HC,
Manasanch EM, Olsem J, Morphey A, Huo XJ, Thomas SK, Bashir Q, et
al: Safety and efficacy of combination maintenance therapy with
ixazomib and lenalidomide in patients with posttransplant myeloma.
Clin Cancer Res. 28:1277–1284. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Richardson PG, Kumar SK, Masszi T, Grzasko
N, Bahlis NJ, Hansson M, Pour L, Sandhu I, Ganly P, Baker BW, et
al: Final overall survival analysis of the TOURMALINE-MM1 phase III
trial of ixazomib, lenalidomide, and dexamethasone in patients with
relapsed or refractory multiple myeloma. J Clin Oncol.
39:2430–2442. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Takakuwa T, Yamamura R, Ohta K, Kaneko H,
Imada K, Nakaya A, Fuchida SI, Shibayama H, Matsuda M, Shimazu Y,
et al: Outcomes of ixazomib/lenalidomide/dexamethasone for multiple
myeloma: A multicenter retrospective analysis. Eur J Haematol.
106:555–562. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jakubowiak A, Usmani SZ, Krishnan A,
Lonial S, Comenzo RL, Wang J, de Boer C, Deraedt W, Weiss BM,
Schecter JM and Chari A: Daratumumab plus carfilzomib,
lenalidomide, and dexamethasone in patients with newly diagnosed
multiple myeloma. Clin Lymphoma Myeloma Leuk. 21:701–710.
2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kumar SK, Jacobus SJ, Cohen AD, Weiss M,
Callander N, Singh AK, Parker TL, Menter A, Yang X, Parsons B, et
al: Carfilzomib or bortezomib in combination with lenalidomide and
dexamethasone for patients with newly diagnosed multiple myeloma
without intention for immediate autologous stem-cell
transplantation (ENDURANCE): A multicentre, open-label, phase 3,
randomised, controlled trial. Lancet Oncol. 21:1317–1330.
2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Jasielec JK, Kubicki T, Raje N, Vij R,
Reece D, Berdeja J, Derman BA, Rosenbaum CA, Richardson P,
Gurbuxani S, et al: Carfilzomib, lenalidomide, and dexamethasone
plus transplant in newly diagnosed multiple myeloma. Blood.
136:2513–2523. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Roussel M, Lauwers-Cances V, Wuilleme S,
Belhadj K, Manier S, Garderet L, Escoffre-Barbe M, Mariette C,
Benboubker L, Caillot D, et al: Up-front carfilzomib, lenalidomide,
and dexamethasone with transplant for patients with multiple
myeloma: the IFM KRd final results. Blood. 138:113–121.
2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Siegel DS, Dimopoulos MA, Ludwig H, Facon
T, Goldschmidt H, Jakubowiak A, San-Miguel J, Obreja M, Blaedel J
and Stewart AK: Improvement in overall survival with carfilzomib,
lenalidomide, and dexamethasone in patients with relapsed or
refractory multiple myeloma. J Clin Oncol. 36:728–734.
2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Onda Y, Kanda J, Kaneko H, Shimura Y,
Fuchida SI, Nakaya A, Itou T, Yamamura R, Tanaka H, Shibayama H, et
al: Real-world effectiveness and safety analysis of
carfilzomib-lenalidomide-dexamethasone and
carfilzomib-dexamethasone in relapsed/refractory multiple myeloma:
a multicenter retrospective analysis. Ther Adv Hematol.
13(20406207221104584)2022.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Baertsch MA, Fougereau M, Hielscher T,
Sauer S, Breitkreutz I, Jordan K, Müller-Tidow C, Goldschmidt H,
Raab MS, Hillengass J and Giesen N: Carfilzomib, lenalidomide, and
dexamethasone followed by salvage autologous stem cell transplant
with or without maintenance for relapsed or refractory multiple
myeloma. Cancers (Basel). 13(4706)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Bazarbachi AH, Al Hamed R, Malard F,
Bazarbachi A, Harousseau JL and Mohty M: Induction therapy prior to
autologous stem cell transplantation (ASCT) in newly diagnosed
multiple myeloma: An update. Blood Cancer J. 12(47)2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Pulte ED, Dmytrijuk A, Nie L, Goldberg KB,
McKee AE, Farrell AT and Pazdur R: FDA approval summary:
Lenalidomide as maintenance therapy after autologous stem cell
transplant in newly diagnosed multiple myeloma. Oncologist.
23:734–739. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Liu J, Zhao R, Jiang X, Li Z and Zhang B:
Progress on the application of bortezomib and bortezomib-based
nanoformulations. Biomolecules. 12(51)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Yang MH, Jung SH, Chinnathambi A, Alahmadi
TA, Alharbi SA, Sethi G and Ahn KS: Attenuation of STAT3 signaling
cascade by daidzin can enhance the apoptotic potential of
bortezomib against multiple myeloma. Biomolecules.
10(23)2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Medhekar R, Ran T, Fu AZ, Patel S and
Kaila S: Real-world patient characteristics and treatment outcomes
among nontransplanted multiple myeloma patients who received
Bortezomib in combination with Lenalidomide and Dexamethasone as
first line of therapy in the United States. BMC Cancer.
22(901)2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Harousseau JL and Mohty M: Daratumumab in
transplant regimens for myeloma? Blood. 136:917–918.
2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Goicoechea I, Puig N, Cedena MT, Burgos L,
Cordón L, Vidriales MB, Flores-Montero J, Gutierrez NC, Calasanz
MJ, Ramos MM, et al: Deep MRD profiling defines outcome and unveils
different modes of treatment resistance in standard- and high-risk
myeloma. Blood. 137:49–60. 2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Shirley M: Ixazomib: First global
approval. Drugs. 76:405–411. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Arastu-Kapur S, Anderl JL, Kraus M,
Parlati F, Shenk KD, Lee SJ, Muchamuel T, Bennett MK, Driessen C,
Ball AJ and Kirk CJ: Nonproteasomal targets of the proteasome
inhibitors bortezomib and carfilzomib: A link to clinical adverse
events. Clin Cancer Res. 17:2734–2743. 2011.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Jayaweera SPE, Wanigasinghe Kanakanamge
SP, Rajalingam D and Silva GN: Carfilzomib: A promising proteasome
inhibitor for the treatment of relapsed and refractory multiple
myeloma. Front Oncol. 11(740796)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Jackson GH, Davies FE, Pawlyn C, Cairns
DA, Striha A, Collett C, Hockaday A, Jones JR, Kishore B, Garg M,
et al: Lenalidomide maintenance versus observation for patients
with newly diagnosed multiple myeloma (Myeloma XI): A multicentre,
open-label, randomised, phase 3 trial. Lancet Oncol. 20:57–73.
2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Hamnvik OP, Larsen PR and Marqusee E:
Thyroid dysfunction from antineoplastic agents. J Natl Cancer Inst.
103:1572–1587. 2011.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Koeppen S: Treatment of multiple myeloma:
Thalidomide-, bortezomib-, and lenalidomide-induced peripheral
neuropathy. Oncol Res Treat. 37:506–513. 2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Dalla Torre C, Zambello R, Cacciavillani
M, Campagnolo M, Berno T, Salvalaggio A, De March E, Barilà G, Lico
A, Lucchetta M, et al: Lenalidomide long-term neurotoxicity:
Clinical and neurophysiologic prospective study. Neurology.
87:1161–1166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Patrizi A, Venturi M, Dika E, Maibach H,
Tacchetti P and Brandi G: Cutaneous adverse reactions linked to
targeted anticancer therapies bortezomib and lenalidomide for
multiple myeloma: new drugs, old side effects. Cutan Ocul Toxicol.
33:1–6. 2014.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Tinsley SM, Kurtin SE and Ridgeway JA:
Practical management of lenalidomide-related rash. Clin Lymphoma
Myeloma Leuk. 15 (Suppl):S64–S69. 2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Shanbhag A, Pritchard ER, Chatterjee K and
Hammond DA: highly probable drug reaction with eosinophilia and
systemic symptoms syndrome associated with lenalidomide. Hosp
Pharm. 52:408–411. 2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Dimopoulos MA, Richardson PG, Brandenburg
N, Yu Z, Weber DM, Niesvizky R and Morgan GJ: A review of second
primary malignancy in patients with relapsed or refractory multiple
myeloma treated with lenalidomide. Blood. 119:2764–2767.
2012.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Nojkov B, Signori C, Konda A and Fontana
RJ: Lenalidomide-associated hepatotoxicity-a case report and
literature review. Anticancer Res. 32:4117–4119. 2012.PubMed/NCBI
|
|
81
|
Hussain S, Browne R, Chen J and Parekh S:
Lenalidomide-induced severe hepatotoxicity. Blood.
110(3814)2007.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Facon T, Dimopoulos MA, Dispenzieri A,
Catalano JV, Belch A, Cavo M, Pinto A, Weisel K, Ludwig H, Bahlis
NJ, et al: Final analysis of survival outcomes in the phase 3 FIRST
trial of up-front treatment for multiple myeloma. Blood.
131:301–310. 2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Benboubker L, Dimopoulos MA, Dispenzieri
A, Catalano J, Belch AR, Cavo M, Pinto A, Weisel K, Ludwig H,
Bahlis N, et al: Lenalidomide and dexamethasone in
transplant-ineligible patients with myeloma. N Engl J Med.
371:906–917. 2014.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Richardson PG, Jacobus SJ, Weller EA,
Hassoun H, Lonial S, Raje NS, Medvedova E, McCarthy PL, Libby EN,
Voorhees PM, et al: Triplet Therapy, transplantation, and
maintenance until progression in myeloma. N Engl J Med.
387:132–147. 2022.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Nucci M and Anaissie E: Infections in
patients with multiple myeloma in the era of high-dose therapy and
novel agents. Clin Infect Dis. 49:1211–1225. 2009.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Kikuchi T, Kusumoto S, Tanaka Y, Oshima Y,
Fujinami H, Suzuki T, Totani H, Kinoshita S, Asao Y, Narita T, et
al: Hepatitis B virus reactivation in a myeloma patient with
resolved infection who received daratumumab-containing salvage
chemotherapy. J Clin Exp Hematop. 60:51–54. 2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Kekre N and Connors JM: Venous
thromboembolism incidence in hematologic malignancies. Blood Rev.
33:24–32. 2019.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Piedra K, Peterson T, Tan C, Orozco J,
Hultcrantz M, Hassoun H, Mailankody S, Lesokhin A, Shah U, Lu S, et
al: Comparison of venous thromboembolism incidence in newly
diagnosed multiple myeloma patients receiving bortezomib,
lenalidomide, dexamethasone (RVD) or carfilzomib, lenalidomide,
dexamethasone (KRD) with aspirin or rivaroxaban thromboprophylaxis.
Br J Haematol. 196:105–109. 2022.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Bradbury CA, Craig Z, Cook G, Pawlyn C,
Cairns DA, Hockaday A, Paterson A, Jenner MW, Jones JR, Drayson MT,
et al: Thrombosis in patients with myeloma treated in the Myeloma
IX and Myeloma XI phase 3 randomized controlled trials. Blood.
136:1091–1104. 2020.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Bwire R, Freeman J and Houn F: Managing
the teratogenic risk of thalidomide and lenalidomide: An industry
perspective. Expert Opin Drug Saf. 10:3–8. 2011.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Somers GS: Thalidomide and congenital
abnormalities. Lancet. 1:912–913. 1962.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Mueller M and Lewis DJ: Implementation of
a pregnancy prevention programme (PPP) with a controlled
distribution system (CDS) for the generic teratogenic phthalimides
thalidomide, lenalidomide and pomalidomide. Ther Innov Regul Sci.
55:1155–1164. 2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Hui JY, Fuchs A and Kumar G: Embryo-fetal
exposure and developmental outcome of lenalidomide following oral
administration to pregnant cynomolgus monkeys. Reprod Toxicol.
114:57–65. 2022.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Zhu YX, Shi CX, Bruins LA, Wang X, Riggs
DL, Porter B, Ahmann JM, de Campos CB, Braggio E, Bergsagel PL and
Stewart AK: Identification of lenalidomide resistance pathways in
myeloma and targeted resensitization using cereblon replacement,
inhibition of STAT3 or targeting of IRF4. Blood Cancer J.
9(19)2019.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Cohen YC, Zada M, Wang SY, Bornstein C,
David E, Moshe A, Li B, Shlomi-Loubaton S, Gatt ME, Gur C, et al:
Identification of resistance pathways and therapeutic targets in
relapsed multiple myeloma patients through single-cell sequencing.
Nat Med. 27:491–503. 2021.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Bukowski K, Kciuk M and Kontek R:
Mechanisms of multidrug resistance in cancer chemotherapy. Int J
Mol Sci. 21(3233)2020.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Mikhael J, Manola J, Dueck AC, Hayman S,
Oettel K, Kanate AS, Lonial S and Rajkumar SV: Lenalidomide and
dexamethasone in patients with relapsed multiple myeloma and
impaired renal function: PrE1003, a PrECOG study. Blood Cancer J.
8(86)2018.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Chen N, Zhou S and Palmisano M: Clinical
pharmacokinetics and pharmacodynamics of lenalidomide. Clin
Pharmacokinet. 56:139–152. 2017.PubMed/NCBI View Article : Google Scholar
|















