Introduction
Lung cancer is one of the most common types of
cancer, accounting for ~11.6% of all types of cancer. Small cell
lung cancer (SCLC), as a subtype, accounts for 15% of lung cancer
(1,2) SCLC is a rapidly progressing and
highly aggressive neuroendocrine cancer with a 5-year survival rate
of only 7% and is sensitive to initial chemotherapy and
radiotherapy (3-5).
Patients with SCLC are divided into limited stage and extensive
stage. Limited stage refers to the lesion being confined to one
side of the chest cavity and the cancer spreading to the pleural
effusion and lymph nodes on the same side. Extensive stage refers
to lesion spread beyond the same chest cavity, including malignant
pleural effusions and pericardial effusions, lymph node metastases
on the contralateral hilar or clavicle, or other parts of the body.
The dichotomized staging system and TNM staging are important
predictors for the prognosis of SCLC. Some clinical variables such
as performance status, age, weight loss, stage, and serum lactate
dehydrogenase (LDH) are also considered to predict the prognosis of
SCLC. Some researchers have studied deep into the gene level to
explore the targets associated with lung cancer (6). However, there are no standardized
prognostic parameters (7).
Therefore, it is important to explore accurate prognostic factors
for SCLC. According to previous studies (8-10),
liver function may be an important factor in the prognosis of
various malignancies. Currently, the Child-Pugh score is the most
important scoring system for evaluation of liver function (11). The Child-Pugh score was based on
the total bilirubin, albumin, prothrombin time, and the clinical
findings of encephalopathy and ascites. It was graded as 5-6 points
for Child-Pugh-A; 7-9 points for Child-Pugh-B; and 10-15 points for
Child-Pugh-C. However, it is not suitable for patients with SCLC,
as most patients will merely be assigned to Child Pugh-A (12). Albumin-bilirubin (ALBI) grade,
which has been used to evaluate liver function, was first described
by Johnson et al (12) in
2015 as an indicator of liver dysfunction in patients with
hepatocellular carcinoma. Several studies have demonstrated the
prognostic value of ALBI grade in hepatocellular carcinoma
(8,12,13),
as well as in intrahepatic cholangiocarcinomas, pancreatic cancer,
and gastric cancer (9,10,14).
Furthermore, there has been a study describing the significance of
ALBI grade in non-small cell lung cancer (15). However, the significance of ALBI
grade in SCLC has not yet been elucidated. The present study aimed
to explore the prognostic impact of ALBI grade in patients with
SCLC.
Patients and methods
Patient and data collection
The present study retrospectively analyzed all
patients with SCLC treated at the Department of Thoracic Surgery in
Hebei General Hospital between April 2015 and August 2021. The
patients were followed up throughout the clinical course for at
least four months, and the cutoff date for data collection was
December 31, 2021. Pre-treatment clinical information and social
history were extracted from the hospital's electronic medical
records. All the patients included in the present study were
pathologically diagnosed with SCLC and there were no other
malignant tumors and immune-related serious diseases or adverse
factors affecting blood routine or biochemical indexes such as
hematologic diseases, liver diseases and kidney diseases before
treatment. The clinical data of the patients before receiving
treatment were obtained before chemotherapy and surgery. Patients
with incomplete test index results, inaccurate clinical data and
failure of follow-up were excluded. End point of assessment was
patient overall survival (OS), which is the time from diagnosis of
SCLC to mortality and the secondary endpoint was progression free
survival (PFS), PFS is defined as the time from initiation of
therapy to disease progression. Patients with significant
radiographic progression, markedly elevated tumor markers, or
distant metastases were considered for PFS analyses and a total of
135 patients were included in the sample.
The present study conducted follow-up visits through
outpatient clinics, hospitalizations and phone calls. The follow-up
interval was 1 month. Follow-up rate was 96.3% and two consecutive
losses to follow-up were defined as death with the date of death
defined as the date of the last follow-up. The clinicopathological
variables including sex, age, smoking status, TNM staging, body
mass index (BMI), PS, Charlson comorbidity index (CCI) and whether
undergoing surgery, chemotherapy or radiotherapy were recorded by
the electronic medical record system. Laboratory parameters
including lactate dehydrogenase (LDH), neutrophil to lymphocyte
ratio (NLR), systemic inflammation index (SII), platelet to
lymphocyte ratio (PLR), prognostic nutrition index (PNI),
carcinoembryonic antigen (CEA) and neuron-specific enolase (NSE)
were obtained from the clinical laboratory of Hebei General
Hospital.
Statistical analysis
ALBI grade was calculated by the following formula:
0.66x log10 [total bilirubin (µmol/l)]-0.085 [albumin (ALB)
(g/l)]11. SII was calculated as PLT x NLR (16). PNI was calculated as 10x serum
albumin level (g/dl) +0.005x total lymphocyte count (per
mm3) (17). In a
previous study, ALBI scores were divided into three scales: grade 1
(ALBI score ≤-2.60), grade 2 (-2.60<ALBI score ≤-1.39), and
grade 3 (-1.39<ALBI score)14. As far as the original
cut-off value is specified according to liver cancer, it is
necessary to find a cut-off value which is more suitable for SCLC.
Therefore, cut-off values for ALBI grade, LDH, NLR, SII, PLR, CEA,
NSE were determined using receiver operating characteristic (ROC)
curve analysis, which can estimate optimal sensitivity,
specificity, and the area under the curve (AUC) for prediction of
mortality from all causes. Pearson correlation, Chi-square test and
Fisher exact test were used to compare continuous and categorical
variables. Cumulative cancer specific survival curves were
calculated using the Kaplan-Meier method, and differences were
assessed using Log rank test. The Cox proportional hazard model was
used to evaluate the predictive power of potential prognostic
variables, and the hazard ratios (HR) estimated from the Cox
analysis reported as relative risks with corresponding 95%
confidence intervals. To eliminate the influence of confounding
factors, propensity score matching (PSM) was applied. Statistical
analyses were performed using the IBM SPSS statistics software
program, version 22.0 (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
In the present study, 135 patients pathologically
diagnosed as SCLC were enrolled. The median age was 65 years
(64.24±9.71; range=14-82 years). A total of 102 patients (76%) were
male and 82 patients (61%) had a history of smoking. A total of 54
patients (40%) were in limited stage. A total of 77 patients (57%)
had a BMI of less than 25. As for PS scores, 1 patient (1%) had a
PS score of 0, 85 patients (63%) of 1, and 43 patients (32%) scored
2. A total of 64 patients (47%) scored CCI as 0, and 68 patients
(50%) scored as 1-2.
Clinicopathological characteristics
associated with ALBI grade
The optimal cutoff point resulted from ROC curve
analysis of ALBI grade for the layering of OS in SCLC was
determined to be-2.55 (Fig. 1A),
which was in close conformity with the ALBI grade 1 and 2
boundaries (-2.60). Thus, the patients were classified as follows:
Group 1 (n=87, 64.4%) with pre-treatment ALBI grade ≤-2.55 for an
improved hepatic reserve and group 2 (n=48, 35.6%) with ALBI grade
>-2.55. Optimal cutoff points of LDH, NLR, SII, PLR, CEA, NSE
were 191.45, 3.519, 874.428, 281.896, 10.29, 23.84, respectively
(Fig. 1B). The relationship
between baseline characteristics and ALBI grade are shown in
Table I. There was a significant
association between ALBI grade and age, LDH, NLR, PNI and NSE. No
significant differences were observed in terms of sex, smoking,
staging, BMI, PS, CCI, surgery, SII, PLR, chemotherapy,
radiotherapy and CEA.
 | Figure 1The results of ROC curve. (A) ROC
curve of ALBI. (B) ROC curves of LDH, NLR, SII, PLR, CEA, NSE. ROC,
receiver operating characteristic; ALBI, albumin-bilirubin; LDH,
lactate dehydrogenase; NLR, neutrophil to lymphocyte ratio; SII,
systemic inflammation index; PLR, platelet to lymphocyte ratio;
CEA, carcinoembryonic antigen; NSE, neuron-specific enolase. |
 | Table IRelationship between patient
characteristics and ALBI grade. |
Table I
Relationship between patient
characteristics and ALBI grade.
| Characteristic | ALBI ≤-2.55 n=87
(%) | ALBI >-2.55 n=48
(%) | P-value |
|---|
| Sex | | | 0.300 |
|
Male | 63 (61.8) | 39 (38.2) | |
|
Female | 24 (72.7) | 9 (27.3) | |
| Age | | | <0.001 |
|
<65years | 53 (84.1) | 10 (15.9) | |
|
≥65years | 34 (47.2) | 38 (52.8) | |
| Smoking | | | 0.715 |
|
Yes | 54 (65.9) | 28 (34.1) | |
|
No | 33 (62.3) | 20 (37.7) | |
| Staging | | | 0.068 |
|
Limited
stage | 40 (74.1) | 14 (25.9) | |
|
Extensive
stage | 47 (58.0) | 34 (42.0) | |
| BMI | | | 0.208 |
|
<25 | 46 (59.7) | 31 (10.3) | |
|
≥25 | 41 (70.7) | 17 (29.3) | |
| PS | | | 0.476 |
|
0 | 1(100) | 0 (0) | |
|
1 | 58 (68.2) | 27 (31.8) | |
|
2 | 24 (55.8) | 19 (44.2) | |
|
3 | 4 (66.7) | 2 (33.3) | |
| CCI | | | 0.904 |
|
0 | 40 (62.5) | 24 (37.5) | |
|
1-2 | 45 (66.2) | 23 (33.8) | |
|
≥3 | 2 (66.7) | 1 (33.1) | |
| Surgery | | | 0.110 |
|
Yes | 28 (75.7) | 9 (24.3) | |
|
No | 59 (60.2) | 39 (39.8) | |
| LDH | | | 0.008 |
|
<191.45 | 52 (75.4) | 17 (24.6) | |
|
≥191.45 | 35 (53.0) | 31 (47.0) | |
| NLR | | | 0.026 |
|
<3.519 | 60 (72.3) | 23 (27.7) | |
|
≥3.519 | 27 (51.9) | 25 (48.1) | |
| SII | | | 0.143 |
|
<874.428 | 57 (69.5) | 25 (30.5) | |
|
≥874.428 | 30 (56.6) | 23 (43.4) | |
| PLR | | | 0.607 |
|
<281.896 | 76 (65.5) | 40 (34.5) | |
|
≥281.896 | 11 (57.9) | 8 (42.1) | |
| PNI | | | <0.001 |
|
<40 | 0 (0) | 11(100) | |
|
≥40 | 87 (70.2) | 37 (29.8) | |
| Chemotherapy | | | 0.444 |
|
Yes | 61 (67.0) | 30 (33.0) | |
|
No | 26 (59.1) | 18 (40.9) | |
| Radiotherapy | | | 0.060 |
|
Yes | 34 (75.6) | 11 (24.4) | |
|
No | 53 (58.9) | 37 (41.1) | |
| CEA | | | 0.089 |
|
Normal | 77 (67.5) | 37 (32.5) | |
|
High | 10 (47.6) | 11 (52.4) | |
| NSE | | | 0.012 |
|
Normal | 49 (75.4) | 16 (24.6) | |
|
High | 38 (54.3) | 32 (45.7) | |
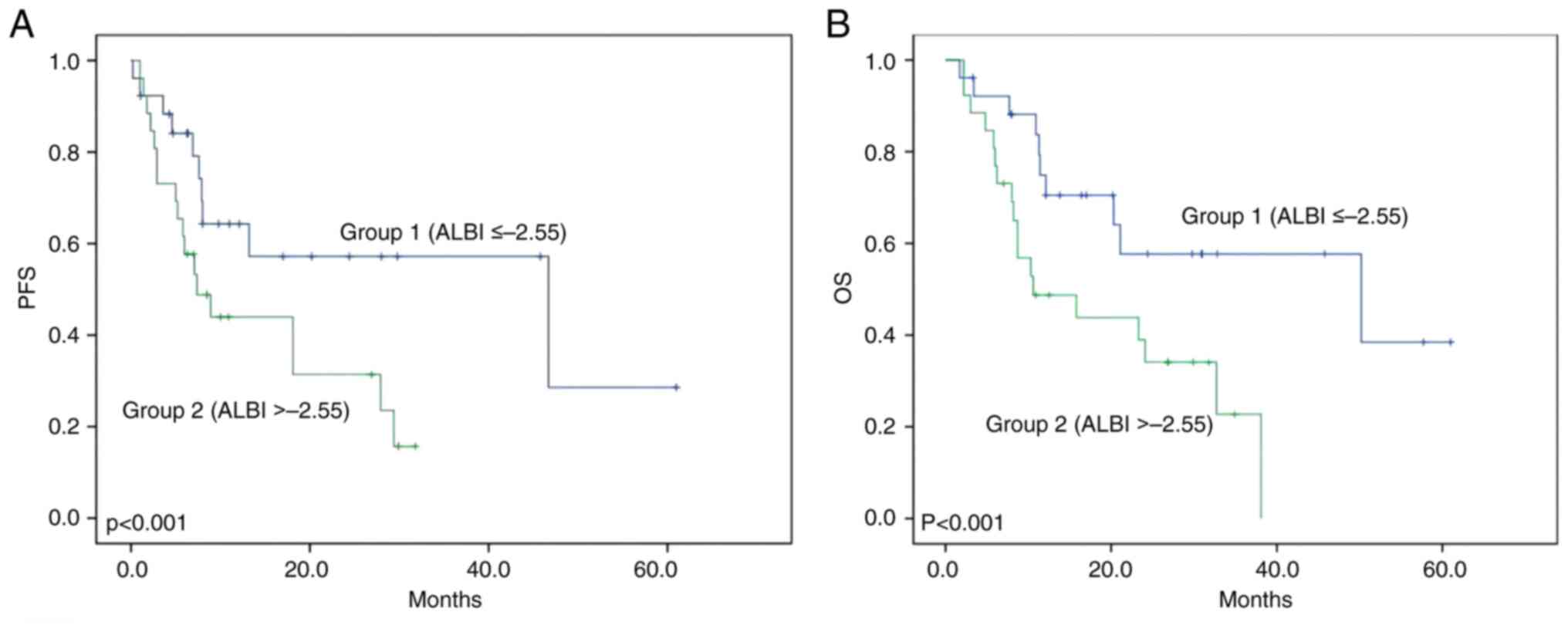
The median PFS rates in group 1 and group 2 were 8.4
months and 5.9 months, respectively. PFS was significantly improved
in group 1 than in group 2 (P<0.001 using the log-rank test,
Fig. 2A). The median OS rates in
group 1 and group 2 were 14.6 months and 9.2 months, respectively.
OS was significantly improved in group 1 compared with in group 2
(P<0.001 using the log-rank test, Fig. 2B).
Univariate and multivariate analysis
of PFS and OS
Univariate analysis revealed sex, age, smoking,
staging, BMI, surgery, LDH, NLR, PLR, chemotherapy, CEA, NSE and
ALBI grade as significant factors for PFS. Multivariate analysis
revealed that sex, surgery, LDH, chemotherapy and ALBI grade are
independent risk factors for PFS (Table II). Univariate analyses showed
that sex, age, smoking, staging, BMI, surgery, LDH, PLR,
Chemotherapy, CEA, NSE, PNI and ALBI grade are significant factors
for OS while multivariate analysis revealed surgery, LDH, BMI,
chemotherapy and ALBI grade as independent risk factors for OS
(Table III).
 | Table IIUnivariate and multivariate analysis
for PFS. |
Table II
Univariate and multivariate analysis
for PFS.
| | Univariable
analysis | Multivariable
analysis |
|---|
| | | 95% CI | | 95% CI |
|---|
| Characteristic | P-value | HR | LL | UL | P-value | HR | LL | UL |
|---|
| Sex | 0.022 | 0.497 | 0.270 | 0.915 | 0.024 | 0.405 | 0.185 | 0.888 |
| Age | 0.018 | 1.720 | 1.090 | 2.714 | 0.326 | 0.776 | 0.467 | 1.288 |
| Smoking | 0.046 | 1.609 | 1.005 | 2.576 | 0.671 | 1.129 | 0.645 | 1.977 |
| Staging | <0.001 | 2.636 | 1.612 | 4.310 | 0.911 | 1.040 | 0.524 | 2.066 |
| BMI | 0.031 | 0.605 | 0.381 | 0.960 | 0.075 | 0.617 | 0.363 | 1.049 |
| PS | | | | | | | | |
|
0 | 0.141 | | | | | | | |
|
1 | 0.649 | 1.749 | 0.157 | 19.484 | | | | |
|
2 | 0.203 | 0.394 | 0.094 | 1.654 | | | | |
|
3 | 0.462 | 0.580 | 0.136 | 2.477 | | | | |
| CCI | | | | | | | | |
|
0 | 0.970 | | | | | | | |
|
1-2 | 0.804 | 1.286 | 0.176 | 9.390 | | | | |
|
≥3 | 0.807 | 1.281 | 0.175 | 9.358 | | | | |
| Surgery | <0.001 | 0.287 | 0.154 | 0.535 | 0.050 | 0.400 | 0.160 | 1.002 |
| LDH | <0.001 | 2.322 | 1.468 | 3.671 | 0.038 | 1.788 | 1.034 | 3.091 |
| NLR | 0.031 | 1.634 | 1.043 | 2.560 | 0.795 | 0.935 | 0.562 | 1.556 |
| SII | 0.117 | 1.430 | 0.913 | 2.240 | | | | |
| PLR | 0.002 | 2.352 | 1.329 | 4.162 | 0.193 | 1.639 | 0.779 | 3.448 |
| Chemotherapy | 0.017 | 0.564 | 0.351 | 0.907 | 0.038 | 0.545 | 0.307 | 0.966 |
| Radiotherapy | 0.706 | 1.091 | 0.693 | 1.720 | | | | |
| CEA | 0.001 | 2.619 | 1.459 | 4.702 | 0.475 | 1.292 | 0.640 | 2.610 |
| NSE | <0.001 | 3.170 | 1.964 | 5.117 | 0.170 | 1.642 | 0.809 | 3.333 |
| PNI | 0.061 | 0.516 | 0.255 | 1.044 | | | | |
| ALBI | <0.001 | 2.259 | 1.433 | 3.562 | 0.028 | 1.807 | 1.067 | 3.060 |
 | Table IIIUnivariate and multivariate analysis
for OS. |
Table III
Univariate and multivariate analysis
for OS.
| | Univariable
analysis | Multivariable
analysis |
|---|
| | | 95% CI | | 95% CI |
|---|
| Characteristic | P-value | HR | LL | UL | P-value | HR | LL | UL |
|---|
| Sex | 0.036 | 1.883 | 1.032 | 3.437 | 0.132 | 0.540 | 0.242 | 1.203 |
| Age | 0.011 | 0.556 | 0.351 | 0.881 | 0.499 | 0.835 | 0.495 | 1.409 |
| Smoking | 0.011 | 0.534 | 0.327 | 0.874 | 0.191 | 1.484 | 0.821 | 2.684 |
| Staging | <0.001 | 0.338 | 0.207 | 0.553 | 0.643 | 1.173 | 0.597 | 2.305 |
| BMI | 0.028 | 1.677 | 1.052 | 2.674 | 0.033 | 0.566 | 0.335 | 0.955 |
| PS | | | | | | | | |
|
0 | 0.250 | | | | | | | |
|
1 | 0.550 | 2.083 | 0.188 | 23.126 | | | | |
|
2 | 0.782 | 0.818 | 0.198 | 3.382 | | | | |
|
3 | 0.733 | 1.284 | 0.305 | 5.398 | | | | |
| CCI | | | | | | | | |
|
0 | 0.763 | | | | | | | |
|
1-2 | 0.699 | 1.481 | 0.202 | 10.838 | | | | |
|
≥3 | 0.604 | 1.692 | 0.232 | 12.357 | | | | |
| Surgery | <0.001 | 3.711 | 1.989 | 6.923 | 0.013 | 0.306 | 0.120 | 0.782 |
| LDH | <0.001 | 2.407 | 1.514 | 3.828 | 0.039 | 1.820 | 1.032 | 3.211 |
| NLR | 0.051 | 0.640 | 0.408 | 1.006 | | | | |
| SII | 0.166 | 0.727 | 0.462 | 1.144 | | | | |
| PLR | 0.015 | 0.502 | 0.285 | 0.886 | 0.788 | 0.900 | 0.419 | 1.936 |
| Chemotherapy | 0.003 | 2.046 | 1.267 | 3.305 | 0.009 | 0.451 | 0.248 | 0.821 |
| Radiotherapy | 0.348 | 1.247 | 0.785 | 1.981 | | | | |
| CEA | <0.001 | 0.365 | 0.204 | 0.655 | 0.841 | 1.075 | 0.530 | 2.179 |
| NSE | <0.001 | 0.309 | 0.191 | 0.498 | 0.167 | 1.630 | 0.816 | 3.257 |
| PNI | 0.019 | 2.276 | 1.124 | 4.610 | 0.423 | 1.408 | 0.610 | 3.246 |
| ALBI | <0.001 | 0.409 | 0.258 | 0.648 | 0.013 | 2.011 | 1.159 | 3.490 |
ALBI grade and survival in propensity
score matching analysis
To further validate the impact of ALBI grade on
survival results in SCLC, a PSM analysis was employed to equalize
background information of the patients. The caliper value was set
as 0.15. As a result, 26 paired patients were extracted from the
two groups. The relationship between baseline characteristics and
ALBI grade after PSM are shown in Table IV. There were no differences in
characteristics of the patients among the two groups. Univariate
analysis showed that group 1 had a significantly longer PFS (HR
2.258, 95% CI 1.013-5.034, P=0.041, Fig. 3A) and OS (HR 2.591, 95% CI
1.154-5.814, P=0.017, Fig. 3B)
than group 2. Multivariate analysis suggested that ALBI grade after
PSM is an independent prognostic factor of PFS (HR 2.379, 95% CI
1.045-5.412, P=0.039, Table V) and
OS (HR 3.496, 95% CI 1.416-8.635, P=0.007, Table VI).
 | Table IVRelationship between patient
characteristics and ALBI grade following PSM. |
Table IV
Relationship between patient
characteristics and ALBI grade following PSM.
| Characteristic | Baseline | ALBI ≤-2.55 n=26
(%) | ALBI >-2.55 n=26
(%) | P-value |
|---|
| Sex | | | | 1.000 |
|
Male | | 20 (48.8) | 21 (51.2) | |
|
Female | | 6 (54.5) | 5 (45.5) | |
| Age | | | | 0.779 |
|
<65
years | | 12 (54.5) | 10 (45.5) | |
|
≥65
years | | 14 (46.7) | 16 (53.3) | |
| Smoking | | | | 0.776 |
|
Yes | | 15 (46.9) | 17 (53.1) | |
|
No | | 11 (55.0) | 9 (45.0) | |
| Staging | | | | 1.000 |
|
Limited
stage | | 11 (52.4) | 10 (47.6) | |
|
Extensive
stage | | 15 (48.4) | 16 (61.6) | |
| BMI | | | | 1.000 |
|
<25 | | 15 (51.7) | 14 (48.3) | |
|
≥25 | | 11 (47.8) | 12 (52.2) | |
| PS | | | | 0.949 |
|
0 | | 0 (0) | 0 (0) | |
|
1 | | 18 (51.4) | 17 (48.6) | |
|
2 | | 6 (46.2) | 7 (53.8) | |
|
3 | | 2 (50.0) | 2 (50.0) | |
| CCI | | | | 0.404 |
|
0 | | 14 (58.3) | 10 (41.7) | |
|
1-2 | | 12 (42.9) | 16 (57.1) | |
|
≥3 | | 0 (0) | 0 (0) | |
| Surgery | | | | 1.000 |
|
Yes | | 8 (53.3) | 7 (46.7) | |
|
No | | 18 (48.6) | 19 (51.4) | |
| LDH | | | | 1.000 |
|
<191.45 | | 12 (50.0) | 12 (50.0) | |
|
≥191.45 | | 14 (50.0) | 14 (50.0) | |
| NLR | | | | 1.000 |
|
<3.519 | | 16 (48.5) | 17 (51.5) | |
|
≥3.519 | | 10 (52.6) | 9 (47.4) | |
| SII | | | | 1.000 |
|
<874.428 | | 16 (48.5) | 17 (51.5) | |
|
≥874.428 | | 10 (52.6) | 9 (47.4) | |
| PLR | | | | 1.000 |
|
<281.896 | | 23 (50.0) | 23 (50.0) | |
|
≥281.896 | | 3 (50.0) | 3 (50.0) | |
| PNI | | | | - |
|
<40 | | 0 (0) | 0 (0) | |
|
≥40 | | 26 (50.0) | 26 (50.0) | |
| Chemotherapy | | | | 0.771 |
|
Yes | | 18 (52.9) | 16 (47.1) | |
|
No | | 8 (44.4) | 10 (55.6) | |
| Radiotherapy | | | | 1.000 |
|
Yes | | 7 (50.0) | 7 (50.0) | |
|
No | | 19 (50.0) | 19 (50.0) | |
| CEA | | | | 1.000 |
|
Normal | | 23 (51.1) | 22 (48.9) | |
|
High | | 3 (42.9) | 4 (57.1) | |
| NSE | | | | 1.000 |
|
Normal | | 11 (47.8) | 12 (52.2) | |
|
High | | 15 (51.7) | 14 (48.3) | |
 | Table VUnivariate and multivariate analysis
for PFS after PSM. |
Table V
Univariate and multivariate analysis
for PFS after PSM.
| | Univariable
analysis | Multivariable
analysis |
|---|
| | | 95% CI | | 95% CI |
|---|
| Characteristic | P-value | HR | LL | UL | P-value | HR | LL | UL |
|---|
| Sex | 0.205 | 2.147 | 0.641 | 7.189 | | | | |
| Age | 0.878 | 1.061 | 0.500 | 2.249 | | | | |
| Smoking | 0.148 | 1.825 | 0.798 | 4.177 | | | | |
| Staging | 0.001 | 4.474 | 1.786 | 11.205 | 0.232 | 2.118 | 0.618 | 7.260 |
| BMI | 0.263 | 1.540 | 0.719 | 3.299 | | | | |
| PS | | | | | | | | |
|
0 | | | | | | | | |
|
1 | 0.091 | | | | | | | |
|
2 | 0.118 | 0.296 | 0.065 | 1.360 | | | | |
|
3 | 0.553 | 0.622 | 0.130 | 2.983 | | | | |
| CCI | 0.064 | 0.477 | 0.214 | 1.060 | | | | |
| Surgery | 0.002 | 0.201 | 0.068 | 0.597 | 0.474 | 0.562 | 0.116 | 2.723 |
| LDH | 0.057 | 2.143 | 0.960 | 4.784 | | | | |
| NLR | 0.368 | 1.431 | 0.653 | 3.136 | | | | |
| SII | 0.931 | 1.036 | 0.465 | 2.308 | | | | |
| PLR | <0.001 | 5.921 | 1.968 | 17.815 | 0.009 | 4.714 | 1.462 | 15.197 |
| Chemotherapy | 0.075 | 0.502 | 0.232 | 1.088 | | | | |
| Radiotherapy | 0.921 | 1.041 | 0.469 | 2.311 | | | | |
| CEA | 0.030 | 2.895 | 1.062 | 7.895 | 0.125 | 2.384 | 0.785 | 7.235 |
| NSE | 0.003 | 3.304 | 1.433 | 7.615 | 0.574 | 1.423 | 0.417 | 4.855 |
| PNI | | | | | | | | |
| ALBI | 0.041 | 2.258 | 1.013 | 5.034 | 0.039 | 2.379 | 1.045 | 5.412 |
 | Table VIUnivariate and multivariate analysis
for OS after PSM. |
Table VI
Univariate and multivariate analysis
for OS after PSM.
| | Univariable
analysis | Multivariable
analysis |
|---|
| | | 95% CI | | 95% CI |
|---|
| Characteristic | P-value | HR | LL | UL | P-value | HR | LL | UL |
|---|
| Sex | 0.143 | 0.448 | 0.150 | 1.342 | | | | |
| Age | 0.713 | 1.152 | 0.542 | 2.451 | | | | |
| Smoking | 0.055 | 2.248 | 0.964 | 5.244 | | | | |
| Staging | <0.001 | 5.126 | 1.962 | 13.394 | 0.116 | 2.710 | 0.782 | 9.399 |
| BMI | 0.228 | 0.627 | 0.291 | 1.349 | | | | |
| PS | | | | | | | | |
|
0 | | | | | | | | |
|
1 | 0.138 | | | | | | | |
|
2 | 0.471 | 0.581 | 0.133 | 2.543 | | | | |
|
3 | 0.738 | 1.299 | 0.280 | 6.019 | | | | |
| CCI | 0.096 | 1.922 | 0.881 | 4.193 | | | | |
| Surgery | 0.001 | 0.179 | 0.060 | 0.538 | 0.189 | 0.357 | 0.076 | 1.663 |
| LDH | 0.031 | 2.358 | 1.059 | 5.251 | 0.137 | 2.049 | 0.797 | 5.270 |
| NLR | 0.422 | 1.383 | 0.625 | 3.060 | | | | |
| SII | 0.957 | 1.023 | 0.454 | 2.304 | | | | |
| PLR | 0.021 | 3.020 | 1.125 | 8.107 | 0.255 | 1.936 | 0.620 | 6.044 |
| Chemotherapy | 0.054 | 0.474 | 0.218 | 1.030 | | | | |
| Radiotherapy | 0.598 | 0.805 | 0.359 | 1.804 | | | | |
| CEA | 0.027 | 2.965 | 1.080 | 8.142 | 0.652 | 1.288 | 0.429 | 3.865 |
| NSE | 0.002 | 3.464 | 1.498 | 8.010 | 0.780 | 0.844 | 0.257 | 2.769 |
| PNI | | | | | | | | |
| ALBI | 0.017 | 2.591 | 1.154 | 5.814 | 0.007 | 3.496 | 1.416 | 8.635 |
Discussion
The present study retrospectively investigated the
impact of pre-treatment ALBI grade on the prognosis of SCLC. It
clarified that ALBI grade is an important prognostic factor of PFS
and OS in univariate and multivariate analysis. To the best of the
authors' knowledge, this is the first study to show the prognostic
importance of ALBI grade in patients with SCLC. The results showed
that ALBI grade was highly associated with age and LDH. The two
factors showed prognostic power in patients with SCLC, which may be
as confounding factors and cause a bias in the present study. In
order to eliminate the influence of confounding factors, PSM was
performed. After matching, ALBI grade proved to be an independent
prognostic factor for the prognosis of SCLC. Sex, age, smoking, BMI
and several clinical parameters were indicated to have prognostic
power in patients with SCLC from univariate analysis before PSM.
However, those factors showed no statistical difference after PSM,
which may be due to the synergy with other factors including
ALBI.
The liver can be partly regarded as an immune organ
as it contains a large number of immune cells (18). Previous studies have indicated that
impaired liver function has important effects on the systemic
immune response in alcoholic liver injury and viral hepatitis
(19,20). It is reported that decreased liver
function can cause changes in T cell repertoires which play an
important role in cellular immunity, and the effect may take place
from the early stage of cirrhosis (20,21).
As a result, the anti-tumor immune response of patients with liver
disease may be weaker than normal patients. ALBI grade, as an
indicator of liver function, can closely reflect the immune status
of the whole body (22,23). Thus, ALBI grade may have a
predictive power on anti-tumor immune response. In addition,
studies have proved that a decrease of albumin, which compose the
ALBI, can be an indicator of decreased liver reserve and increased
inflammatory response in the tumor microenvironment (24,25).
Hypoalbuminemia has been reported to indicate inflammation and
prognosis in patients with non-small cell lung cancer (NSCLC)
(26). Inflammation and immunity
can affect the tumor microenvironment by influencing the formation
of blood vessels (27,28), thereby further affecting the
prognosis of SCLC. Therefore, the immune inflammatory response is
considered to have prognostic power on patients with SCLC, which
could be one of the mechanisms of the prognostic effect of ALBI
grade (15,29).
Several inflammatory indicators were recorded and
analyzed including NLR, SII and PLR, which have been proved to be
important in predicting the prognosis of lung cancer, according to
previous reports (30-32).
NLR showed predictive effect of PFS in univariate analysis and PLR
showed predictive effect of both PFS and OS. However, neither of
the two factors had statistical significance in multivariate
analysis. The results indicated that although immunity and
inflammation have a predictive effect on the prognosis of SCLC,
they may not have independent prognostic power. There may be
synergistic factors that interact with immune inflammatory
responses. This also reflects that there are other mechanisms for
the prognostic effect of ALBI on patients with SCLC.
Nutrition and metabolism play an important role in
tumor progression. Malnutrition in cancer patients can impair
quality of life and response to treatment (33). BMI, PNI and ALB, which can reflect
nutrition and metabolism to a certain extent, have been proved to
be important parameters for assessing nutritional status (34-37).
According to previous studies, these three factors are closely
associated with the survival rate of advanced lung cancer (26,35-38).
Therefore, they may have prognostic use for patients with SCLC.
Previous studies have shown that weight loss in patients with
advanced cancer may increase the risk of mortality (39,40).
In the present study, BMI ≥25 indicated longer PFS and OS, and PNI
≥40 indicated longer OS. This confirmed that nutritional status has
a certain effect on the prognosis of patients with SCLC. In
addition, liver function can also reflect nutrition and metabolism
(41). Bilirubin plays an
important role in liver metabolism. Li et al (42) reported that elevated serum
bilirubin levels are associated with improved survival in patients
with NSCLC. There is evidence that serum bilirubin levels are
associated with incidence and mortality of lung cancer in smokers
(43). Therefore, to a large
extent, bilirubin may be able to evaluate the prognosis of patients
with SCLC. ALBI grade, consisting of albumin and bilirubin, may
reflect the nutrition and metabolism status in patients with SCLC,
which may be a mechanism of the prognostic effect.
One of the most important indicators in ALBI is ALB,
which can directly affect the value of ALBI. ALB can bind and
transport various endogenous and exogenous substances and promote
their transport in the circulation (44). In addition, ALB can bind to a
variety of drugs, affecting their release in target tissues
(45). Previous studies (46,47)
showed that ALB levels may affect the benefit of chemotherapy in
elderly cancer patients. The present study also found that higher
ALB levels and lower ALBI levels were associated with longer PFS
and OS.
In addition, LDH has been reported as a prognostic
indicator of SCLC and it can also predict the response to treatment
of patients with SCLC (48). This
may be due to the estimation ability of LDH on tumor burden. In the
present study, LDH showed independent prognostic power for both PFS
and OS of patients with SCLC. The results of the present study also
indicated that LDH is strongly correlated with ALBI grade.
Following PSM, LDH showed no statistical significance in
multivariate analysis, which indicated that LDH may have a similar
mechanism to ALBI grade in affecting the prognosis of SCLC.
Therefore, it is hypothesized that ALBI grade can predict the
effect of medication on patients with SCLC. Chemotherapy is
currently one of the most important medical treatments for SCLC.
The present study confirmed that chemotherapy can be an independent
prognostic factor for SCLC. In addition, the importance of ALBI
grade to predict the therapeutic effect of chemotherapy has been
previously reported in hepatocellular carcinomas and gastric cancer
(13,14,49)
Therefore, ALBI grade may be predictive of the effectiveness of
chemotherapy or post-recurrence chemotherapy on patients with SCLC
to achieve prognostic evaluation effect.
In the present study, a total of 81 patients had
distant metastases, mostly bone metastases, brain metastases and
abdominal organ metastases. Only 15 patients had liver metastases,
although ALBI values may have an effect in these patients with
liver metastases. However, the main purpose of the present study
was to discuss the relationship between ALBI and the prognosis of
patients with SCLC, so it was considered that this would not affect
the final results of the present study.
However, there are several limitations to the
present study. First, this is a single-center retrospective study
and there may be bias on patient selection and data collection.
Second, the small number of samples may lead to poor credibility of
the hypothesis. Large-scale prospective studies and experiments are
needed to consolidate the conclusion of the present study and
further explore the mechanism.
The present study showed that pre-treatment ALBI
grade can be an independent prognostic factor in SCLC, of which the
mechanisms may be associated with the immune inflammatory
responses, nutrition and the response to chemotherapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL carried out experimental design and data
statistics. QZ provided experimental guidance and result analysis.
ZW was a major contributor to writing the manuscript. XZ conducted
experimental design and helped write the manuscript. SL and ZB
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The Ethical Committee of Hebei General Hospital
approved the present study and informed consent was waived
(approval no. 2022061). The authors confirm the confidentiality of
the data maintained and compliance with the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang D, Guo D, Shi F, Zhu Y, Li A, Kong L,
Teng F and Yu J: The predictive effect of the systemic
immune-inflammation index for patients with small-cell lung cancer.
Future Oncol. 15:3367–3379. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Frese KK, Simpson KL and Dive C: Small
cell lung cancer enters the era of precision medicine. Cancer Cell.
39:297–299. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
van Meerbeeck JP, Fennell DA and De
Ruysscher DK: Small-cell lung cancer. Lancet. 378:1741–1755.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhan W, Liu Y, Gao Y, Gong R, Wang W,
Zhang R, Wu Y, Kang T and Wei D: RMI2 plays crucial roles in growth
and metastasis of lung cancer. Signal Transduct Target Ther.
5(188)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Go SI, Jeon H, Park SW, Kang MH, Kim HG
and Lee GW: Low pre-treatment nutritional index is significantly
related to poor outcomes in small cell lung cancer. Thorac Cancer.
9:1483–1491. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee SK, Song MJ, Kim SH and Park M:
Comparing various scoring system for predicting overall survival
according to treatment modalities in hepatocellular carcinoma
focused on Platelet-albumin-bilirubin (PALBI) and albumin-bilirubin
(ALBI) grade: A nationwide cohort study. PLoS One.
14(e0216173)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tsilimigras DI, Hyer JM, Moris D, Sahara
K, Bagante F, Guglielmi A, Aldrighetti L, Alexandrescu S, Marques
HP, Shen F, et al: Prognostic utility of Albumin-bilirubin grade
for short- and long-term outcomes after hepatic resection for
intrahepatic cholangiocarcinoma: A multi-institutional analysis of
706 patients. J Surg Oncol. 120:206–213. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kanda M, Tanaka C, Kobayashi D, Uda H,
Inaoka K, Tanaka Y, Hayashi M, Iwata N, Yamada S, Fujii T, et al:
Preoperative Albumin-bilirubin grade predicts recurrences after
radical gastrectomy in patients with pT2-4 gastric cancer. World J
Surg. 42:773–781. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Albers I, Hartmann H, Bircher J and
Creutzfeldt W: Superiority of the Child-pugh classification to
quantitative liver function tests for assessing prognosis of liver
cirrhosis. Scand J Gastroenterol. 24:269–276. 1989.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Johnson PJ, Berhane S, Kagebayashi C,
Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A,
Palmer D, et al: Assessment of liver function in patients with
hepatocellular carcinoma: A new evidence-based approach-the ALBI
grade. J Clin Oncol. 33:550–558. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao S, Zhang T, Li H, Wang M, Xu K, Zheng
D, Du X and Liu L: Comparison of albumin-bilirubin grade versus
Child-Pugh score in predicting the outcome of transarterial
chemoembolization for hepatocellular carcinoma using time-dependent
ROC. Ann Transl Med. 8(538)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yagyu T, Saito H, Sakamoto T, Uchinaka EI,
Morimoto M, Amisaki M, Watanabe J, Tokuyasu N, Honjo S, Ashida K
and Fujiwara Y: Preoperative Albumin-bilirubin grade as a useful
prognostic indicator in patients with pancreatic cancer. Anticancer
Res. 39:1441–1446. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kinoshita F, Yamashita T, Oku Y, Kosai K,
Ono Y, Wakasu S, Haratake N, Toyokawa G, Takenaka T, Tagawa T, et
al: Prognostic impact of Albumin-bilirubin (ALBI) Grade on
Non-small lung cell carcinoma: A Propensity-score matched analysis.
Anticancer Res. 41:1621–1628. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB,
Peng JJ, Chen CQ, He YL and Cai SR: Systemic immune-inflammation
index for predicting prognosis of colorectal cancer. World J
Gastroenterol. 23:6261–6272. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cadwell JB, Afonso AM and Shahrokni A:
Prognostic nutritional index (PNI), independent of frailty is
associated with six-month postoperative mortality. J Geriatr Oncol.
11:880–884. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Racanelli V and Rehermann B: The liver as
an immunological organ. Hepatology. 43 (2 Suppl 1):S54–S62.
2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tuchendler E, Tuchendler PK and Madej G:
Immunodeficiency caused by cirrhosis. Clin Exp Hepatol. 4:158–164.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Albillos A, Lario M and Álvarez-Mon M:
Cirrhosis-associated immune dysfunction: Distinctive features and
clinical relevance. J Hepatol. 61:1385–1396. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Irvine KM, Ratnasekera I, Powell EE and
Hume DA: Causes and consequences of innate immune dysfunction in
cirrhosis. Front Immunol. 10(293)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Robinson MW, Harmon C and O'Farrelly C:
Liver immunology and its role in inflammation and homeostasis. Cell
Mol Immunol. 13:267–276. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Noor MT and Manoria P: Immune dysfunction
in cirrhosis. J Clin Transl Hepatol. 5:50–58. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lipschitz DA: Protein-energy malnutrition.
Hosp Pract (Off Ed). 23:87–99. 1988.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Harimoto N, Yoshizumi T, Sakata K, Nagatsu
A, Motomura T, Itoh S, Harada N, Ikegami T, Uchiyama H, Soejima Y
and Maehara Y: Prognostic significance of preoperative controlling
nutritional status (CONUT) score in patients undergoing hepatic
resection for hepatocellular carcinoma. World J Surg. 41:2805–2812.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tomita M, Ayabe T, Chosa E and Nakamura K:
Prognostic significance of pre- and postoperative glasgow
prognostic score for patients with non-small cell lung cancer.
Anticancer Res. 34:3137–3140. 2014.PubMed/NCBI
|
|
27
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444.
2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pinato DJ, Sharma R, Citti C, Platt H,
Ventura-Cots M, Allara E, Chen TY, Dalla Pria A, Jain M, Mínguez B,
et al: The albumin-bilirubin grade uncovers the prognostic
relationship between hepatic reserve and immune dysfunction in
HIV-associated hepatocellular carcinoma. Aliment Pharmacol Ther.
47:95–103. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Guo W, Cai S, Zhang F, Shao F, Zhang G,
Zhou Y, Zhao L, Tan F, Gao S and He J: Systemic immune-inflammation
index (SII) is useful to predict survival outcomes in patients with
surgically resected non-small cell lung cancer. Thorac Cancer.
10:761–768. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Thompson D, Perry LA, Renouf J, Vodanovich
D, Hong Lee AH, Dimiri J and Wright G: Prognostic utility of
inflammation-based biomarkers, neutrophil-lymphocyte ratio and
change in neutrophil-lymphocyte ratio, in surgically resected lung
cancers. Ann Thorac Med. 16:148–155. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Han Y, Wang J, Hong L, Sun L, Zhuang H,
Sun B, Wang H, Zhang X and Ren X: Platelet-lymphocyte ratio is an
independent prognostic factor in patients with ALK-positive
non-small-cell lung cancer. Future Oncol. 13:51–61. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classification of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jin Y, Zhao L and Peng F: Prognostic
impact of serum albumin levels on the recurrence of stage I
non-small cell lung cancer. Clinics. 68:686–693. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nakagawa T, Toyazaki T, Chiba N, Ueda Y
and Gotoh M: Prognostic value of body mass index and change in body
weight in postoperative outcomes of lung cancer surgery. Interact
Cardiovasc Thorac Surg. 23:560–566. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tewari N, Martin-Ucar AE, Black E, Beggs
L, Beggs FD, Duffy JP and Morgan WE: Nutritional status affects
long term survival after lobectomy for lung cancer. Lung Cancer.
57:389–394. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Watanabe H, Yamada T, Komori K, Hara K,
Kano K, Takahashi K, Kumazu Y, Fujikawa H, Numata M, Aoyama T, et
al: Effect of prognostic nutrition index in gastric or
Gastro-oesophageal junction cancer patients undergoing nivolumab
monotherapy. In Vivo. 35:563–569. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yotsukura M, Ohtsuka T, Kaseda K, Kamiyama
I, Hayashi Y and Asamura H: Value of the glasgow prognostic score
as a prognostic factor in resectable non-small cell lung cancer. J
Thorac Oncol. 11:1311–1318. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
McMillan DC: An inflammation-based
prognostic score and its role in the nutrition-based management of
patients with cancer. Proc Nutr Soc. 67:257–262. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shepshelovich D, Xu W, Lu L, Fares A, Yang
P, Christiani D, Zhang J, Shiraishi K, Ryan BM, Chen C, et al: Body
Mass Index (BMI), BMI change, and overall survival in patients with
SCLC and NSCLC: A pooled analysis of the international lung cancer
consortium. J Thorac Oncol. 14:1594–1607. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kotoh Y, Saeki I, Yamasaki T, Sasaki R,
Tanabe N, Oono T, Maeda M, Hidaka I, Ishikawa T, Takami T and
Sakaida I: Albumin-bilirubin score as a useful predictor of energy
malnutrition in patients with hepatocellular carcinoma. Clin Nutr.
40:3585–3591. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li N, Xu M, Cai MY, Zhou F, Li CF, Wang
BX, Ou W and Wang SY: Elevated serum bilirubin levels are
associated with improved survival in patients with curatively
resected non-small-cell lung cancer. Cancer Epidemiol. 39:763–768.
2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wen CP, Zhang F, Liang D, Wen C, Gu J,
Skinner H, Chow WH, Ye Y, Pu X, Hildebrandt MA, et al: The ability
of bilirubin in identifying smokers with higher risk of lung
cancer: A large cohort study in conjunction with global metabolomic
profiling. Clin Cancer Res. 21:193–200. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Otagiri M: A molecular functional study on
the interactions of drugs with plasma proteins. Drug Metab
Pharmacokinet. 20:309–323. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kragh-Hansen U, Chuang VT and Otagiri M:
Practical aspects of the ligand-binding and enzymatic properties of
human serum albumin. Biol Pharm Bull. 25:695–704. 2002.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ikeda S, Yoshioka H, Ikeo S, Morita M,
Sone N, Niwa T, Nishiyama A, Yokoyama T, Sekine A, Ogura T and
Ishida T: Serum albumin level as a potential marker for deciding
chemotherapy or best supportive care in elderly, advanced non-small
cell lung cancer patients with poor performance status. BMC Cancer.
17(797)2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ito S, Ito H, Sato N, Hirayama Y, Kusakabe
T, Terui T and Ishitani K: Clinical factors associated with the
therapeutic outcome of chemotherapy in very elderly cancer
patients. Int J Clin Oncol. 24:596–601. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hsieh AH, Tahkar H, Koczwara B,
Kichenadasse G, Beckmann K, Karapetis C and Sukumaran S:
Pre-treatment serum lactate dehydrogenase as a biomarker in small
cell lung cancer. Asia Pac J Clin Oncol. 14:e64–e70.
2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Pinato DJ, Sharma R, Allara E, Yen C,
Arizumi T, Kubota K, Bettinger D, Jang JW, Smirne C, Kim YW, et al:
The ALBI grade provides objective hepatic reserve estimation across
each BCLC stage of hepatocellular carcinoma. J Hepatol. 66:338–346.
2017.PubMed/NCBI View Article : Google Scholar
|