Introduction
According to World Health Organization cancer
statistics, gastric cancer is the second most common malignancy and
the fourth most common cause of cancer mortality worldwide
(1). Prognosis has gradually
improved because of advances in chemotherapy regimens, but is not
yet satisfactory, and a permanent cure is rarely achieved.
Currently, the standard treatment for unresectable
or metastatic gastric cancer is systemic chemotherapy. In Japan,
combined induction chemotherapy with fluorouracil (5FU) and
platinum is the current first-line standard therapy for
unresectable or metastatic gastric cancer (2-5).
For second-line therapy, the fourth edition of the Gastric Cancer
Treatment Guidelines recommends three anticancer monotherapies,
viz. paclitaxel (PTX), irinotecan, and docetaxel, as second-line
therapy (6). PTX plus ramucirumab
(RAM) showed additional efficacy compared with PTX monotherapy in
the RAINBOW trial (7), making it
the standard of care in the fifth edition of the Gastric Cancer
Treatment Guidelines (8).
Chemotherapy has been shown to prolong survival in
patients who received first- and second-line treatments for
unresectable advanced/recurrent gastric cancer; however, no
treatment has shown sufficient efficacy after third-line therapy.
Previously, PTX and irinotecan as monotherapy were recommended for
second- line therapy based on the results of the WJOG4007 trial
(9). The recommended third-line
regimen include nivolumab and irinotecan monotherapies (8). However, since PTX + RAM has become
the standard of care for second-line therapy, irinotecan has been
used as third-line treatment. Currently, no trials comparing
irinotecan and nivolumab exist; therefore, it is unclear which drug
should be administered first.
Nivolumab, a human IgG4 monoclonal antibody against
the immune checkpoint molecule, programmed cell death-1 receptor,
has shown efficacy and safety in various cancer types. It
significantly prolonged survival compared with placebo in patients
with unresectable advanced or recurrent gastric cancer, who were
treated with two or more chemotherapy regimens in the phase III
ATTRACTION-2 trial (10). The
subgroup analysis of this trial revealed that prior treatment with
RAM, a vascular endothelial growth factor (VEGF) inhibitor,
affected the therapeutic effect of nivolumab (11). VEGF signaling alters the tumor
microenvironment and may affect the efficacy of immunotherapy
(12-14).
Therefore, this study reviewed patients with
unresectable or metastatic gastric cancer who were treated with
second-line chemotherapy using PTX + RAM followed by nivolumab.
Furthermore, we evaluated the outcomes of nivolumab treatment in
selected patients who responded well to PTX + RAM treatment.
Patients and methods
Patients
Twenty-nine patients with metastatic gastric cancer
were recruited for the present retrospective study. They were
treated with PTX (80 mg/m2) + RAM (8 mg/m2)
as second-line treatment, followed by nivolumab monotherapy (240
mg/body) as third-line treatment between January 2017 and October
2020 at the Saitama Medical Center, Jichi Medical University,
Japan. The patients were >18 years old, and their Eastern
Cooperative Oncology Group performance status (ECOG PS) was 0, 1,
or 2. This study was approved by the Research Ethics Committee of
Jichi Medical University (GC07-13). Written informed consent was
obtained from all patients before receiving chemotherapy according
to the Institutional Review Board instructions of Jichi Medical
University. In addition to the ethical approval and informed
consents, all methods were performed in accordance with the
Declaration of Helsinki on ethical principles in conducting human
research.
Efficacy and safety assessment
The incidences of adverse events, progression-free
survival (PFS), and overall survival (OS) were assessed. PFS was
defined as the time from the start of nivolumab therapy to either
disease progression or death. OS was defined as the time from the
start of nivolumab therapy to death from any cause. Tumors were
evaluated every 2 or 3 months using computed tomography (CT) or
positron emission tomography/CT imaging that was initially used to
stage the tumor. Tumor response and progression were evaluated
according to the Response Evaluation Criteria in Solid Tumors
(RECIST) version 1.1. Adverse events were graded according to the
Common Terminology Criteria for Adverse Events version 4.0.
Treatment was continued until disease progression, unacceptable
toxicity, deterioration of the ECOG PS to >2, or withdrawal of
patient consent.
Response criteria for target
lesions
Response was assessed after two cycles of
chemotherapy. Measurable tumors were evaluated according to the
RECIST. Complete response (CR) was defined as the disappearance of
all non-nodal target lesions, with each nodal target lesion having
a reduction in the short axis of <10 mm. When nodal target
lesions are selected at baseline, the sum of the diameters may not
be 0 mm, even if the target lesion response is CR. Partial response
(PR) was defined as a decrease of at least 30% in the sum of target
lesion diameters, taking the baseline sum of diameters as
reference. Progressive disease (PD) was defined as an increase of
at least 20% in the sum of target lesion diameters, taking the
smallest sum of diameters as reference (this includes the baseline
sum if that is the smallest in the study). Stable disease (SD) was
defined as insufficient tumor shrinkage to qualify as PR and
insufficient tumor growth relative to the sum of the smallest
longest diameters to qualify as PD. Owing to the absence of
measurable lesions, no evaluation (NE) is difficult to determine.
The sum of the diameters must demonstrate an absolute increase of
at least 5 mm.
Statistical analysis
Statistical analyses were performed using StatView
5.0.1 (SAS Institute Inc., NC, USA). The OS and PFS curves were
analyzed using the Kaplan-Meier method, and the differences between
the groups were compared using the log-rank test. Prognostic
factors, including, age, sex, ECOG PS, site of metastasis, previous
gastrectomy, HER2 and MSI status, first-line chemotherapy, and PTX
+ RAM and nivolumab response, were analyzed for survival by
multivariate analysis using the Cox proportional hazards model. All
reported P-values were two-sided, and P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
The characteristics of the 29 patients (22 males and
7 females) are detailed in Table
I. The median age of the patients was 68 years (range 45-82
years). ECOG PS 0, 1, and 2 were observed in eight, 17, and four
patients, respectively. Gastrectomy was performed in 12 patients.
Human epidermal growth factor receptor 2 (HER2) positivity was
identified in six patients. High microsatellite instability (MSI-H)
was observed in two patients. All patients were treated with a
single regimen prior to PTX + RAM. All HER2-positive patients
received combination therapy with trastuzumab. Approximately all
HER2-negative patients received 5FU and platinum anti-tumor
agents.
 | Table IBaseline patient characteristics. |
Table I
Baseline patient characteristics.
| Characteristic | Value |
|---|
| Median age, years
(range) | 68 (45-82) |
| Sex
(Male/Female) | 22/7 |
| ECOG PS
(0/1/2/3) | 8/17/4/0 |
| Site of
metastases | |
|
Lymph
node | 3 |
|
Peritoneum | 14 |
|
Liver | 6 |
|
Lung | 1 |
|
Pleura | 0 |
|
Bone | 1 |
|
Other | 1 |
| Previous
gastrectomy | |
|
No | 17 |
|
Yes | 12 |
| HER2 | |
|
Positive | 6 |
|
Negative | 23 |
| MSI | |
|
High | 2 |
|
Low | 27 |
| Previous
treatment | |
|
Any | 29 |
|
Pyrimidine
analogs | 28 |
|
Platinum | 27 |
|
Taxane | 1 |
|
Trastuzumab | 6 |
Efficacy
Nivolumab treatment showed CR, PR, SD, PD, and NE in
2 (7.0%), 4 (13.8%), 12 (41.4%), 8 (27.6%), and 3 patients (10.3%),
respectively. The objective response rate (CR + PR) was 20.7%, and
the disease control rate (CR + PR + SD) was 62.1%. The median PFS
and OS were 4.4 months (3.3-7.1) and 14.9 months (9.9-24.0),
respectively (Fig. 1).
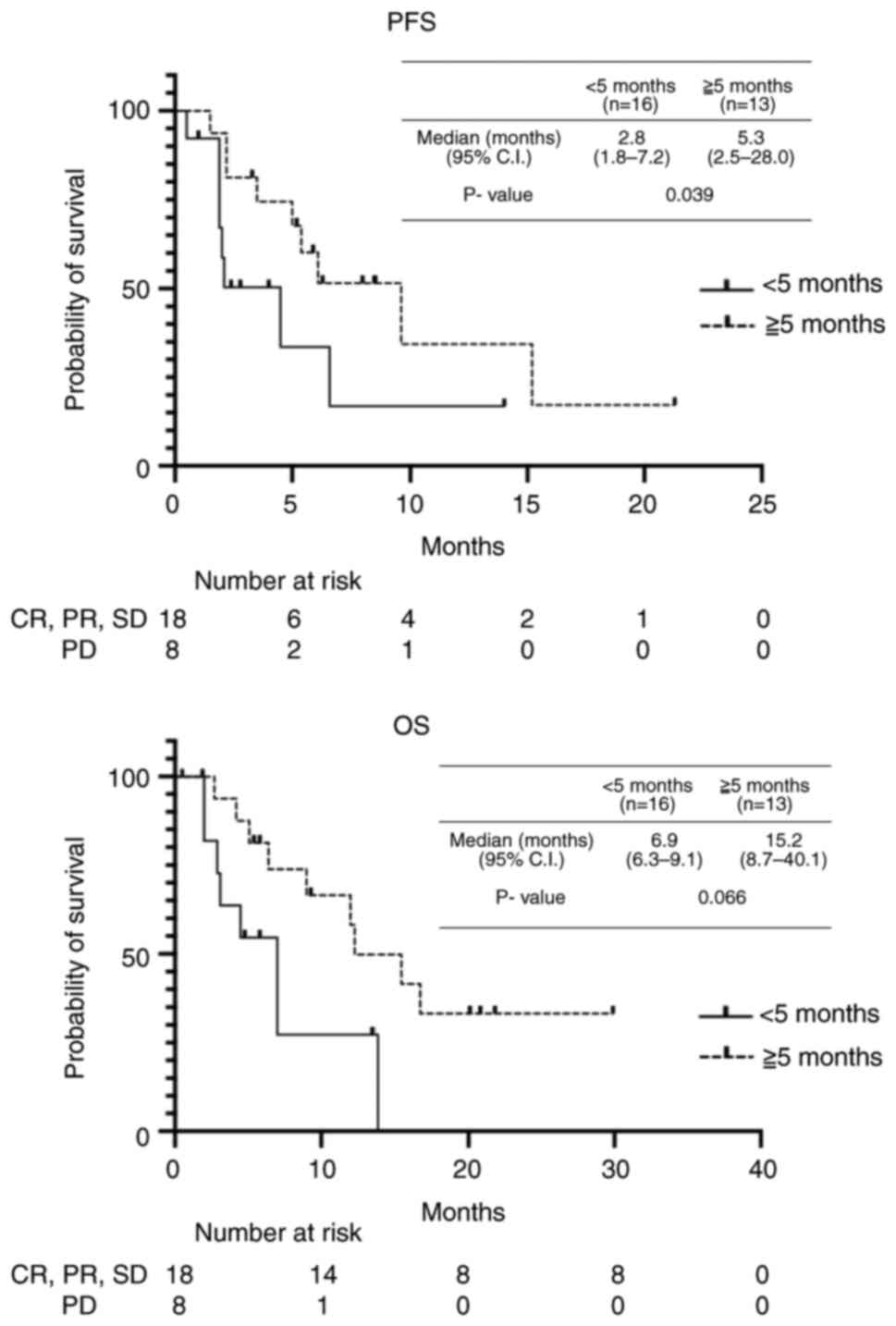
The median PFS in patients after PTX + RAM treatment
was 5.1 months (0.5-19.6); we analyzed the therapeutic effect of
nivolumab in two groups: poor response (PFS <5 months after PTX
+ RAM therapy) and good response (PFS >5 months after PTX + RAM
therapy) groups. The poor and good response groups included 13 and
16 patients, respectively. The good response group showed better
outcome in both PFS and OS after nivolumab treatment than the poor
response group (PFS: 5.3 vs. 2.8 months, P=0.039; OS: 15.2 vs. 6.9
months, P=0.066) (Fig. 2). In
addition, we analyzed the therapeutic effect of nivolumab in two
groups; poor response and good response to the first-line
chemotherapy (PFS <9 months vs. PFS >9 months). The median
PFS during first-line chemotherapy was 9.1 months (0.9-35.9) and
the poor and good response groups included 14 and 15 patients,
respectively. No difference in OS after nivolumab treatment was
seen between the poor and good response group (10.1. vs. 11.6
months, P=0.566), which indicated that the first-line chemotherapy
was not involved in the therapeutic effect of nivolumab.
Multivariate analyses showed a good response to PTX
+ RAM [hazard ratio (HR) 0.116, 95% confidence interval (CI)
0.037-0.742, P=0.019] as an independent prognostic factor for
survival (Table II). Age, sex,
ECOG PS, metastatic site, previous gastrectomy, HER2 status, MSI
status, first-line chemotherapy, and nivolumab were not
significantly associated with survival.
 | Table IIResults of multivariate analyses of
associations between patient characteristics and survival. |
Table II
Results of multivariate analyses of
associations between patient characteristics and survival.
| Variable | P-value |
|---|
| PTX + RAM response
(+/-) | 0.019 |
| Age (≤59/≥60
years) | 0.990 |
| Sex
(male/female) | 0.711 |
| ECOG PS
(0/1/2/3) | 0.348 |
| Site of metastases
(peritoneum/others) | 0.665 |
| Previous gastrectomy
(+/-) | 0.816 |
| HER2
(positive/negative) | 0.278 |
| MSI (high/low) | 0.761 |
| Response of
first-line chemotherapy (response/no response) | 0.543 |
Discussion
In this study, the efficacy of nivolumab as a
third-line treatment option was associated with the efficacy of
prior PTX + RAM treatment in patients with advanced gastric cancer.
The longer the PFS after pretreatment with PTX + RAM, the better
the therapeutic effect of nivolumab. To our knowledge, this
retrospective analysis is the first to show a therapeutic
association between second-line PTX + RAM and third-line nivolumab
treatment. This study on the therapeutic effect of PTX + RAM is a
useful tool in actual clinical practice. Currently, the Japanese
guidelines recommend nivolumab, CPT-11, and trastuzumab deruxtecan
as third-line therapies for HER2-positive patients (8). Therefore, patients with HER2-positive
gastric cancer were excluded from this study.
In the Japanese subgroup analysis of the
ATTRACTION-2 trial, the overall PFS and OS were 1.7 and 5.4 months,
respectively (11). In contrast,
in this study, the overall PFS and OS were 4.4 and 14.9 months,
respectively. Although a simple comparison could not be made, the
PFS in the present study was more than twice the PFS observed in
the Japanese subgroup analysis. Herein, all patients were treated
with RAM + PTX as second-line treatment, followed by nivolumab
monotherapy as third-line treatment. In contrast, in the
above-mentioned Japanese subgroup analysis, several patients in the
RAM + PTX group received nivolumab after treatment with irinotecan
or other drugs. This may have affected the results. For those who
respond well to PTX + RAM, nivolumab consecutively before using
irinotecan may be valuable.
Recently, several trials including CheckMate 649 and
ATTRACTION-4 demonstrated the benefit of first-line chemotherapy
with nivolumab and cytotoxic anticancer drugs in patients without
HER amplification (12,13). These findings led to an increase in
the use of combination therapy with nivolumab in first-line
chemotherapy, resulting in a decrease in the use of nivolumab
monotherapy in third-line chemotherapy. However, patients who
received first-line chemotherapy without nivolumab would be good
candidates for third-line nivolumab monotherapy if they responded
well to RAM + PTX during the second-line chemotherapy.
The development and clinical application of immune
checkpoint inhibitors (ICIs), such as nivolumab and pembrolizumab,
have dramatically improved the outcome of cancer chemotherapy. In
addition, as the analysis of tumor immunity has progressed,
attention has focused on the dynamic and complex mutual involvement
of many protein molecules in the biological processes of
angiogenesis and tumor immunity. Previous reports have provided
information on the relationship between angiogenesis and tumor
immunity (14-19).
VEGF suppresses dendritic cell maturation and T-cell function and
migration and promotes suppressive T-cell activation, all of which
promote tumor immune responses (14,17).
RAM is a monoclonal antibody that binds to the VEGF receptor
(VEGFR)-2 and primarily inhibits the VEGF-A/VEGFR-2-mediated
angiogenic signaling cascade. Moreover, angiogenesis inhibitors
such as ramucirumab are expected to promote anti-tumor immune
responses by regulating immunosuppressive activity. This suggests
that the combined use of angiogenesis inhibitors and
immunotherapies, including ICIs, may exhibit synergistic antitumor
effects. Various reports exist on predicting nivolumab efficacy;
however, no biomarkers have been identified that can be used in all
cases. Programmed death-ligand 1 expression is a promising
biomarker. In our study, we postulated that RAM reactivated the
tumor immune response and enhanced nivolumab efficacy. The enhanced
effect of an ICI in combination with angiogenesis inhibitors has
already been reported in lung, renal, and hepatocellular carcinomas
(18,19).
In the near future, we may narrow down the
characteristics of the patient group that can benefit from this
combination therapy and obtain important information on the
appropriate timing and dosage of the combination therapy. This may
generate significant evidence for combination therapy with a
cytotoxic anticancer drug + an angiogenesis inhibitor + an ICI.
Currently, a clinical trial of nivolumab combined with PTX + RAM as
second-line treatment for gastric cancer is being conducted
(20).
Several limitations of the present study should be
acknowledged. First, the study was conducted with a retrospective
design at a single center. Second, all enrolled patients with
advanced gastric cancer were Japanese, and the sample size was
small. Therefore, confirmation in a large-scale prospective study is
required.
In conclusion, RAM may enhance nivolumab efficacy as
the therapeutic effect of nivolumab was found to be associated with
PTX + RAM pretreatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MS acted as a guarantor of the integrity of the
study, conceived the study concept AND designed the study. ST, YE
and FW performed the literature research. ST, YE, FW, YK and IA
performed data acquisition, data analysis and statistical analysis,
and prepared and edited the manuscript. KS and TR reviewed the
manuscript. MS, TR and KS confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Ethics
Committee of Jichi Medical University (approval no. GC07-13).
Written informed consent was obtained from all patients before
receiving chemotherapy.
Patient consent for publication
Written informed consent was obtained from all
patients for publication of this paper. In addition, all methods
were performed in accordance with the Declaration of Helsinki on
ethical principles in conducting human research.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
No authors listed. WORLD Health
Organization. Cancer registration and statistics. J Am Med Womens
Assoc. 6(142)1951.PubMed/NCBI
|
|
2
|
Cunningham D, Starling N, Rao S, Iveson T,
Nicolson M, Coxon F, Middleton G, Daniel F, Oates J and Norman AR:
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom. Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yamada Y, Higuchi K, Nishikawa K, Gotoh M,
Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, et al:
Phase III study comparing oxaliplatin plus S-1 with cisplatin plus
S-1 in chemotherapy-naïve patients with advanced gastric cancer.
Ann Oncol. 26:141–148. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yoon HH, Bendell JC, Braiteh FS, Firdaus
I, Philip PA, Cohn AL, Lewis N, Anderson DM, Arrowsmith E, Schwartz
JD, et al: Ramucirumab combined with FOLFOX as front-line therapy
for advanced esophageal, gastroesophageal junction, or gastric
adenocarcinoma: A randomized, double-blind, multicenter phase II
trial. Ann Oncol. 27:2196–2203. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Japanese Gastric Cancer Association.
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric
Cancer. 20:1–19. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Japanese Gastric Cancer Association.
Japanese gastric cancer treatment guidelines 2018 (5th edition).
Gastric Cancer. 24:1–21. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hironaka S, Ueda S, Yasui H, Nishina T,
Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki
T, et al: Randomized, open-label, phase III study comparing
irinotecan with paclitaxel in patients with advanced gastric cancer
without severe peritoneal metastasis after failure of prior
combination chemotherapy using fluoropyrimidine plus platinum: WJOG
4007 trial. J Clin Oncol. 31:4438–4444. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kato K, Satoh T, Muro K, Yoshikawa T,
Tamura T, Hamamoto Y, Chin K, Minashi K, Tsuda M, Yamaguchi K, et
al: A subanalysis of Japanese patients in a randomized,
double-blind, placebo-controlled, phase 3 trial of nivolumab for
patients with advanced gastric or gastro-esophageal junction cancer
refractory to, or intolerant of, at least two previous chemotherapy
regimens (ONO-4538-12, ATTRACTION-2). Gastric Cancer. 22:344–354.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos Bragagnoli A, et al: First-line nivolumab plus chemotherapy
versus chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC,
Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, et al:
Nivolumab plus chemotherapy versus placebo plus chemotherapy in
patients with HER2-negative, untreated, unresectable advanced or
recurrent gastric or gastro-oesophageal junction cancer
(ATTRACTION-4): A randomised, multicentre, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 23:234–247.
2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fukuoka S, Hara H, Takahashi N, Kojima T,
Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, et
al: Regorafenib plus nivolumab in patients with advanced gastric or
colorectal cancer: An open-label, dose-escalation, and
dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol.
38:2053–2061. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Herbst RS, Arkenau HT, Santana-Davila R,
Calvo E, Paz-Ares L, Cassier PA, Bendell J, Penel N, Krebs MG,
Martin-Liberal J, et al: Ramucirumab plus pembrolizumab in patients
with previously treated advanced non-small-cell lung cancer,
gastro-oesophageal cancer, or urothelial carcinomas (JVDF): A
multicohort, non-randomised, open-label, phase 1a/b trial. Lancet
Oncol. 20:1109–1123. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim J, Byeon S, Kim H, Yeo JH, Hong JY,
Lee J, Lim HY, Kang WK and Kim ST: Impact of prior ramucirumab use
on treatment outcomes of checkpoint inhibitors in advanced gastric
cancer patients. Targeted Oncol. 15:203–209. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ohm JE, Gabrilovich DI, Sempowski GD,
Kisseleva E, Parman KS, Nadaf S and Carbone DP: VEGF inhibits
T-cell development and may contribute to tumor-induced immune
suppression. Blood. 101:4878–4886. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tada Y, Togashi Y, Kotani D, Kuwata T,
Sato E, Kawazoe A, Doi T, Wada H, Nishikawa H and Shitara K:
Targeting VEGFR2 with Ramucirumab strongly impacts
effector/activated regulatory T cells and CD8+ T cells
in the tumor microenvironment. J Immunother Cancer.
6(106)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Voron T, Colussi O, Marcheteau E, Pernot
S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N,
Tanchot C, et al: VEGF-A modulates expression of inhibitory
checkpoints on CD8+ T cells in tumors. J Exp Med. 212:139–148.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nakajima TE, Kadowaki S, Minashi K,
Nishina T, Yamanaka T, Hayashi Y, Izawa N, Muro K, Hironaka S,
Kajiwara T and Kawakami Y: Multicenter phase I/II study of
nivolumab combined with paclitaxel plus ramucirumab as second-line
treatment in patients with advanced gastric cancer. Clin Cancer
Res. 27:1029–1036. 2021.PubMed/NCBI View Article : Google Scholar
|