Introduction
The advent of immune checkpoint inhibitors (ICIs)
represents a groundbreaking development in cancer treatment. ICIs
have found extensive application in various forms of cancer
therapy, including palliative treatment, neoadjuvant therapy and
adjuvant therapy. Nevertheless, the efficacy of ICIs as a
monotherapy is limited, as evidenced by a response rate of 20% or
lower in patients with cancer (1-3).
It has been reported in several preclinical studies that the
combination of ICIs with radiotherapy or chemotherapy is
increasingly being employed since it can lead to the release of
tumor antigens and result in a therapeutic synergistic effect
(4,5). As of November 2023, more than 600
clinical trials of radiotherapy in combination with ICIs had been
registered in the National Institutes of Health clinical trials
(clinicaltrials.gov). However, due to the
potential overlap in pulmonary toxicity induced by thoracic
radiotherapy and ICIs, studies have also reported an elevated
incidence of pneumonitis (6,7). In
a comprehensive examination of 1,113 patients diagnosed with
non-small cell lung cancer across 11 clinical studies, it was
observed that concurrent treatment exhibited a higher occurrence of
adverse pneumonitis at all grades (25.8 vs. 21.3%) compared with
sequential therapy. Therefore, modifying the timing of medication
administration has emerged as a potential strategy for mitigating
lung injury.
The neutrophil-to-lymphocyte ratio (NLR) serves as a
marker of systemic inflammation. It has previously been used as a
robust indicator to assess the severity of community-acquired
pneumonia (8), chronic obstructive
pulmonary disease (9) and
COVID-19(10). Moreover, a
previous study has revealed that during thoracic radiotherapy, NLR
levels were elevated in patients who developed pneumonitis
following radiotherapy (11).
Additionally, increased NLR levels during ICIs treatment also
served as a biomarker for early diagnosis of checkpoint
inhibitor-related pneumonitis (CIP) in a recent study (12).
As the combination of thoracic radiotherapy and ICIs
becomes more widely used, it is imperative to address two
noteworthy aspects: The optimal timing for administering these
treatments and the non-invasive methodology for assessing lung
injury. To that end, patients that underwent thoracic radiotherapy
plus immunotherapy were retrospectively analyzed and animal models
were established to evaluate the effect of timing of combination
therapy on the occurrence of pneumonitis, and it was observed that
NLR is a promising predictor of lung inflammation caused by ICIs
combined with radiotherapy.
Materials and methods
Patients
Patients who underwent thoracic radiotherapy
combined with ICIs treatments at the Central Hospital Affiliated to
Shandong First Medical University (Jinan, China) between January
2019 and May 2022 were reviewed. The sites targeted for
radiotherapy encompassed intrapulmonary or mediastinal tumors, such
as primary lung cancer, metastatic lung cancer and esophageal
cancer, among others. All patients were treated with
intensity-modulated radiotherapy using a linear accelerator
(Synergy/Infinity, Elekta, Sweden). The purpose of radiotherapy
included radical or palliative therapy. The present study was
approved (approval no. R202303060092) by the Ethical Committee of
Central Hospital Affiliated to Shandong First Medical University
(Jinan, China). Oral and written informed consents were obtained
from patients and their surrogates in person.
Herein, ICIs agents included antibodies targeting
programmed cell death protein 1 (PD-1) or programmed death-ligand
1. There exist two distinct classifications of sequential approach.
The first category bore resemblance to the study of Antonia et
al (13), wherein patients
were administered ICIs subsequent to the completion of a
radiotherapy course. The second category entailed the suspension of
ICIs during the initiation of radiotherapy. Meanwhile, the
concurrent treatment approach followed that of the ETOP NICOLAS
trial (14) and Keynote-799 study
(15), with ICIs being used during
radiotherapy.
Data collection and outcome
assessment
Patients and treatment characteristics were
retrospectively collected from each patient's medical records,
including patient demographics, smoking history, tumor types and
Eastern Cooperative Oncology Group Performance Status (ECOG PS).
Lung V20 (the percentage of lung volume received >20 Gy), the
mean lung dose (MLD) and total dose were evaluated on the treatment
planning workstation.
Diagnosis of treatment-related
pneumonitis (TRP)
The patients underwent evaluation one month
following radiotherapy, with subsequent evaluations conducted
concurrently with tumor assessment. Additional examinations were
conducted in patients exhibiting respiratory disease-related
symptoms. TRP was diagnosed by experienced pulmonologists and
radiologists. TRP was defined as new-onset infiltrates on thoracic
imaging and/or clinical symptoms such as cough, shortness of
breath, or wheezing, while excluding other etiologies (disease
progression, infection, or heart failure). Pneumonitis severity was
graded using the Common Toxicity Criteria for Adverse Events
version 5.0 (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf).
NLR was collected from a patient's blood routine at the time of
diagnosis of TRP.
Establishment of the acute radiation
lung injury mice model
A total of 16 male C57BL/6 mice (6-8 weeks old,
weighing ~18-22 g) were purchased from SiPeiFu Biotechnology Co.,
Ltd (Beijing, China). Mice were housed under standard light-dark
cycle (12/12-h light/dark cycle) and temperature (22±2˚C)
conditions with sterilized food and water provided ad
libitum, following institutional and office of laboratory
animal welfare guidelines. All animal experiments were approved
(approval no. JNCHIACUC2021-26) by the Ethical Committee of Central
Hospital Affiliated to Shandong First Medical University (Jinan,
China).
Irradiation
Mice were anesthetized by intraperitoneal injection
of pentobarbital sodium (50 mg/kg). The acute radiation pneumonitis
model was established following established protocols (16). Mice were anesthetized and exposed
to whole thorax radiation by X-ray at a dose rate of 600.00 cGy/min
and a cumulative radiation dose of 18 Gy from a linear accelerator
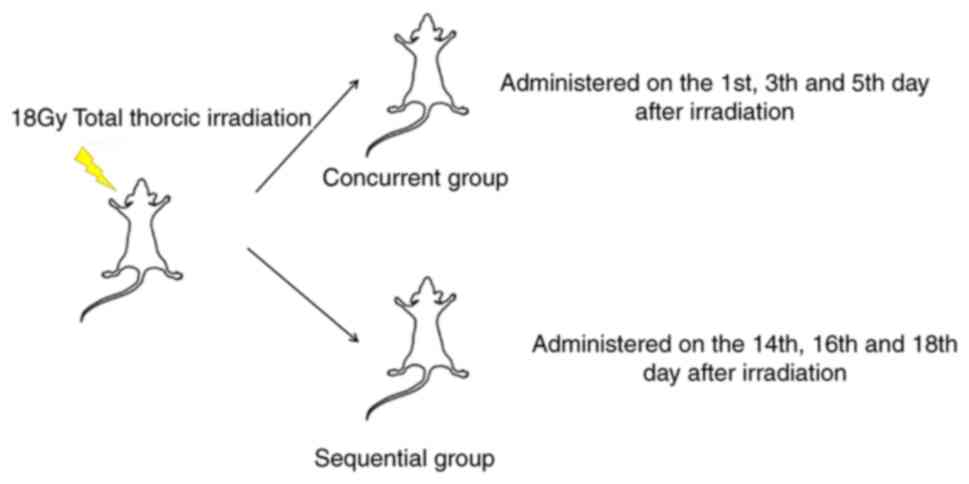
(Elekta Synergy, Sweden) at our institution. Mice were randomly
assigned to two groups prior to irradiation initiation: The
concurrent group and the sequential group (n=8 in each group).
PD-1 blockade is administered at
different intervals after irradiation
The two dosing strategies used in the present study
are demonstrated in Fig. 1. In the
concurrent group, the anti-PD-1 antibody (200 µg/mouse; Bio X Cell,
cat. no. BE0146; 1:12.5 resuspended with fresh PBS) was
administered intraperitoneally on days 1, 3 and 5 after
irradiation. In the sequential group, the anti-PD-1 antibody was
injected intraperitoneally on days 14, 16, and 18 after
irradiation.
Sample collection
Mice were sacrificed by cervical dislocation on the
28th day after irradiation. The lungs were then removed and
immersed in 4% paraformaldehyde for 48 h before being embedded in
paraffin. Peripheral blood samples were collected from the mice
after euthanasia via the orbital sinus using
ethylenediaminetetraacetic acid tubes. All mice were euthanized by
dislocating cervical vertebra under general anesthesia at the end
of experiments or if a humane endpoint was reached. Humane endpoint
was defined as the occurrence of severe dyspnea, vomiting,
inability to ambulate or rise for food and water, or a loss of
>15% of body weight. However, none of the animals reached these
humane endpoints.
Histopathological analysis
Histopathological changes were evaluated following
H&E staining. Alveolar congestion, hemorrhage, aggregation of
inflammatory cells in airspaces or vessel walls and the thickness
of the alveolar walls were assessed using a 0-4-point
semi-quantitative histological analysis method (17) (4: Extremely serious; 3: Serious; 2:
Middle; 1: Slight; 0: Normal). In total, five fields of view were
randomly selected, and the histology score of each sample was
determined using an average of all the scores.
Circulating leukocytes were analyzed
using flow cytometry
Neutrophil counts were determined by quantifying
Ly6G+ CD11b+ cells in peripheral blood using
flow cytometric analysis, following established methodologies
(18). For the analysis of
lymphocyte populations in peripheral blood, a lymphoid gate
(low-side scatter) was applied to exclude cells of monocytic origin
(19). The following antibodies
were utilized: APC-Cy7 anti-mouse CD45 (cat. no. 557659; BD
Biosciences), PE anti-mouse CD11b (cat. no. 24965) and PerCP-cy5.5
anti-mouse ly6G (cat. no. 63460) both from Cell Signaling
Technology, Inc.). Cell counts were analyzed using a BD Canto II
flow cytometer, and the analysis was performed with FACS Diva
software (version 6.1.2; BD Biosciences).
Statistical analysis
Univariate and multivariate logistic regression
analyses were conducted to identify the independent risk factors
for TRP. In addition, the proportional hazard ratio (HR) and 95%
confidence intervals (CIs) were also calculated. Receiver operating
characteristic (ROC) curves were employed to assess the effects of
lung V20, total dose, MLD and NLR on TRP. Propensity score matching
(PSM) was adopted to match subjects in the concurrent and
sequential groups. A paired Student's t-test was performed after
matching. All data represent the mean ± standard error of Mean
(SEM) from at least three independent experiments. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were conducted using SPSS 27.0 software (IBM
Corp.) and/or Prism GraphPad 8.0 (Dotmatics).
Results
Patient characteristics
The clinicopathological characteristics of the 80
patients included in the present study are listed in Table I. Among them, 31 patients were
<70 years old, while the remaining were >70 years old. There
were 21 (26.25%) patients with an ECOG PS of 0, 42 (52.5%) patients
with a PS of 1, and 17 (21.25%) patients with a PS of 2. In terms
of tumor categories, a total of 31 instances of intrapulmonary
tumors were observed, constituting 38.75% of the cases,
predominantly manifesting as lung metastases. Additionally, there
were 29 occurrences of mediastinal tumors, encompassing esophageal
cancer, gastroesophageal junction tumors and mediastinal lymph node
metastases. A total of 20 cases involved both intrapulmonary and
mediastinal lymph node metastases, accounting for 25.00% of the
total. In total, three dose-volumetric parameters were selected:
Whole lung V20, total dose and MLD. Of the total, 59 patients had
V20 <20% (73.75%), while 21 patients had V20 ≥20% (26.25%). For
MLD, 63 patients (78.75%) had MLD <10 Gy, and 17 patients
(21.25%) had MLD ≥10 Gy. Regarding treatment approach, 14 patients
(17.50%) received concurrent ICIs and radiotherapy, while 66
patients did not undergo concurrent administration of thoracic
radiotherapy and ICIs. On peripheral blood testing onset of
pneumonitis (Mindary Blood Cell Analyzer), 44 (55.00%) had NLR
<5 and 36 (45.00%) had NLR ≥5. During follow-up period, a total
of 14 patients (17.50%) developed grade ≥2 pneumonitis.
 | Table IClinicopathological characteristics of
patients. |
Table I
Clinicopathological characteristics of
patients.
| Clinicopathological
characteristics | Total number
(n=80) | Percentage (%) |
|---|
| Age | | |
|
<70 | 31 | 38.75 |
|
≥70 | 49 | 61.25 |
| Sex | | |
|
Male | 52 | 65.00 |
|
Female | 28 | 35.00 |
| Smoking History | | |
|
None | 38 | 47.50 |
|
Yes | 42 | 52.50 |
| ECOG | | |
|
0 | 21 | 26.25 |
|
1 | 42 | 52.50 |
|
2 | 17 | 21.25 |
| Tumor types | | |
|
Intrapulmonary | 31 | 38.75 |
|
Mediastinal | 29 | 36.25 |
|
Both | 20 | 25.00 |
| V20 | | |
|
<20% | 59 | 73.75 |
|
≥20% | 21 | 26.25 |
| Therapeutic
modalities | | |
|
Concurrent | 14 | 17.50 |
|
Sequential | 66 | 82.50 |
|
Neutrophil-to-lymphocyte ratio | | |
|
<5 | 44 | 55.00 |
|
≥5 | 36 | 45.00 |
| Mean lung dose | | |
|
<10
Gy | 63 | 78.75 |
|
≥10 Gy | 17 | 21.25 |
| Grade ≥2
pneumonitis | | |
|
Yes | 14 | 17.50 |
|
No | 66 | 82.50 |
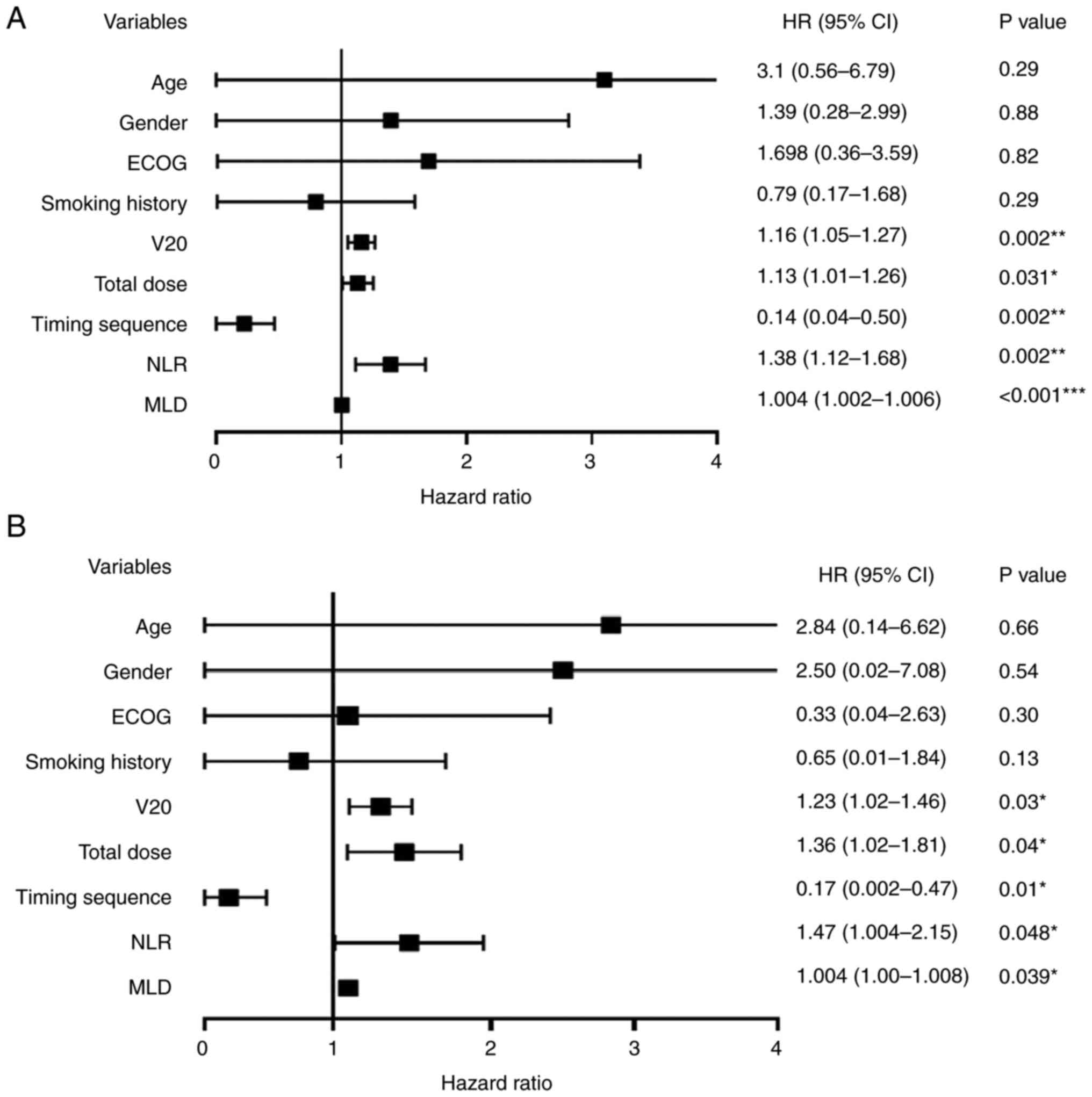
Univariate and multivariate analysis
of TRP
The relationships between TRP and clinical
characteristics are revealed in Fig.
2. In the univariate analysis, V20, total dose, MLD, sequence
of administration and NLR were found to be independent predictive
factors for TRP. Univariate logistic regression identified that the
HR value of whole lung V20 was 1.16 (95% CI, 1.05-1.27; P=0.002),
the HR value of MLD was 1.004 (95% CI, 1.002-1.006; P<0.001),
the HR value of total dose was 1.13 (95% CI, 1.01-1.26; P=0.031),
the HR value for administration sequence was 0.14 (95% CI,
0.04-0.5; P=0.002) and the HR value of NLR was 1.38 (95% CI,
1.12-1.68; P=0.002).
In the multivariate analysis, V20, MLD, total dose,
administered sequence and NLR remained independent predictive
factors for TRP. Multivariate logistic regression revealed that the
HR value of whole lung V20 was 1.23 (95% CI, 1.02-1.46; P=0.03),
the HR value of MLD was 1.004 (95% CI, 1.002-1.008; P=0.039), the
HR value of total dose was 1.36 (95% CI, 1.02-1.81; P=0.04), the HR
value of administration sequence was 0.17 (95% CI, 0.002-0.47;
P=0.01) and the HR value of NLR was 1.47 (95% CI, 1.004-2.15;
P=0.048).
ROC curve analyses were constructed to determine the
area under the ROC curve (AUC) for each variable (Fig. 3). Based on the results of the ROC
curve test, the AUC for V20 was 0.769 (22.87% was used as the
cutoff value). The AUC for total dose was 0.683, with 54 Gy used as
the cutoff value. The AUC for MLD was 0.786, with 10.47 Gy used as
the cutoff value. The AUC for NLR was 0.796, with 6.08 used as the
cutoff value.
Effect of combination therapy on lung
injury with different time intervals
To evaluate pathological changes, H&E staining
was utilized in mouse models (Fig.
4). The staining was quantified by pathologists to assess lung
injury. Notably, the concurrent treatment group exhibited more
severe lung tissue injury compared with the sequential treatment
group, with a score of 3.2±0.2 in the concurrent treatment group
vs. 2.2±0.3 in the sequential treatment group (P<0.05).
NLR in patients and animal models with
different treatment timing sequence
In the patients reviewed, baseline characteristics
were not initially balanced. To address this, PSM was performed
using SPSS software (version 27.0; IBM Corp.). Ultimately, 26
patients were successfully matched. A paired Student's t-test
revealed that the NLR in the concurrent treatment group was
significantly higher compared with the sequential group (T=2.27,
P=0.043) (Fig. 5A). In the animal
model of combination treatment, the NLR was 0.58±0.08 in the
concurrent treatment group and 0.26±0.06 in the sequential
treatment group (P<0.01) (Fig.
5B).
Discussion
The treatment approach of ICIs combined with
radiotherapy in several types of cancer has demonstrated
significant clinical benefits. Studies have reported that
irradiation may increase non-synonymous mutation burden and trigger
neoantigen production in cancer cells, possibly favoring in
situ vaccine development and tumor microenvironment (TME)
reprogramming (20). The
combination of ICIs with radiotherapy has been revealed to reverse
the suppressive TME, potentially making a significant difference in
cancer treatment. Currently, two combination therapy strategies are
widely employed clinically, namely sequential utilization of
radiotherapy and ICIs, and concurrent implementation of both
modalities. Previously, the therapeutic strategy of radiotherapy
combined with ICIs for thoracic cancer was centered on
understanding the influence of radiotherapy on the immune response
in patients (21,22). Nevertheless, it is essential to
acknowledge the deleterious effects induced by combination therapy
on patients' quality of life. Pneumonitis is a common and
potentially lethal complication in the treatment of patients with
thoracic tumors using radiotherapy or ICIs. Radiation-induced
pneumonitis stands out as a significant toxicity in thoracic
radiotherapy, occurring in ~5-15% of patients (23,24).
Although CIP is not frequently observed in patients treated with
ICIs, it remains the leading cause of ICI-related death, with a
fatality rate ranging from 10-17% (25). Due to the overlapping toxicity
profiles of the two treatment modalities in the lungs, interest has
been drawn to the approach of reducing lung toxicity when combining
these two treatments.
In the present study, an escalation was observed in
the severity of pulmonary inflammation when thoracic irradiation
and ICIs were administered concurrently. The collected data
revealed that among the examined cases, 50% (7/14) of patients in
the concurrent treatment group experienced grade 2 or higher
pneumonitis. By contrast, only 10.6% (7/66) of patients in the
sequential treatment group exhibited the same level of pneumonitis.
Furthermore, in mouse models of acute radiation pneumonitis, the
group receiving PD-1 within 14 days exhibited more severe lung
injury compared with the group receiving PD-1 after 14 days.
As the mechanism of pneumonitis in combination
therapy remains unclear, it is known that radiation induces
inflammatory cell infiltration (26), DNA damage and reactive oxygen
species generation, which further leads to the release of various
cytokines to promote inflammation (27,28).
It was reasoned that ICIs administered in this inflammatory
environment can lead to an immune-boosting effect through a series
of processes involving autoreactive lymphocytes, autoantibodies and
cytokines, such as IL-3, -6, -10 and -17, TNF-α and TGF-β (29). It was concluded that the possible
crosstalk among signaling pathways was inflammatory cell
infiltration and numerous cytokines released. Besides, the
administration of ICIs could also amplify the inflammatory response
in irradiated healthy tissues. After a certain period of time, the
local inflammatory response was reduced, using of ICIs might not
lead to the aforementioned inflammatory cascade. This could
potentially account for the relatively low prevalence of
pneumonitis observed in patients undergoing sequential
treatment.
In addition, the current investigation also delved
into the potential of NLR as a non-invasive measure for detecting
treatment-related lung injury. The present findings revealed that
V20, total dose, MLD and NLR were significant independent
predictors of treatment-associated pneumonitis. Based on the ROC
curves for V20, total dose, MLD and NLR, the optimal cut-off values
in the present study were determined to be 22.87%, 54 Gy, 10.47 Gy
and 6.08, respectively. Notably, a previous study reported
consistent cut-off values of 24% for V20 and 12.26 Gy for MLD in
relation to grade ≥2 radiation pneumonitis (30). Alongside dosimetric parameters, the
present study revealed that the NLR is an independent prognostic
factor for the development of treatment-associated pneumonitis in
combination therapies. Importantly, this was the inaugural
identification of such associations.
Previous studies have established that the NLR can
partially reflect the systemic inflammation status (31-33).
Given its demonstrated ability to reflect the severity of radiation
pneumonitis and predict the occurrence of CIP, it was hypothesized
that NLR could serve as a highly efficient indicator for
pneumonitis in patients receiving combination therapy. Herein, the
NLR value ≥6.08 was used as a reference to reflect lung injury in
patients treated with thoracic radiotherapy and ICIs. Furthermore,
in vivo models were used to further demonstrate that NLR
could effectively serve as an indicator of pulmonary damage
resulting from the administration of combination therapy.
Nonetheless, the present study has certain
limitations. Firstly, the study sample size was small, and patients
were not randomized. Following the matching process, the concurrent
and sequential groups consisted of only 26 patients each.
Additionally, the investigation did not explore the optimal timing
for administering ICIs in patients undergoing thoracic
radiotherapy. The study also lacked a dynamic observation of NLR,
preventing the establishment of a correlation between pre- and
post-treatment NLR values and pneumonitis occurrence. Lastly, while
it has been documented in numerous studies that NLR can serve as an
independent indicator for evaluating inflammatory status (34,35),
a more precise determination of inflammatory states can potentially
be achieved by combining NLR with comprehensive markers of
inflammation, including C-reactive protein, IL-6, IL-10, IL-17 and
other inflammatory cytokines.
In conclusion, the findings of the present study
indicated that the simultaneous administration of ICIs and thoracic
radiation therapy may elevate the likelihood of grade 2 and higher
pneumonitis. Additionally, the NLR exhibited promise as a
non-invasive method for monitoring lung damage in real-time during
combination therapy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Youth Project of
Natural Science Foundation of Shandong (grant no. ZR2022QH187), the
Shandong Medical and Health Technology Development Project (grant
no. 202104080454) and the Shandong Traditional Chinese Medicine
Science and Technology Project (grant no. Q-2022004).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AT conducted the data analysis and authored the
original draft. ZW was responsible for the collection and
visualization of clinical data. SW and QJ assumed oversight and
leadership in the research. XL generated pathology slides and
interpretation. FL and PY designed the study. PY and ZW performed
the animal experiments. PY provided funding. AT and PY confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
R202303060092) by the Ethical Committee of Central Hospital
Affiliated to Shandong First Medical University (Jinan, China).
Oral and written informed consents were obtained from patients and
their surrogates in person. The present study followed the
guidelines of the Declaration of Helsinki. All animal experiments
were approved (approval no. JNCHIACUC2021-26) by the Ethical
Committee of Central Hospital Affiliated to Shandong First Medical
University (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shitara K, Özgüroğlu M, Bang YJ, Di
Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic
C, Chung HC, et al: Pembrolizumab versus paclitaxel for previously
treated, advanced gastric or gastro-oesophageal junction cancer
(KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial.
Lancet. 392:123–133. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ikeda S, Goodman AM, Cohen PR, Jensen TJ,
Ellison CK, Frampton G, Miller V, Patel SP and Kurzrock R:
Metastatic basal cell carcinoma with amplification of PD-L1:
Exceptional response to anti-PD1 therapy. NPJ Genom Med.
1(16037)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sharabi AB, Nirschl CJ, Kochel CM, Nirschl
TR, Francica BJ, Velarde E, Deweese TL and Drake CG: Stereotactic
radiation therapy augments antigen-specific PD-1-Mediated antitumor
immune responses via cross-presentation of tumor antigen. Cancer
Immunol Res. 3:345–355. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Deng L, Liang H, Burnette B, Beckett M,
Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1
treatment synergistically promote antitumor immunity in mice. J
Clin Invest. 124:687–695. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Shaverdian N, Lisberg AE, Bornazyan K,
Veruttipong D, Goldman JW, Formenti SC, Garon EB and Lee P:
Previous radiotherapy and the clinical activity and toxicity of
pembrolizumab in the treatment of non-small-cell lung cancer: A
secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol.
18:895–903. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Theelen WSME, Peulen HMU, Lalezari F, van
der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I,
Niemeijer AN, de Langen AJ, et al: Effect of pembrolizumab after
stereotactic body radiotherapy vs pembrolizumab alone on tumor
response in patients with advanced non-small cell lung cancer:
Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA
Oncol. 5:1276–1282. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee H, Kim I, Kang BH and Um SJ:
Prognostic value of serial neutrophil-to-lymphocyte ratio
measurements in hospitalized community-acquired pneumonia. PLoS
One. 16(e0250067)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu J, Liu J and Zou Y: Relationship
between neutrophil-lymphocyte ratio and short-term prognosis in the
chronic obstructive pulmonary patients with acute exacerbation.
Biosci Rep. 39(BSR20190675)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li
C, Zhang M, Tan J, Xu Y, Song R, et al: Neutrophil-to-lymphocyte
ratio predicts critical illness patients with 2019 coronavirus
disease in the early stage. J Transl Med. 18(206)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee YH, Choi HS, Jeong H, Kang KM, Song
JH, Lee WS, Lee GW, Song HN, Kim HG, Kang MH, et al:
Neutrophil-lymphocyte ratio and a dosimetric factor for predicting
symptomatic radiation pneumonitis in non-small-cell lung cancer
patients treated with concurrent chemoradiotherapy. Clin Respir J.
12:1264–1273. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lin X, Deng H, Yang Y, Wu J, Qiu G, Li S,
Xie X, Liu M, Xie Z, Qin Y, et al: Peripheral blood biomarkers for
early diagnosis, severity, and prognosis of checkpoint
inhibitor-related pneumonitis in patients with lung cancer. Front
Oncol. 11(698832)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et
al: Overall survival with durvalumab after chemoradiotherapy in
stage III NSCLC. N Engl J Med. 379:2342–2350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Peters S, Felip E, Dafni U, Belka C,
Guckenberger M, Irigoyen A, Nadal E, Becker A, Vees H, Pless M, et
al: Safety evaluation of nivolumab added concurrently to
radiotherapy in a standard first line chemo-radiotherapy regimen in
stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung
Cancer. 133:83–87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jabbour SK, Lee KH, Frost N, Breder V,
Kowalski DM, Pollock T, Levchenko E, Reguart N, Martinez-Marti A,
Houghton B, et al: Pembrolizumab plus concurrent chemoradiation
therapy in patients with unresectable, locally advanced, stage III
non-small cell lung cancer: The phase 2 KEYNOTE-799 nonrandomized
trial. JAMA Oncol. 7:1–9. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gao J, Peng S, Shan X, Deng G, Shen L, Sun
J, Jiang C, Yang X, Chang Z, Sun X, et al: Inhibition of AIM2
inflammasome-mediated pyroptosis by Andrographolide contributes to
amelioration of radiation-induced lung inflammation and fibrosis.
Cell Death Dis. 10(957)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mikawa K, Nishina K, Takao Y and Obara H:
ONO-1714, a nitric oxide synthase inhibitor, attenuates
endotoxin-induced acute lung injury in rabbits. Anesth Analg.
97:1751–1755. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dave MN, Silva JE, Eliçabe RJ, Jeréz MB,
Filippa VP, Gorlino CV, Autenrieth S, Autenrieth IB and Di Genaro
MS: Yersinia enterocolitica YopH-Deficient strain activates
neutrophil recruitment to Peyer's patches and promotes clearance of
the virulent strain. Infect Immun. 84:3172–3181. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Loken MR, Brosnan JM, Bach BA and Ault KA:
Establishing optimal lymphocyte gates for immunophenotyping by flow
cytometry. Cytometry. 11:453–459. 1990.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Herrera FG, Bourhis J and Coukos G:
Radiotherapy combination opportunities leveraging immunity for the
next oncology practice. CA Cancer J Clin. 67:65–85. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Suzuki Y, Mimura K, Yoshimoto Y, Watanabe
M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T and Kono K:
Immunogenic tumor cell death induced by chemoradiotherapy in
patients with esophageal squamous cell carcinoma. Cancer Res.
72:3967–3976. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ma JL, Jin L, Li YD, He CC, Guo XJ, Liu R,
Yang YY and Han SX: The intensity of radiotherapy-elicited immune
response is associated with esophageal cancer clearance. J Immunol
Res. 2014(794249)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Segawa Y, Takigawa N, Kataoka M, Takata I,
Fujimoto N and Ueoka H: Risk factors for development of radiation
pneumonitis following radiation therapy with or without
chemotherapy for lung cancer. Int J Radiat Oncol Biol Phys.
39:91–98. 1997.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kong FM, Hayman JA, Griffith KA,
Kalemkerian GP, Arenberg D, Lyons S, Turrisi A, Lichter A, Fraass
B, Eisbruch A, et al: Final toxicity results of a radiation-dose
escalation study in patients with non-small-cell lung cancer
(NSCLC): Predictors for radiation pneumonitis and fibrosis. Int J
Radiat Oncol Biol Phys. 65:1075–1086. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cadranel J, Canellas A, Matton L, Darrason
M, Parrot A, Naccache JM, Lavolé A, Ruppert AM and Fallet V:
Pulmonary complications of immune checkpoint inhibitors in patients
with nonsmall cell lung cancer. Eur Respir Rev.
28(190058)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Klein D, Steens J, Wiesemann A, Schulz F,
Kaschani F, Röck K, Yamaguchi M, Wirsdörfer F, Kaiser M, Fischer
JW, et al: Mesenchymal stem cell therapy protects lungs from
radiation-induced endothelial cell loss by restoring superoxide
dismutase 1 expression. Antioxid Redox Signal. 26:563–582.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Azzam EI, Jay-Gerin JP and Pain D:
Ionizing radiation-induced metabolic oxidative stress and prolonged
cell injury. Cancer Lett. 327:48–60. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Abratt RP, Morgan GW, Silvestri G and
Willcox P: Pulmonary complications of radiation therapy. Clin Chest
Med. 25:167–177. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhai X, Zhang J, Tian Y, Li J, Jing W, Guo
H and Zhu H: The mechanism and risk factors for immune checkpoint
inhibitor pneumonitis in non-small cell lung cancer patients.
Cancer Biol Med. 17:599–611. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sheng L, Cui X, Cheng L, Chen Y and Du X:
Risk factors of grade ≥ 2 radiation pneumonitis after gemcitabine
induction chemotherapy for patients with non-small cell lung
cancer. Radiat Oncol. 14(229)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yuan C, Pan Y and Ning Y: Predictive Value
of IL-6 Combined with NLR in inflammation and cancer. Cancer
Invest. 39:489–504. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cheng H, Luo G, Lu Y, Jin K, Guo M, Xu J,
Long J, Liu L, Yu X and Liu C: The combination of systemic
inflammation-based marker NLR and circulating regulatory T cells
predicts the prognosis of resectable pancreatic cancer patients.
Pancreatology. 16:1080–1084. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mundama M, Van Cauter M, Detrembleur C,
Cornu O, Dubuc JE and Yombi JC: Neutrophil-to-lymphocyte ratio
(NLR) distribution shows an advantage compared to C-reactive
protein (CRP) for the early inflammation monitoring after total hip
arthroplasty. Acta Orthop Belg. 86:405–411. 2020.PubMed/NCBI
|
|
34
|
Huang Z, Fu Z, Huang W and Huang K:
Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A
meta-analysis. Am J Emerg Med. 38:641–647. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Buonacera A, Stancanelli B, Colaci M and
Malatino L: Neutrophil to lymphocyte ratio: An emerging marker of
the relationships between the immune system and diseases. Int J Mol
Sci. 23(3636)2022.PubMed/NCBI View Article : Google Scholar
|