Introduction
Low-grade serous ovarian cancer (LGSOC) is an
understudied rare disease that is a distinct pathological and
clinical entity representing 2% of all epithelial ovarian cancer
cases and 4.7% of serous ovarian cancer cases globally (1). LGSOC is associated with reduced
aggressive biological behavior, a lower sensitivity to chemotherapy
and a more prolonged overall survival (OS) time compared with
high-grade serous ovarian carcinoma (HGSOC) (2). Due to changes in the diagnostic
criteria and the consequent diagnostic shift from LGSOC to serous
borderline ovarian tumors (SBOTs), the proportion of LGSOC
diagnoses has decreased by 3-4% per year while the proportion of
SBOTs has increased (3).
Microscopically, LGSOC is characterized by a
consistent population of cuboidal, low columnar and occasionally
flattened cells with an amphophilic or lightly eosinophilic
cytoplasm. In addition, LGSOC is associated with mild to moderate
atypia, with ≤12 mitoses per 10 high-power fields. Destructive
invasion by neoplastic cells can be detected in a ≥3.0 mm area
(linear dimension) of the tumor/ovarian stroma or in an area with
desmoplasia. Furthermore, psammoma bodies occur often in LGSOC and
with high frequency (4). The
presence of invasive implants in patients with ovarian serous
borderline neoplasms is now classified as LGSOC due to similar
overall survival times (5).
Age at diagnosis, body mass index (BMI), smoking
status and stage at diagnosis are all considered important
prognostic factors in women diagnosed with LGSOC (6-8).
According to Shvartsman et al (9), women with de novo LGSOC have a
similar survival time to those who had a prior SBOT that underwent
a malignant transformation to LGSOC.
Optimal cytoreductive surgery is the cornerstone in
the primary management of all stages of LGSOC. If unresectable
disease is found or if the patient is not fit for surgery (due to
comorbidities, age or nutritional status), then treatment with
neoadjuvant chemotherapy and subsequent interval debulking surgery
may be considered once the disease has been histologically
confirmed (10). Adjuvant
therapies are not indicated for stage IA-IB after comprehensive
surgical staging (11). Notably,
LGSOC has an indolent behavior and appears to be less responsive to
chemotherapy, both as a first-line treatment and when used to treat
recurrence, compared with HGSOC (1,8,12-15).
However, women with LGSOC may benefit from hormonal treatment
(16-19).
Although it is relatively chemoresistant, adjuvant
platinum-based chemotherapy is still the standard of care for
LGSOC, while hormonal maintenance therapy following adjuvant
chemotherapy can confer an improved outcome. Disease recurrence may
be treated using secondary cytoreductive surgery, hormonal therapy,
chemotherapy, targeted therapy and therapies in clinical trials.
Notably, genomic studies and targeted therapies are expected to
bring about enhancements for the overall treatment of LGSOC
(4).
The present retrospective study aims to present the
experience of a single institute with regard to the management and
survival of a cohort of women diagnosed with LGSOC.
Patients and methods
Study design
The present study is a retrospective study with the
aim of reporting real-world, single-institution experiences of
LGSOC management, to evaluate the clinicopathological
characteristics, treatment and long-term survival of LGSOC, and to
determine prognostic factors affecting survival.
The primary objectives were to evaluate the efficacy
of treatment modalities used in cases of LGSOC in terms of
progression-free survival (PFS) and OS. The secondary objectives
were to assess baseline characteristics and prognostic factors
affecting survival.
Procedure and data collection
The medical records of patients with histologically
proven LGSOC diagnosed and treated at King Faisal Specialist
Hospital and Research Center (KFSHRC) (Riyadh, Saudi Arabia)
between January 2003 and December 2019 were reviewed. All patients
were diagnosed histologically at the institution, and those cases
that were initially diagnosed outside, but treated inside, the
institution were pathologically reviewed by pathologists of the
institution to confirm the diagnosis. Retrospectively, the
electronic charts of the patients were examined, and the following
information was entered in RedCap (https://redcap.kfshrc.edu.sa): Patient demographics,
presenting symptoms and signs, International Federation of
Gynecology and Obstetrics (FIGO) stage (20), histology, subsequent management and
outcome.
Patient characteristics were collected, such as age,
clinical presentation, parity, Eastern Cooperative Oncology Group
Performance Status (ECOG PS) score (21), BMI as per World Health scale
(underweight, <18.5 kg/m2; normal weight, 18.5-24.9
kg/m2; overweight, 25-29.9 kg/m2; obese,
30.0-30.9 kg/m2; and extremely obese, ≥40
kg/m2) (22),
Tumor-Node-Metastasis staging (23), FIGO staging at initial
presentation, surgery, surgical outcome, adjuvant therapy, response
rate, disease progression, and survival outcome. Radiographic
responses were assessed retrospectively by a radiologist according
to Response Evaluation Criteria in Solid Tumors v1.1(24). The response in patients with
non-measurable disease was categorized according to the decision
made by the treating physician.
Statistical analysis
Patient characteristics are presented using
frequencies with percentages for categorical variables and medians
with interquartile ranges for continuous variables. Fisher's exact
test was performed to test the distribution of different risk
factors among treatment groups. Probabilities of OS were summarized
using the Kaplan-Meier estimator with variance calculated using the
Greenwood formula. Survival curves were compared using the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference. All statistics were performed using
SPSS® Version 20 for Windows (IBM Corp.).
PFS time was calculated from the start of treatment
until the date of radiological progression, death, or last
follow-up. OS time was calculated from the start of treatment to
death or last follow-up. Patients lost to follow-up were censored
at the date of their last follow-up. PFS and OS were analyzed
according to age, BMI (<25 or ≥25), primary site (unilateral
ovarian, bilateral ovarian or primary peritoneal cancer), cancer
antigen 125 (CA125) level (normal ≤35 U/ml or high >35 U/ml),
optimal surgery (<1 cm residual) vs. suboptimal surgery (>1
cm residual), and presence of lymphovascular invasion (LVI) or
perineural invasion (PNI).
Ethical considerations
This project was conducted according to the
principles of the Declaration of Helsinki (2000), Good Clinical
Practice Guidelines, and the policies and guidelines of the
Research Advisory Council at KFSHRC, and was approved by the
Medical Ethics Committee (approval no. 2231168). The identities of
the patients remained anonymous, since no identifying data or
protected health information was recorded. All data were
password-secured to safeguard the confidentiality of the collected
patient data. This research was approved for publication by the
Office of Research Affairs, and as per the internal regulations of
KFSHRC, all authors read and approved the final manuscript.
Results
A total of 23 female patients diagnosed with LGSOC
and treated at KFSHRC were identified. The patient characteristics
are shown in Table I. The median
age at diagnosis was 45.5 years (range, 26-66 years) and the median
BMI was 26.1 kg/m2 (range, 18-43 kg/m2).
Notably, most patients (78.3%) had an ECOG PS of 0-1 at diagnosis.
Most of the patients (73.9%) presented with abdominal pain. In
addition, 5 patients (21.7%) presented with constitutional
symptoms, 2 patients (8.7%) with urinary symptoms, 1 patient (4.3%)
with dysmenorrhea, 2 patients (8.7%) with infertility, 2 patients
(8.7%) with pelvic symptoms, 1 patient (4.3%) with gastric outlet
obstruction and 1 patient (4.3%) with pulmonary embolism. A total
of 9 patients (39.1%) were asymptomatic and were diagnosed
incidentally, and those patients underwent optimal debulking
surgery. A total of 21 patients (91.3%) had de novo LGSOC,
whereas only 2 patients (8.7%) had LGSOC that had transformed from
SBOT and recurred. A total of 8 patients (34.8%) were diagnosed
with FIGO stage IV, and 3 (13.0%), 3 (13.0%) and 9 (39.1%) were
diagnosed with stages I, II and III, respectively. Fisher's exact
test was performed to test the distribution of different risk
factors among treatment groups. However, no significant differences
were shown (Table II).
 | Table IPatient characteristics (n=23). |
Table I
Patient characteristics (n=23).
| Characteristic | Value |
|---|
| Age, years | |
|
Median | 45.5 |
|
Range | 26-66 |
| BMI,
kg/m2 | |
|
Median | 26.1 |
|
Range | 18-43 |
| ECOG PS, n (%) | |
|
0 | 8 (34.8) |
|
1 | 10 (43.5) |
|
2 | 3 (13.0) |
|
Unknown | 2 (8.7) |
| Presenting
symptoms, n (%) | |
|
Abdominal
pain | 17 (73.9) |
|
Constitutional
symptoms | 5 (21.7) |
|
Pelvic
symptoms | 2 (8.7) |
|
Urinary
symptoms | 2 (8.7) |
|
Dysmenorrhea | 1 (4.3) |
|
Infertility | 2 (8.6) |
|
Gastric
outlet obstruction | 1 (4.3) |
|
Pulmonary
Embolism | 1 (4.3) |
|
Incidental
finding (asymptomatic) | 9 (39.1) |
| Parity, n (%) | |
|
Nulliparous | 6 (26.1) |
|
Multiparous | 13 (56.5) |
|
Unknown | 4 (17.4) |
| Comorbidities, n
(%) | |
|
PCOS | 1 (4.3) |
|
Hypertension | 4 (17.4) |
|
DM | 7 (30.4) |
|
Primary
infertility | 5 (21.7) |
| Ascites, n (%) | |
|
No | 8 (34.8) |
|
Yes | 11 (47.8) |
|
Unknown | 4 (17.4) |
| Primary site, n
(%) | |
|
Unilateral
ovarian | 9 (39.1) |
|
Bilateral
ovarian | 7 (30.4) |
|
Primary
peritoneal | 7 (30.4) |
| Baseline CA125,
U/ml | |
|
Median | 275.5 |
|
Range | 10.3-9482 |
| Anemia, n (%) | |
|
Yes | 7 (30.4) |
|
No | 16 (69.6) |
| Surgical outcome, n
(%) | |
|
Optimal
debulking <1 cm residual | 13 (56.5) |
|
Non-optimal
debulking >1 cm residual | 10 (43.5) |
| FIGO stage at
presentation, n (%) | |
|
I | 3 (13.0) |
|
II | 3 (13.0) |
|
III | 9 (39.1) |
|
IV | 8 (34.8) |
| T stage, n (%) | |
|
T1 | 2 (8.7) |
|
T2 | 2 (8.7) |
|
T3 | 7 (30.4) |
|
Tx | 12 (52.2) |
| N stage, n (%) | |
|
N0 | 4 (17.4) |
|
N1 | 1 (4.3) |
|
Nx | 18 (78.3) |
| M (stage), n
(%) | |
|
M0 | 10 (43.5) |
|
M1 | 8 (34.8) |
|
Mx | 5 (21.7) |
| Low-grade serous
type, n (%) | |
|
De novo low
grade | 21 (91.3) |
|
Transformed
from borderline | 2 (8.7) |
| LVI, n (%) | |
|
No | 3 (13.0) |
|
Yes | 3 (13.0) |
|
Unknown | 9 (39.1) |
|
Missing
data | 8 (34.8) |
| Response to first
line chemotherapy, n (%) | |
|
CR | 8 (34.8) |
|
PR | 3 (13.0) |
|
SD | 5 (21.7) |
|
PD | 3 (13.0) |
|
NA | 4 (17.4) |
 | Table IIDistribution of risk factors among
chemotherapy (n=15) and other treatment (n=8) groups. |
Table II
Distribution of risk factors among
chemotherapy (n=15) and other treatment (n=8) groups.
| Factor | Chemotherapy, n
(%) | Others, n (%) | P-value |
|---|
| Age, years | | | 0.612 |
|
≤40 | 8 (53.3) | 3 (37.5) | |
|
<40 | 7 (46.7) | 5 (62.5) | |
| BMI | | | 0.903 |
|
<25 | 6 (40.0) | 3 (37.5) | |
|
≥25 | 9 (60.0) | 5 (62.5) | |
| Stage | | | 0.627 |
|
I-II | 3 (20.0) | 3 (37.5) | |
|
III-IV | 12 (80.0) | 5 (62.5) | |
| CA125,
U/mla | | | |
|
≤35 | 0 (0.0) | 1 (14.3) | 0.433 |
|
>35 | 8 (100.0) | 6 (85.7) | |
| Primary tumor
sitea | | | 0.990 |
|
Bilateral | 2 (25.0) | 2 (28.6) | |
|
Unilateral | 6 (75.0) | 5 (71.4) | |
| Paritya | | | 0.992 |
|
Nulliparous | 3 (37.5) | 1 (25.0) | |
|
Multiparous | 5 (62.5) | 3 (75.0) | |
At a median follow-up time of 34 months [95%
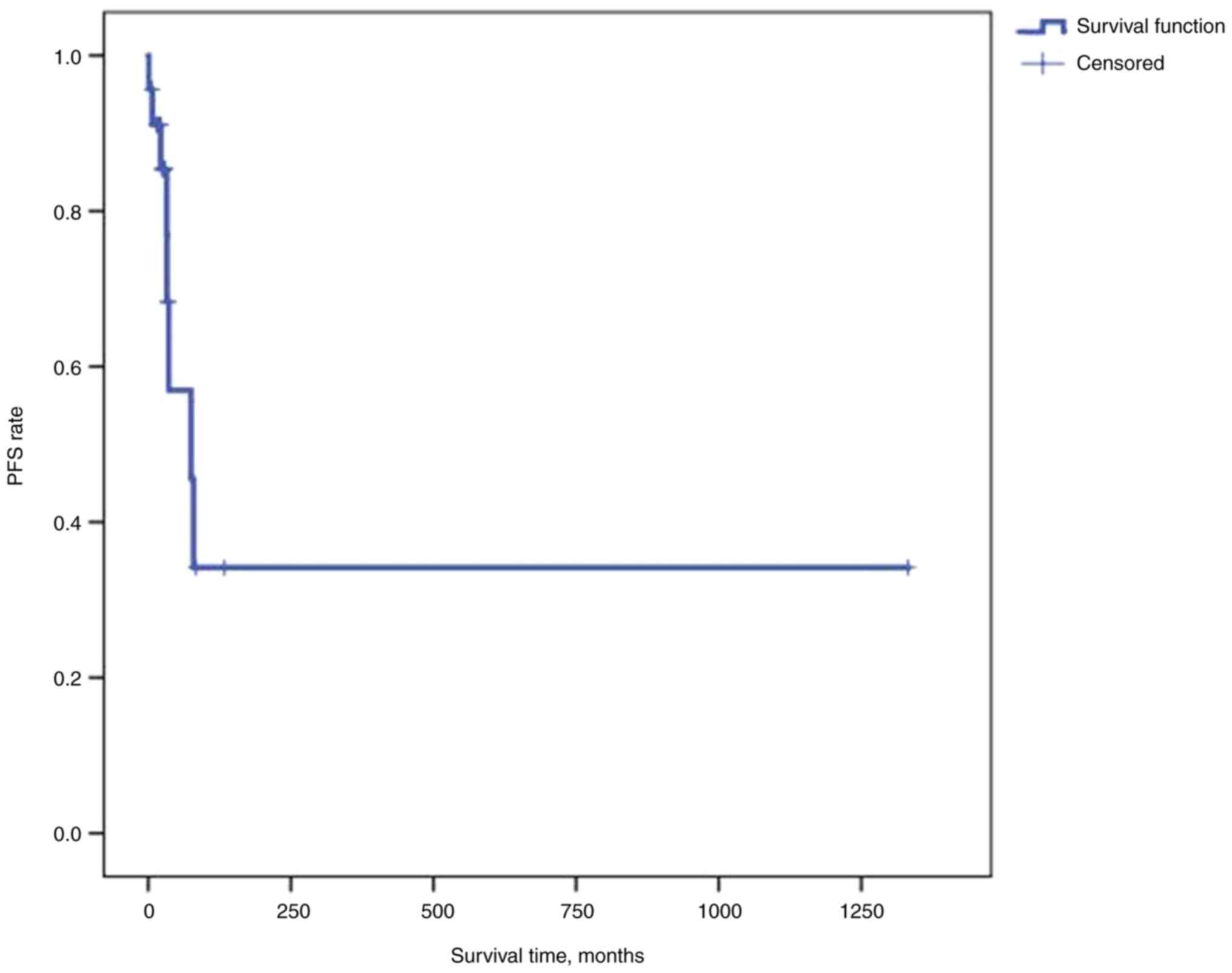
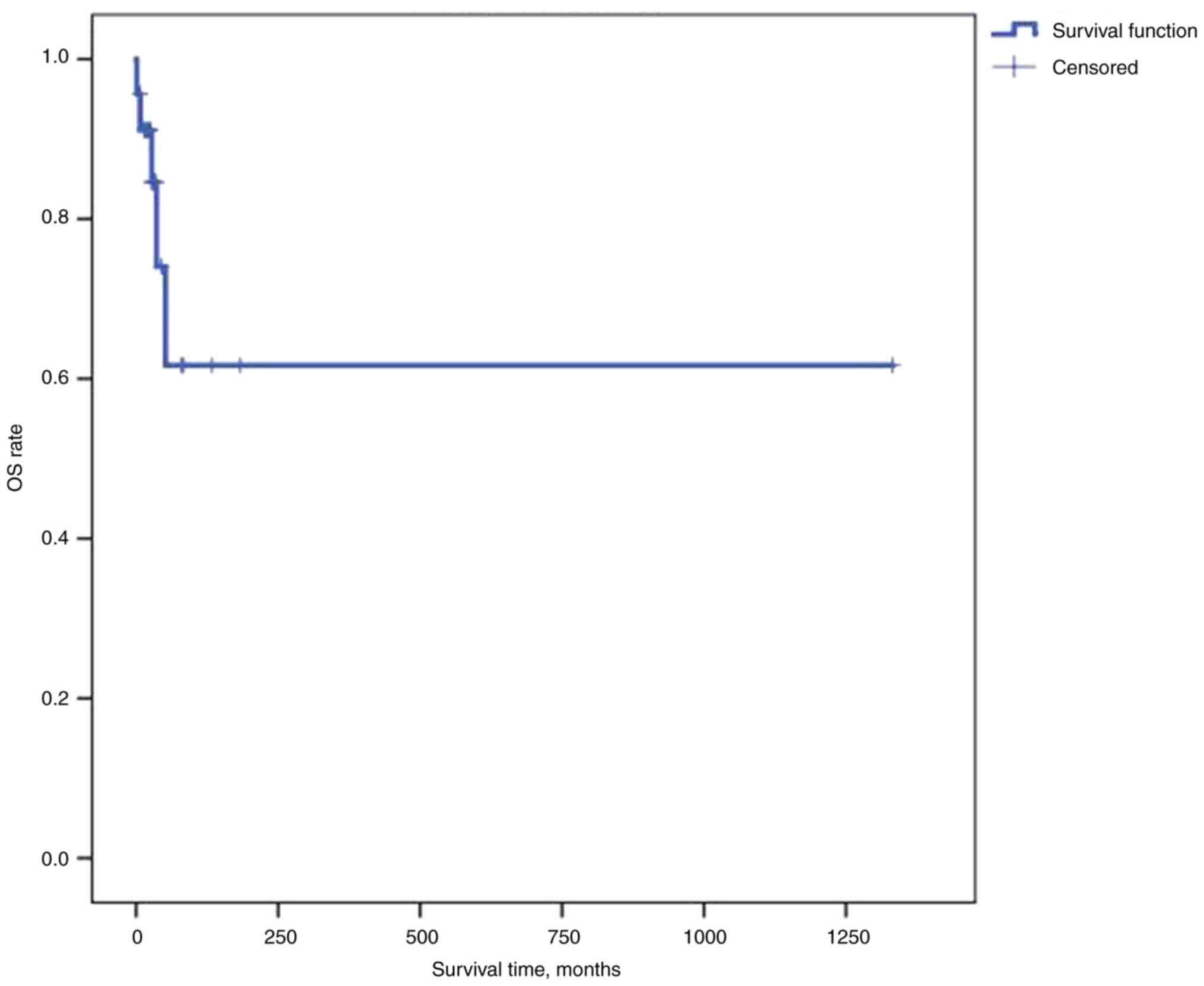
confidence (CI), 25.32-42.69], the median PFS time was 75.2 months
(95% CI, 17.35-133.05) and the median OS time was not reached
(Figs. 1 and 2). Univariate analysis of different
subgroups, including age, BMI (<25 or ≥25 kg/m2),
primary site (unilateral ovarian, bilateral ovarian, or primary
peritoneal cancer), CA125 (normal ≤35 U/ml or high >35 U/ml),
optimal surgery (<1 cm residual) vs. suboptimal surgery (>1
cm residual), and presence of LVI or PNI, showed no statistical
significance for PFS and OS. PFS time was significantly higher in
patients who received neoadjuvant chemotherapy (P=0.017); however,
a significant difference was not achieved for OS (Table III). Multivariate analysis was
not performed, as none of the proposed risk factors were
significant.
 | Table IIIUnivariate analysis of different
prognostic factors in patients with low-grade serous ovarian
cancer. |
Table III
Univariate analysis of different
prognostic factors in patients with low-grade serous ovarian
cancer.
| | PFS | OS |
|---|
| Factor | Median (95% CI),
months | P-value | Median OS (95% CI),
months | P-value |
|---|
| Chemotherapy | | 0.017 | | 0.251 |
|
Neoadjuvant | NR (-) | | NR (-) | |
|
Adjuvant | 32.361
(10.864-53.859) | | 51.647
(14.517-88.777) | |
| Surgery | | 0.801 | | 0.466 |
|
Optimal
debulking | 75.203
(32.228-118.175) | | NR (-) | |
|
Non-optimal
debulking | 36.074
(28.734-43.414) | | NR (-) | |
| BMI | | 0.804 | | 0.338 |
|
<25 | 36.074
(30.815-41.333) | | 51.647
(26.467-76.827) | |
|
≥25 | 4.400
(4.260-4.540) | | NR (-) | |
| CA125, U/ml | | 0.324 | | 0.232 |
|
<35 | 21.881a | | 27.335a | |
|
≥35 | 75.203
(18.000-132.400) | | NR (-) | |
| Laterality | | 0.190 | | 0.343 |
|
Bilateral | 75.203a | | NR (-) | |
|
Unilateral | 32.821
(0.000-75.824) | | NR (-) | |
| Parity | | 0.682 | | 0.421 |
|
Nulliparous | 75.203a | | NR (-) | |
|
Multiparous | NR (-) | | 51.647a | |
Discussion
LGSOC is a rare histological subtype of ovarian
cancer. Notably, women with this type of cancer are often younger
and exhibit prolonged survival times compared with those with HGSOC
(10). To the best of our
knowledge, the present study is the first to present data on LGSOC
from the Middle East. The median age of the patients at diagnosis
was 45.5 years, which is similar to the retrospective analysis
performed by Di Lorenzo et al (25). Patients with LGSOC can have
different clinical presentations, ranging from an asymptomatic
adnexal mass to abdominal pain and distension, with even more
symptoms often detected in advanced disease (4). In the present study, 9 patients
(39.1%) were asymptomatic; however, 17 (73.9%) had abdominal pain
at the initial presentation. In addition, obesity is associated
with a higher risk of ovarian cancer (26,27)
the median BMI for the patients in the present study was 26.
Different studies have shown that LGSOC is an
indolent disease with prolonged survival times compared with HGSOC
(4,8,28,29,30).
At a median follow-up time of 34 months (95% CI, 25.32-42.69) in
the present study, the median PFS time was 75.2 months (95% CI,
17.35-133.05) and the median OS was not reached. In a previous
retrospective study comparing the outcomes of women with HGSOC and
LGSOC, the median OS time was 40.7 months among patients with
high-grade tumors and 90.8 months among women with low-grade tumors
(29). The median OS time for
stage II-IV LGSOC as previously reported to be 97.8 months based on
information from the MD Anderson LGSOC Database (8). The median OS time for women with
stage III and IV HGSOC as reported to be 39 months in the
Gynecologic Oncology Group (GOG) 218 study (30).
Certain factors influence outcome in patients with
LGSOC, including age, FIGO stage and undergoing optimal
cytoreductive surgery (31,32).
In the present study, 43.5% of women had residual disease after
cytoreductive surgery, and ~87.0% of patients presented with
advanced stage (II-IV) disease. Patients aged ≤40 years comprised
34.8% of the study population. These three factors did not show a
statistically significant effect on PFS and OS in the study,
possibly due to it being an analysis of a rare disease in a small
number of patients. LGSOC of peritoneal origin occurred in 30.4% of
the patients; in general, this is associated with worse outcomes as
compared with LGSOC of ovarian origin (33,34).
LGSOC is considered relatively chemoresistant
compared with HGSOC (34). In one
study, the overall response rate was 4% and stable disease was
observed in >60% of treated patients (4). In the adjuvant setting, the overall
response rate, including complete and partial response, has been
reported to reach 25% in previous studies (15,27).
The complete response rate in the present study was 34.8%, mainly
as the patients underwent optimal surgical debulking and
subsequently received adjuvant chemotherapy. A partial response was
achieved in 13.0% of the patients and stable disease was achieved
in 21.7%.
Numerous studies have shown an increased incidence
of KRAS proto-oncogene GTPase and B-Raf proto-oncogene
serine/threonine kinase mutations with activation of the MAPK
pathway in LGSOC (35,36). MEK inhibitors represent a novel
therapeutic approach for patients with recurrent LGSOC. The GOG 281
study reported significantly improved PFS and response rates when
using trametinib compared with those when using single-agent
chemotherapy (37). Furthermore,
selumetinib showed a 15% response rate and a 65% stable disease
rate in a phase II study (38).
The limitations of the present study include the
retrospective design, long study period and different treatments
regarding systemic therapy and surgery (upfront and interval
debulking surgery). The retrospective nature of the study was a
major limiting factor with regard to sourcing detailed data about
the surgical procedure.
The main strengths of the study are the extended
follow-up, the pathological review conducted by an expert
pathologist in gynecological malignancies and the fact that the
surgery was performed by a talented surgeon in a center that sees a
high volume of gynecological oncology cases.
In conclusion, LGSOC is a rare type of malignant
ovarian cancer, which has a better PFS and OS times than HGSOC as
compared with data from the literature. Notably, there is still a
lack of precise guidance on the best type of systemic treatment
(chemotherapy or hormonal therapy) for LGSOC. In the present study,
optimal cytoreduction showed numerically higher, but
non-significant, PFS and OS times compared with suboptimal
debulking, which is similar to the increased times found in the
literature.
Acknowledgements
The abstract was presented at the European Society
of Gynecological Oncology Congress, March 7-10 2024 in Barcelona,
Spain, and published as abstract 391 in the International Journal
of Gynecological Cancer (39).
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HH, MAE and AB conceived the study and wrote the
proposal. BA, YS, AJ, AQ, ALSH, AYSH and IM collected the data. HH,
MAE, AB, BA, YS, AJ, AQ, ALSH, AYSH, TH and IM analyzed the data.
HH, MAE, AB, BA, YS, AJ, AQ, ALSH, AYSH, TH and IM confirm the
authenticity of all of the raw data. HH and MAE wrote the first
draft of the manuscript. All authors critically revised the
manuscript for important intellectual content, and have read and
approved the final version.
Ethics approval and consent to
participate
All methods followed the relevant guidelines and
regulations. The study was approved, and the requirement for
informed patient consent was waived by, the Research Advisory
Council at King Faisal Specialist Hospital and Research Centre
(Riyadh, Saudi Arabia; approval no. 2231168).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Slomovitz B, Gourley C, Carey MS, Malpica
A, Shih IM, Huntsman D, Fader AN, Grisham RN, Schlumbrecht M, Sun
CC, et al: Low-grade serous ovarian cancer: State of the science.
Gynecol Oncol. 156:715–725. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zwimpfer TA, Tal O, Geissler F, Coelho R,
Rimmer N, Jacob F and Heinzelmann-Schwarz V: Low grade serous
ovarian cancer-a rare disease with increasing therapeutic options.
Cancer Treat Rev. 112(102497)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Matsuo K, Machida H, Grubbs BH, Matsuzaki
S, Klar M, Roman LD, Sood AK and Gershenson DM: Diagnosis-shift
between low-grade serous ovarian cancer and serous borderline
ovarian tumor: A population-based study. Gynecol Oncol. 157:21–28.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Babaier A, Mal H, Alselwi W and Ghatage P:
Low-grade serous carcinoma of the ovary: The current status.
Diagnostics. 12(458)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McKenney JK, Gilks CB, Kalloger S and
Longacre TA: Classification of extraovarian implants in patients
with ovarian serous borderline tumors (tumors of low malignant
potential) based on clinical outcome. Am J Surg Pathol.
40:1155–1164. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kaldawy A, Segev Y, Lavie O, Auslender R,
Sopik V and Narod SA: Low-grade serous ovarian cancer: A review.
Gynecol Oncol. 143:433–438. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Canaz E, Grabowski JP, Richter R, Braicu
EI, Chekerov R and Sehouli J: Survival and prognostic factors in
patients with recurrent low-grade epithelial ovarian cancer: An
analysis of five prospective phase II/III trials of NOGGO metadata
base. Gynecol Oncol. 154:539–546. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gershenson DM, Bodurka DC, Lu KH, Nathan
LC, Milojevic L, Wong KK, Malpica A and Sun CC: Impact of age and
primary disease site on outcome in women with low-grade serous
carcinoma of the ovary or peritoneum: Results of a large
single-institution registry of a rare tumor. J Clin Oncol.
33:2675–2682. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shvartsman HS, Sun CC, Bodurka DC, Mahajan
V, Crispens M, Lu KH, Deavers MT, Malpica A, Silva EG and
Gershenson DM: Comparison of the clinical behavior of newly
diagnosed stages II-IV low-grade serous carcinoma of the ovary with
that of serous ovarian tumors of low malignant potential that recur
as low-grade serous carcinoma. Gynecol Oncol. 105:625–629.
2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ricciardi E, Baert T, Ataseven B, Heitz F,
Prader S, Bommert M, Schneider S, du Bois A and Harter P: Low-grade
Serous ovarian carcinoma. Geburtshilfe Frauenheilkd. 78:972–976.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gadducci A and Cosio S: Therapeutic
approach to low-grade serous ovarian carcinoma: State of art and
perspectives of clinical research. Cancers (Basel).
12(1336)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schlumbrecht MP, Sun CC, Wong KN, Broaddus
RR, Gershenson DM and Bodurka DC: Clinicodemographic factors
influencing outcomes in patients with low-grade serous ovarian
carcinoma. Cancer. 117:3741–3749. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Grabowski JP, Harter P, Heitz F,
Pujade-Lauraine E, Reuss A, Kristensen G, Ray-Coquard I, Heitz J,
Traut A, Pfisterer J and du Bois A: Operability and chemotherapy
responsiveness in advanced low-grade serous ovarian cancer. An
analysis of the AGO Study Group metadatabase. Gynecol Oncol.
140:457–462. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bristow RE, Gossett DR, Shook DR, Zahurak
ML, Tomacruz RS, Armstrong DK and Montz FJ: Recurrent
micropapillary serous ovarian carcinoma. Cancer. 95:791–800.
2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gershenson DM, Sun CC, Bodurka D, Coleman
RL, Lu KH, Sood AK, Deavers M, Malpica AL and Kavanagh JJ:
Recurrent low-grade serous ovarian carcinoma is relatively
chemoresistant. Gynecol Oncol. 114:48–52. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Escobar J, Klimowicz AC, Dean M, Chu P,
Nation JG, Nelson GS, Ghatage P, Kalloger SE and Köbel M:
Quantification of ER/PR expression in ovarian low-grade serous
carcinoma. Gynecol Oncol. 128:371–376. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fader AN, Bergstrom J, Jernigan A, Tanner
EJ III, Roche KL, Stone RL, Levinson KL, Ricci S, Wethingon S, Wang
TL, et al: Primary cytoreductive surgery and adjuvant hormonal
monotherapy in women with advanced low-grade serous ovarian
carcinoma: Reducing overtreatment without compromising survival?
Gynecol Oncol. 147:85–91. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gershenson DM, Bodurka DC, Coleman RL, Lu
KH, Malpica A and Sun CC: Hormonal maintenance therapy for women
with low-grade serous cancer of the ovary or peritoneum. J Clin
Oncol. 35:1103–1111. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tang M, O'Connell RL, Amant F, Beale P,
McNally O, Sjoquist KM, Grant P, Davis A, Sykes P, Mileshkin L, et
al: PARAGON: A phase II study of anastrozole in patients with
estrogen receptor-positive recurrent/metastatic low-grade ovarian
cancers and serous borderline ovarian tumors. Gynecol Oncol.
154:531–538. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Prat J: FIGO's staging classification for
cancer of the ovary, fallopian tube, and peritoneum: Abridged
republication. J Gynecol Oncol. 26(87)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Azam F, Latif MF, Farooq A, Tirmazy SH,
AlShahrani S, Bashir S and Bukhari N: Performance status assessment
by using ECOG (eastern cooperative oncology group) score for cancer
patients by oncology healthcare professionals. Case Rep Oncol.
12:728–736. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Purnell JQ: Definitions, Classification,
and Epidemiology of Obesity, 2000.
|
|
23
|
Olawaiye AB, Baker TP, Washington MK and
Mutch DG: The new (version 9) American joint committee on cancer
tumor, node, metastasis staging for cervical cancer. CA Cancer J
Clin. 71:287–298. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Di Lorenzo P, Conteduca V, Scarpi E,
Adorni M, Multinu F, Garbi A, Betella I, Grassi T, Bianchi T, Di
Martino G, et al: Advanced low grade serous ovarian cancer: A
retrospective analysis of surgical and chemotherapeutic management
in two high volume oncological centers. Front Oncol.
12(970918)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Olsen CM, Green AC, Whiteman DC, Sadeghi
S, Kolahdooz F and Webb PM: Obesity and the risk of epithelial
ovarian cancer: A systematic review and meta-analysis. Eur J
Cancer. 43:690–709. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu Z, Zhang TT, Zhao JJ, Qi SF, Du P, Liu
DW and Tian QB: The association between overweight, obesity and
ovarian cancer: A meta-analysis. Jpn J Clin Oncol. 45:1107–1115.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cobb L and Gershenson D: Novel
therapeutics in low-grade serous ovarian cancer. Int J Gynecol
Cancer. 33:377–384. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gockley A, Melamed A, Bregar AJ, Clemmer
JT, Birrer M, Schorge JO, Del Carmen MG and Rauh-Hain JA: Outcomes
of women with high-grade and low-grade advanced-stage serous
epithelial ovarian cancer. Obstet Gynecol. 129:439–447.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ferriss JS, Java JJ, Bookman MA, Fleming
GF, Monk BJ, Walker JL, Homesley HD, Fowler J, Greer BE, Boente MP
and Burger RA: Ascites predicts treatment benefit of bevacizumab in
front-line therapy of advanced epithelial ovarian, fallopian tube
and peritoneal cancers: An NRG oncology/GOG study. Gynecol Oncol.
139:17–22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fader AN, Java J, Ueda S, Bristow RE,
Armstrong DK, Bookman MA and Gershenson DM: Gynecologic Oncology
Group (GOG)*. Survival in women with grade 1 serous ovarian
carcinoma. Obstet Gynecol. 122:225–232. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kimyon Comert G, Turkmen O, Mesci CG,
Karalok A, Sever O, Sinaci S, Boran N, Basaran D and Turan T:
Maximal cytoreduction is related to improved disease-free survival
in low-grade ovarian serous carcinoma. Tumori. 105:259–264.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Schnack TH, Sørensen RD, Nedergaard L and
Høgdall C: Demographic clinical and prognostic characteristics of
primary ovarian, peritoneal and tubal adenocarcinomas of serous
histology-a prospective comparative study. Gynecol Oncol.
135:278–84. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Usach I, Blansit K, Chen LM, Ueda S,
Brooks R, Kapp DS and Chan JK: Survival differences in women with
serous tubal, ovarian, peritoneal, and uterine carcinomas. Am J
Obstet Gynecol. 212:188.e1–6. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Teneriello MG, Ebina M, Linnoila RI, Henry
M, Nash JD, Park RC and Birrer MJ: p53 and Ki-ras gene mutations in
epithelial ovarian neoplasms. Cancer Res. 53:3103–3108.
1993.PubMed/NCBI
|
|
36
|
Grisham RN, Iyer G, Garg K, Delair D,
Hyman DM, Zhou Q, Iasonos A, Berger MF, Dao F, Spriggs DR, et al:
BRAF Mutation is associated with early stage disease and improved
outcome in patients with low-grade serous ovarian cancer. Cancer.
119:548–554. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gershenson DM, Miller A, Brady WE, Paul J,
Carty K, Rodgers W, Millan D, Coleman RL, Moore KN, Banerjee S, et
al: Trametinib versus standard of care in patients with recurrent
low-grade serous ovarian cancer (GOG 281/LOGS): An international,
randomised, open-label, multicentre, phase 2/3 trial. Lancet.
399:541–553. 2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Farley J, Brady WE, Vathipadiekal V,
Lankes HA, Coleman R, Morgan MA, Mannel R, Yamada SD, Mutch D,
Rodgers WH, et al: Selumetinib in women with recurrent low-grade
serous carcinoma of the ovary or peritoneum: an open-label,
single-arm, phase 2 study. Lancet Oncol. 14:134–140.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Elshenawy MA, Badran A, Juhani AF Al,
Alshamsan B, Alsagaih Y, Alqayidi AA, Sheikh A, Alhassan T,
Maghfoor I, Elshentenawy A and Alhusseini H: 391 outcome and
prognostic factors of low-grade serous ovarian cancers: An
observational retrospective study. Int J Gynecol Cancer. 34 (Suppl
1):A324.2–A324. 2024.
|