Introduction
Although an osteoclast-like giant cell (OGC) tumor
of bone is capable of malignancy, its typical feature is that of a
benign tumor (1). OGC tumors in
other organs, by contrast, are malignant neoplasms. OGC tumors have
been found in several other organs including liver, breast,
gallbladder, and pancreas (2-6).
Among various histological subtypes of urothelial carcinoma (UC),
carcinoma with OGC resembling a giant cell tumor of bone is
extremely rare and only a few reports of urothelial carcinoma of
the bladder with osteoclast-like giant cells (UCOGCs) have been
reported (7-11).
The fifth edition of the WHO Urinary and Male Genital Tumours
classified UCOGCs as a poorly differentiated UC (12). The standard of care for and the
prediction of the prognosis of UCOGCs remain unclear due to limited
number of patients. To date, only one case report of UCOGCs with
genetic testing has been reported. As the clinical utility of
cancer gene panel testing in diagnosis and therapeutic
decision-making is widely recognized, further accumulation of
genomic results of UCOGCs is needed. Therefore, we performed
genomic analysis of invasive UC with OGCs using a cancer panel
test.
Case report
A 75-year-old man presented to Central Japan
International Medical Center in November 2022 with gross hematuria
and pain on voiding. Cystoscopy, computed tomography (CT), and
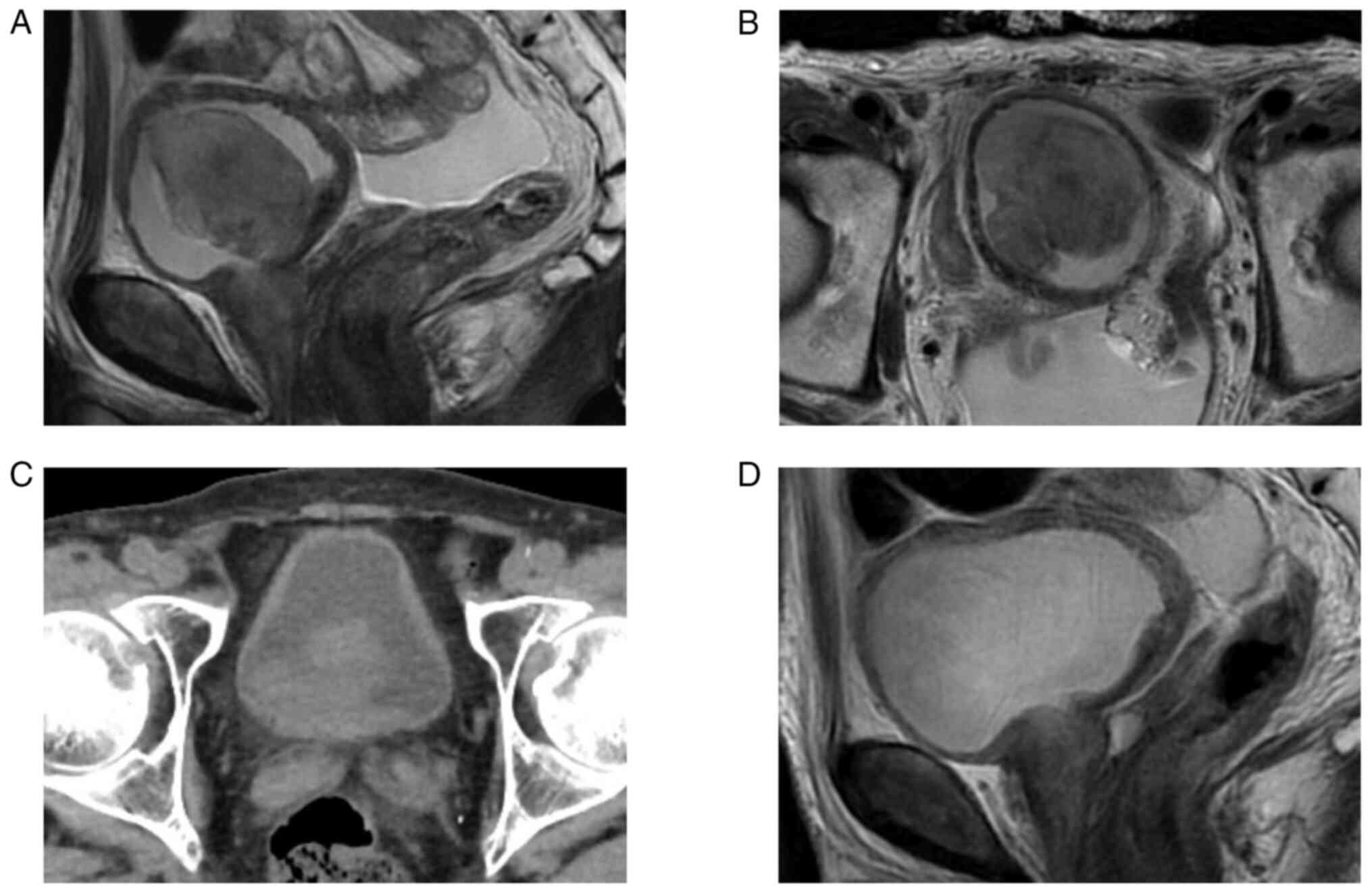
magnetic resonance imaging (MRI) revealed a bladder tumor of 56 mm
in diameter on the right wall of the bladder (Fig. 1A,B). Serum C-reactive protein (CRP)
level was 10.19 mg/l. As no muscle invasion or metastasis was
suspected, transurethral resection of the bladder tumor (TURBT) was
performed (resected tumor weight: 54 g). Hematoxylin and eosin
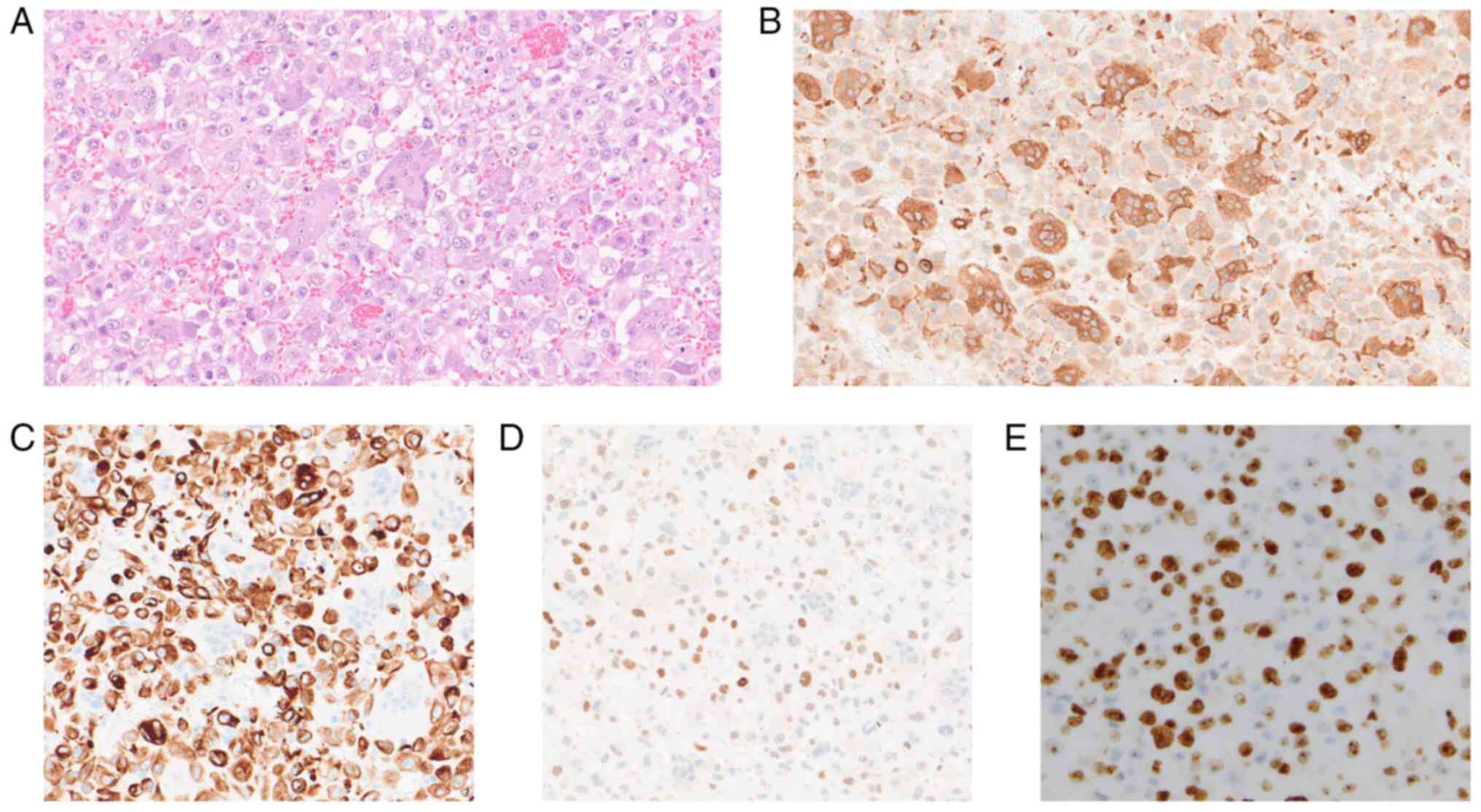
staining revealed that the tumor cells showed significant atypia
and were accompanied by multinucleated cells positive for CD68
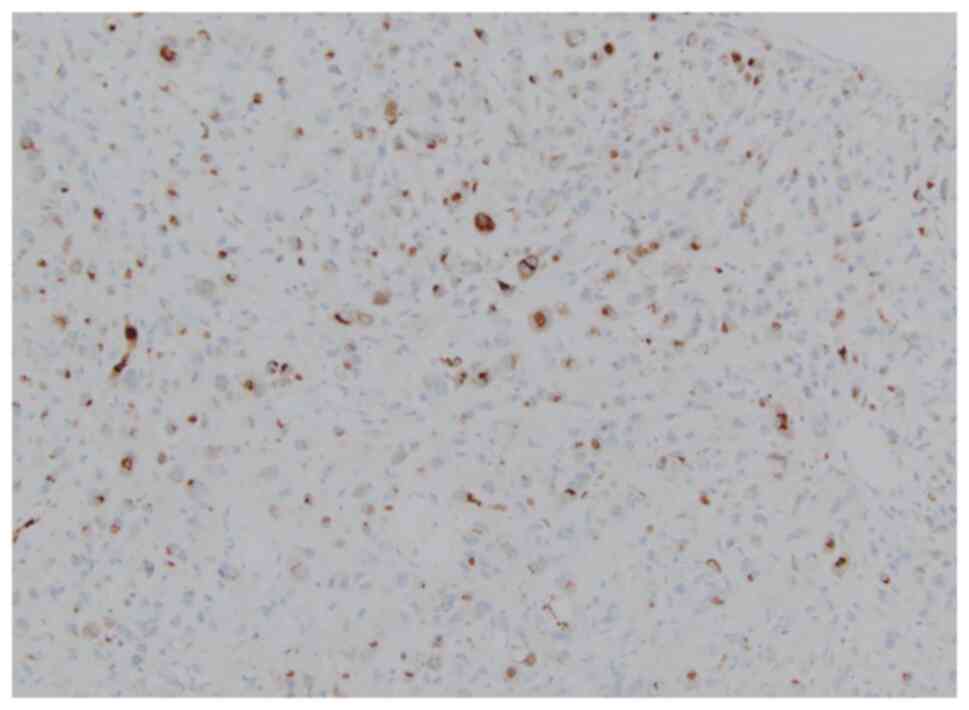
(Ventana Medical Systems, AZ, USA, 518-102425) (Fig. 2A,B). Immunohistochemistry also
showed the tumor cells to be positive for AE1/AE3 (Ventana Medical
Systems, 518-110178), partly positive for GATA3 (Ventana Medical
Systems, 518-111953), and strongly positive for Ki-67 (Ventana
Medical Systems, 518-102456) (Fig.
2C,D,E) but negative for CK7 (Ventana Medical Systems,
518-100902), CK20 (Ventana Medical Systems, 518-101152), CK5/6
(Ventana Medical Systems, 518-109851), and p63 (Ventana Medical
Systems, 518-10961). Eventually, the tumor was pathologically
diagnosed as UCOGCs with muscle invasion. As no standard of care
for UCOGCs has been established, neoadjuvant chemotherapy using
gemcitabine and cisplatin was started prior to radical cystectomy
similar to treatment for usual muscle invasive cancer. A CT scan
showed a large recurrent tumor in the bladder after the second
course of chemotherapy (Fig. 1C),
so radical cystectomy was planned rather than additional
chemotherapy. During the surgical waiting time, at four months
after the first TURBT, the patient suffered from pronounced bladder
tamponade due to bleeding from the tumor, and he underwent
unplanned repeat TURBT (resected tumor volume: 30 g). MRI just
after surgery showed no residual tumor (Fig. 1D). One month later, robot-assisted
radical cystectomy with lymph node dissection and ileal conduit
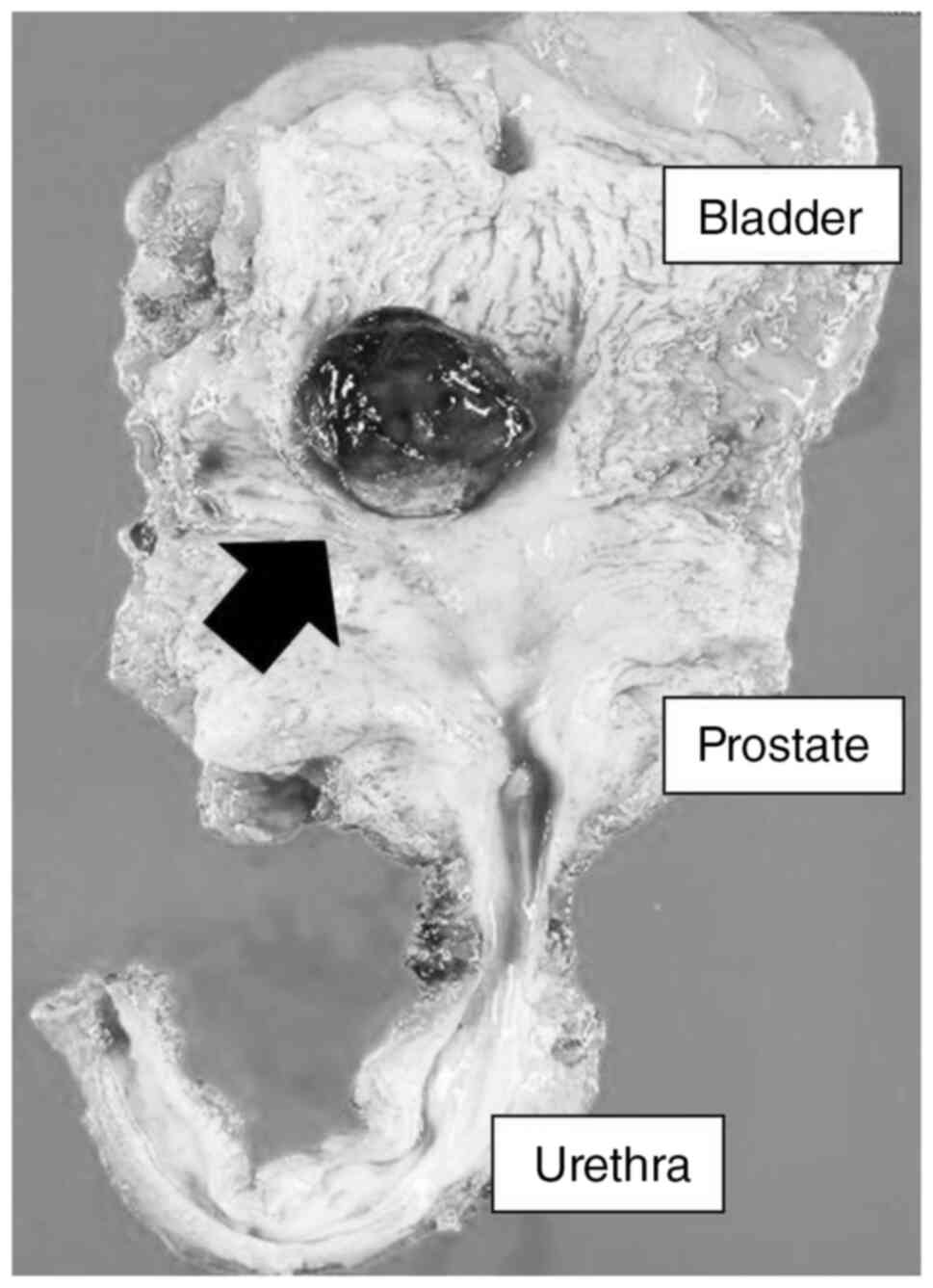
reconstruction were performed. Macroscopically, a large recurrent
tumor of 30 mm in diameter was observed again (Fig. 3), and pathological findings were
invasive UC with OGCs, high grade, pT3b, pN1 (right internal iliac
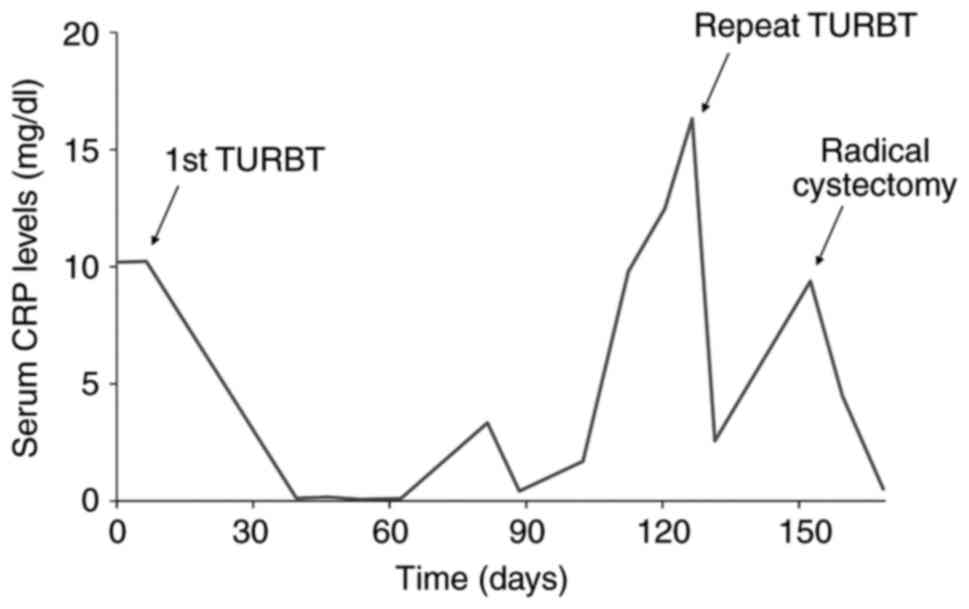
lymph node), RM0. Serum CRP levels were high when a high-volume
tumor was present in the patient (Fig.
4). Additional immunohistochemistry showed tumor cells were
positive for IL-6 (GeneTex, CA, USA, GTX110527) and OGCs were
partly positive (Fig. 5).
Next, we analyzed the genomic features using a
cancer panel test. The area containing predominantly tumor cells
and OGCs was selected by a pathologist, and then the specimen was
macro-dissected from formalin-fixed paraffin-embedded (FFPE) tissue
sections. DNA isolation and sequencing following genome annotation
and curation were performed as previously reported (13). Briefly, DNA was extracted using a
Maxwell RSC DNA FFPE Kit-PKK, Custom (Promega, Fitchburg, WI, USA).
DNA libraries were prepared for subsequent genomic sequencing
following gene amplification using the GeneRead Human Comprehensive
Cancer Panel (160 genes, NGHS-501X; Qiagen). Targeted amplicon
exome sequencing for cancer-related genes was performed using the
Illumina Miseq sequencing platform (Illumina, San Diego, CA, USA).
Genome annotation and curation were performed using GenomeJack
software (Mitsubishi Electric Software Corporation, Tokyo, Japan)
(14). The genes detected with
genetic alterations were as follows: TERT promoter
(-124C>T; ClinVar pathogenic), PIK3CA (E542K; ClinVar
pathogenic), HRAS (G13R; ClinVar pathogenic), ARAF (5
times the copy number amplification), CDKN2A (copy number
loss), TP53 (E285V; ClinVar pathogenic), and ARID1A
(truncate mutation). Tumor mutation burden was 5.9 Mut/Mbp, and
microsatellite instability status was stable.
Discussion
Various pathological subtypes of UC are known. Among
them, UCOGCs is included in the subtype of poorly differentiated UC
(12). Its clinical symptoms are
similar to those of conventional UC (e.g., gross hematuria)
(7-11)
and our patient presented with hematuria and pain on voiding. The
clinical characteristics of the rare subtypes of UC are poorly
understood, and no standard or optimal treatment has been
established due to the limited number of cases (15). In previous reports, surgical
treatment of either TURBT or radical cystectomy was performed for
invasive UC with OGC of the bladder (7-11).
In those cases, both no recurrence of cancer and an aggressive
clinical course with patient deaths were reported. Several subtypes
of UC have been reported as predictive factors for worse clinical
outcomes (16,17). Our patient showed no recurrence
during the 10 months after the surgery without adjuvant
chemotherapy, but the postoperative follow-up period was too short
to evaluate recurrence adequately.
UCOGCs is usually composed of mononuclear carcinoma
cells, host histiocytes, and multinucleated OGCs. A conventional
component of UC often coexists with UCOGCs (12). As shown in our result, OGCs show no
nuclear pleomorphism of the nuclei, and they are positive for CD68
histiocytic markers (12,18).
There are many reports on the relationship between
serum CRP levels and various cancers including bladder cancer
(19,20). The preoperative CRP levels were
reported to be a predictive factor for primary tumor stage, lymph
node metastasis, and cancer-specific and overall survival in muscle
invasive bladder cancer (20). In
the present case, CRP levels were high when tumor volumes were
high. We previously reported that prostate cancer cell lines
secrete IL-6 and IL-8 and that those chemokines promote
CD11b-positive cells to differentiate into osteoclast like
multinuclear cells (21). We infer
that IL-6 secreted from tumor cells may promote OGC formation and
increase CRP levels in our case.
As UCOGCs is a very rare subtype of UC, few genomic
studies have been reported. To the best of our knowledge, only a
single case of genomic testing for UCOGCs has been reported, and it
showed a TP53 mutation (11). UC had a higher rate of genomic
alteration compared to that of other urologic cancers (13). In the present case, we found
pathogenic variants in five genes including TP53 and copy
number alterations in two genes. These genomic alterations were
similar to those in conventional urothelial carcinomas (22-26).
To explain the difference in clinical course between UCOGCs and
conventional UC, further research including whole genome sequencing
will be needed.
There are several reports on genomic analysis of
OGCs other than UC. Mutations in KRAS, BRCA2,
CDKN2A, TP53, SMAD4, and GNAS in
undifferentiated carcinoma with OGCs of the pancreas are reported
(27-29).
A mutation in TP53 was identified in uterine leiomyosarcoma
with OGCs (30). It was also
reported that genetic testing has been linked to treatment for
undifferentiated carcinoma with OGCs of the pancreas. Although
pancreatic cancers generally exhibit a suboptimal response to
immune checkpoint inhibitors, pembrolizumab as a third-line therapy
is more effective for pancreatic cancers of undifferentiated
carcinoma with OGCs showing a high tumor mutation burden (31). Platinum-based chemotherapy is
standard treatment for advanced bladder cancer (32-34).
Mutations in ARID1A, TP53, and MDM2 were
reported as negative predictive factors for platinum-based
chemotherapy (35-38).
In our case, it is possible that some genetic mutation is
responsible for platinum-based drug resistance. Therefore, targeted
therapy based on the result of genomic test could be considered in
next treatment.
In conclusion, we showed genomic alteration in a
patient with UCOGCs. Genetic alterations or IL-6 production may be
associated with increased inflammatory response and OGC formation,
as well as resistance to cisplatin-based chemotherapy. These
results may contribute to further research on UCOGCs to find
precise treatments for this rare disease.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by Gifu Prefecture Medical
Association Research Grant.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KK and KMi conceived the study. KK, KMi, TY, SS,
KMa, KH, YK, HN, SI and TD contributed to data analysis and
interpretation. KK, KMi, SK and SY performed clinical evaluations
and treatment. KK and KMi wrote and edited the manuscript. KK and
KMi confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent participate
This study was approved by the research ethics
committee of Central Japan International Medical Center (approval
no. 2022-013). Written informed consent was obtained from the
patient for this study.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Palmerini E, Picci P, Reichardt P and
Downey G: Malignancy in giant cell tumor of bone: A review of the
literature. Technol Cancer Res Treat.
18(1533033819840000)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Geramizadeh B and Kazemi K:
Osteoclastoma-like giant cell tumor of the liver, an extremely rare
tumor. Hepatitis Monthly. 17(e56097)2017.
|
|
3
|
Angellotti G, Tomasicchio G, Montanaro AE,
Telgrafo M, Mastropasqua MG and Punzo C: Osteoclast-like stromal
giant cells in invasive ductal breast cancer: A case series. Int J
Surg Case Rep. 97(107421)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang YJ, Huang CP, Hong ZJ, Liao GS and Yu
JC: Invasive breast carcinoma with osteoclast-like stromal giant
cells: A case report. World J Clin Cases. 11:1521–1527.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Niwa A, Tomita H, Watanabe N, Kiriyama S,
Hara A and Tanaka T: Case report: A case of gallbladder
carcinosarcoma with osteoclast-like multinucleated giant cells that
was associated with RANK-RANKL signaling. Pathol Oncol Res.
28(1610134)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao N, Mei Y, Yi H, Wang H, Wang Y, Yao Y
and Li C: Case report: Pathological and genetic features of
pancreatic undifferentiated carcinoma with osteoclast-like giant
cells. Pathol Oncol Res. 29(1610983)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Baydar D, Amin MB and Epstein JI:
Osteoclast-rich undifferentiated carcinomas of the urinary tract.
Mod Pathol. 19:161–171. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu PJ, Su CK, Li JR, Yang CR and Chen CL:
Osteoclast-like giant cell carcinoma of the urinary bladder. J Chin
Med Assoc. 72:495–497. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Osman C, Muammer B, Murat O, Tamer A and
Fatih A: Osteoclast-type giant cell carcinoma of the urinary
bladder: An unusual and aggressive variant of urothelial carcinoma.
Urol Case Rep. 23:50–51. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Karasavvidou F, Mitrakas L, Strataki M,
Anastasiou D, Koukoulis G and Tzortzis V: Poorly differentiated
muscle-invasive giant cell tumor of the bladder leads to
unfavorable clinical outcome. J Surg Case Rep.
2022(rjac046)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Satturwar S, Parwani AV, Thomas R,
Bastacky S, Dhir R and Quiroga-Garza GM: The osteoclast-type giant
cell rich carcinoma of urinary bladder: A case series. Pathol Res
Pract. 239(154164)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
WHO Classification of Tumours Editorial
Board: Urinary and male genital tumours 5th edition. International
Agency for Research on Cancer, Lyon, France, 2022.
|
|
13
|
Mizutani K, Hirade K, Sugiyama S, Kato Y,
Nishihara H and Ishihara S: Genomic landscape of treatment-naive
urological cancers using next-generation sequencing-based panel
test in the Japanese population. Int J Urol. 29:909–911.
2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shimozaki K, Hayashi H, Tanishima S, Horie
S, Chida A, Tsugaru K, Togasaki K, Kawasaki K, Aimono E, Hirata K,
et al: Concordance analysis of microsatellite instability status
between polymerase chain reaction based testing and next generation
sequencing for solid tumors. Sci Rep. 11(20003)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Warrick JI: Clinical significance of
histologic variants of bladder cancer. J Natl Compr Canc Netw.
15:1268–1274. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Monn MF, Kaimakliotis HZ, Pedrosa JA, Cary
KC, Bihrle R, Cheng L and Koch MO: Contemporary bladder cancer:
Variant histology may be a significant driver of disease. Urol
Oncol. 33:18.e15–18.e20. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mori K, Abufaraj M, Mostafaei H, Quhal F,
Karakiewicz PI, Briganti A, Kimura S, Egawa S and Shariat SF: A
systematic review and meta-analysis of variant histology in
urothelial carcinoma of the bladder treated with radical
cystectomy. J Urol. 204:1129–1140. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eble JN, Argani P, Grignon DJ and Cheng L:
American Registry of Pathology: Tumors of the kidney, bladder, and
related urinary structures. American Registry of Pathology
Arlington, Virginia, Arlington, Virginia, 2022.
|
|
19
|
Allin KH, Bojesen SE and Nordestgaard BG:
Baseline C-reactive protein is associated with incident cancer and
survival in patients with cancer. J Clin Oncol. 27:2217–2224.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
O'Brian D, Prunty M, Hill A and Shoag J:
The role of C-reactive protein in kidney, bladder, and prostate
cancers. Front Immunol. 12(721989)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mizutani K, Sud S and Pienta KJ: Prostate
cancer promotes CD11b positive cells to differentiate into
osteoclasts. J Cell Biochem. 106:563–569. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nassar AH, Umeton R, Kim J, Lundgren K,
Harshman L, Van Allen EM, Preston M, Dong F, Bellmunt J, Mouw KW,
et al: Mutational analysis of 472 urothelial carcinoma across
grades and anatomic sites. Clin Cancer Res. 25:2458–2470.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Robertson AG, Kim J, Al-Ahmadie H,
Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA,
Akbani R, et al: Comprehensive molecular characterization of
muscle-invasive bladder cancer. Cell. 171:540–556 e525.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pietzak EJ, Bagrodia A, Cha EK, Drill EN,
Iyer G, Isharwal S, Ostrovnaya I, Baez P, Li Q, Berger MF, et al:
Next-generation sequencing of nonmuscle invasive bladder cancer
reveals potential biomarkers and rational therapeutic targets. Eur
Urol. 72:952–959. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Springer SU, Chen CH, Pena MDC, Li L,
Douville C, Wang Y, Cohen JD, Taheri D, Silliman N, Schaefer J, et
al: Non-invasive detection of urothelial cancer through the
analysis of driver gene mutations and aneuploidy. Elife.
7(e32143)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shuai H, Duan X, Zhou JJ, Liu Y and Wu T:
Effect of the TERT mutation on the prognosis of patients with
urothelial carcinoma: A systematic review and meta-analysis. BMC
Urol. 23(177)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang G, Yin J, Ou K, Du Q, Ren W, Jin Y,
Peng L and Yang L: Undifferentiated carcinoma with osteoclast-like
giant cells of the pancreas harboring KRAS and BRCA mutations: Case
report and whole exome sequencing analysis. BMC Gastroenterol.
20(202)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Luchini C, Pea A, Lionheart G, Mafficini
A, Nottegar A, Veronese N, Chianchiano P, Brosens LA, Noë M,
Offerhaus GJA, et al: Pancreatic undifferentiated carcinoma with
osteoclast-like giant cells is genetically similar to, but
clinically distinct from, conventional ductal adenocarcinoma. J
Pathol. 243:148–154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yamamoto S and Sakai Y: A case of
undifferentiated carcinoma with osteoclast-like giant cells of the
pancreas derived from an intraductal papillary mucinous neoplasm.
Clin J Gastroenterol. 14:1263–1268. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen Z, Ji J, Yung E, Martin SE and Walia
S: Uterine leiomyosarcoma with osteoclast-like giant cells: Report
of 2 cases and review of literature. Int J Gynecol Pathol.
43:182–189. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Besaw RJ, Terra AR, Malvar GL, Chapman TR,
Hertan LM and Schlechter BL: Durable response to PD-1 blockade in a
patient with metastatic pancreatic undifferentiated carcinoma with
osteoclast-like giant cells. J Natl Compr Canc Netw. 19:247–252.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Flaig TW, Spiess PE, Abern M, Agarwal N,
Bangs R, Boorjian SA, Buyyounouski MK, Chan K, Chang S, Friedlander
T, et al: NCCN Guidelines® insights: Bladder cancer,
version 2.2022. J Natl Compr Canc Netw. 20:866–878. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cathomas R, Lorch A, Bruins HM, Comperat
EM, Cowan NC, Efstathiou JA, Fietkau R, Gakis G, Hernandez V,
Espinos EL, et al: The 2021 updated European association of urology
guidelines on metastatic urothelial carcinoma. Eur Urol. 81:95–103.
2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Grossman HB, Natale RB, Tangen CM,
Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF,
Wood DP Jr, Raghavan D, et al: Neoadjuvant chemotherapy plus
cystectomy compared with cystectomy alone for locally advanced
bladder cancer. N Engl J Med. 349:859–866. 2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Plimack ER, Dunbrack RL, Brennan TA,
Andrake MD, Zhou Y, Serebriiskii IG, Slifker M, Alpaugh K, Dulaimi
E, Palma N, et al: Defects in DNA repair genes predict response to
neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder
cancer. Eur Urol. 68:959–967. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Teo MY, Bambury RM, Zabor EC, Jordan E,
Al-Ahmadie H, Boyd ME, Bouvier N, Mullane SA, Cha EK, Roper N, et
al: DNA damage response and repair gene alterations are associated
with improved survival in patients with platinum-treated advanced
urothelial carcinoma. Clin Cancer Res. 23:3610–3618.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lee SH, Cheon J, Lee S, Kang B, Kim C,
Shim HS, Park YN, Jung S, Choi SH, Choi HJ, et al: ARID1A mutation
from targeted next-generation sequencing predicts primary
resistance to gemcitabine and cisplatin chemotherapy in advanced
biliary tract cancer. Cancer Res Treat. 55:1291–1302.
2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bagrodia A, Lee BH, Lee W, Cha EK,
Sfakianos JP, Iyer G, Pietzak EJ, Gao SP, Zabor EC, Ostrovnaya I,
et al: Genetic determinants of cisplatin resistance in patients
with advanced germ cell tumors. J Clin Oncol. 34:4000–4007.
2016.PubMed/NCBI View Article : Google Scholar
|